Abstract
The enterocyte is a highly specialized cell of the duodenal epithelium that coordinates iron uptake and transport into the body. Until recently, the molecular mechanisms underlying iron absorption and iron homeostasis have remained a mystery. This review focuses on the proteins and regulatory mechanisms known to be present in the enterocyte precursor cell and in the mature enterocyte. The recent cloning of a basolateral iron transporter and investigations into its regulation provide new insights into possible mechanisms for iron transport and homeostasis. The roles of proteins such as iron regulatory proteins, the hereditary hemochromatosis protein (HFE)–transferrin receptor complex, and hephaestin in regulating this transporter and in regulating iron transport across the intestinal epithelium are discussed. A speculative, but testable, model for the maintenance of iron homeostasis, which incorporates the changes in the iron-related proteins associated with the life cycle of the enterocyte as it journeys from the crypt to the tip of the villous is proposed.
Introduction
Iron is required by almost every organism. The ability of this transition metal to exist in 2 redox states makes it useful at the catalytic center of fundamental biochemical reactions. DNA synthesis, transport of oxygen and electrons, and respiration all require iron. The same properties that make iron useful in each of these reactions also make it toxic. Free iron has the ability to generate oxidative radicals that damage essential biologic components such as lipids, proteins, and DNA. An organism must sense its internal iron load and respond appropriately by altering iron uptake and storage processes. In the case of humans, the amount of iron that is absorbed, rather than the amount that is excreted, is controlled. Inappropriate responses or lack of a response lead to anemia or iron overload.
The most important checkpoint of iron homeostasis in higher organisms is contained in the epithelial cell layer of the duodenum, which is responsible for sensing changes in body iron demands and then adapting to meet them. Within the crypts of the intestine are multipotent precursor cells, some of which migrate onto the villus and differentiate into enterocytes.1,2 The enterocytes are specialized for absorption and transport of iron (Figure1). The precursor cells differ from enterocytes in their expression of proteins related to iron uptake and transport 3-7 (Figure 2). Although its precursor acts only as a sensor of body iron needs, upon differentiation the enterocyte is capable of transporting iron. New proteins required for the absorption, storage, and export of the total dietary iron requirement of the organism are expressed in the enterocyte.
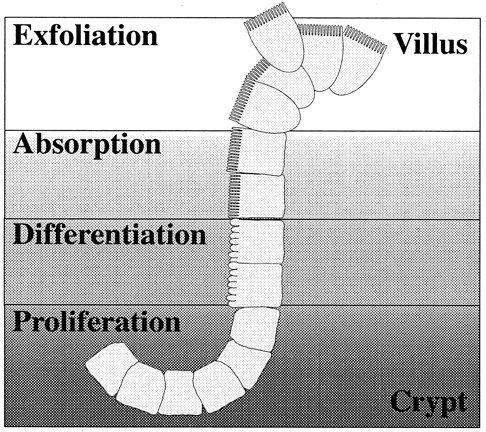
Life cycle of the duodenal enterocyte.
Stem cells present in the crypts of the duodenum proliferate and give rise to precursor cells that further differentiate into absorptive cells or enterocytes. These enterocytes are highly specialized for absorption of micronutrients from the intestinal lumen. Their apical membrane is structured with microvilli and contains enzymes that facilitate transport of nutrients through the epithelial cell layer. The majority of iron absorption is facilitated by these specialized cells. The process of differentiation occurs simultaneously with migration out of the crypt and onto the villus. Zones of cells in similar stages are evident when the crypt/villus junction is viewed in cross section. After the cells have migrated to the villar tip, they are exfoliated and excreted.
Life cycle of the duodenal enterocyte.
Stem cells present in the crypts of the duodenum proliferate and give rise to precursor cells that further differentiate into absorptive cells or enterocytes. These enterocytes are highly specialized for absorption of micronutrients from the intestinal lumen. Their apical membrane is structured with microvilli and contains enzymes that facilitate transport of nutrients through the epithelial cell layer. The majority of iron absorption is facilitated by these specialized cells. The process of differentiation occurs simultaneously with migration out of the crypt and onto the villus. Zones of cells in similar stages are evident when the crypt/villus junction is viewed in cross section. After the cells have migrated to the villar tip, they are exfoliated and excreted.
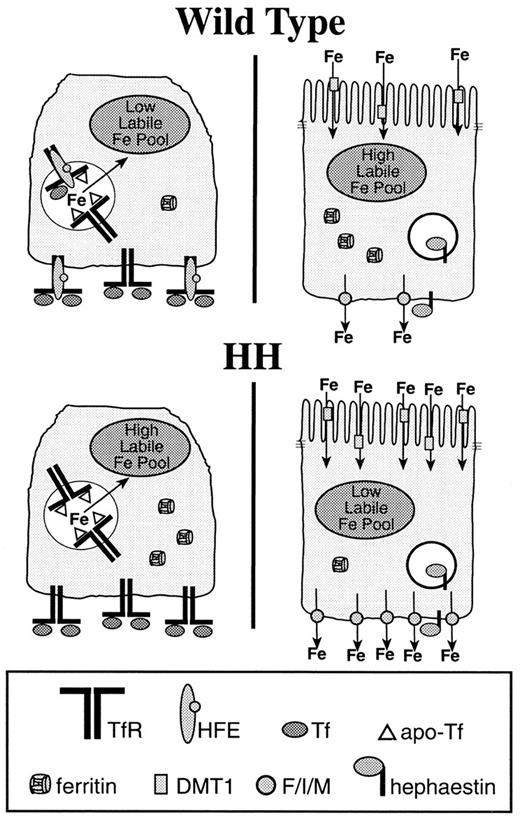
A model for the establishment of the enterocyte iron absorptive set point in the crypt.
Precursor cells (left) present in duodenal crypts express a complement of iron metabolic proteins that are distinct from those of the differentiated enterocyte (right). In the crypt, the transferrin receptor (TfR)–HFE complex facilitates iron uptake from transferrin (Tf) in the plasma and contributes to the labile iron pool. The labile iron pool directly influences iron regulatory protein RNA binding activity and may affect iron-regulated transcription, translation, protein trafficking, and degradation processes. In this way, the iron absorptive capacity, or set point for the enterocyte, may be established in the crypt. When the precursor cell differentiates, the expression of proteins specific to the enterocyte, such as the apical and basolateral iron transporters and hephaestin, is induced. Although the enterocyte precursor absorbs, but does not transport iron, the differentiated enterocyte takes up iron from the duodenum at the apical membrane through the divalent metal transporter (DMT1) and transports it into the plasma. The basolateral iron transporter, Ferroportin1/Ireg1/MTP1 (F/I/M), has been shown to act independently as an iron exporter. The multicopper oxidase, hephaestin, is also required for efficient iron efflux from the enterocyte, but whether it acts through Ferroportin1/Ireg1/MTP1 or another pathway is not yet known.
A model for the establishment of the enterocyte iron absorptive set point in the crypt.
Precursor cells (left) present in duodenal crypts express a complement of iron metabolic proteins that are distinct from those of the differentiated enterocyte (right). In the crypt, the transferrin receptor (TfR)–HFE complex facilitates iron uptake from transferrin (Tf) in the plasma and contributes to the labile iron pool. The labile iron pool directly influences iron regulatory protein RNA binding activity and may affect iron-regulated transcription, translation, protein trafficking, and degradation processes. In this way, the iron absorptive capacity, or set point for the enterocyte, may be established in the crypt. When the precursor cell differentiates, the expression of proteins specific to the enterocyte, such as the apical and basolateral iron transporters and hephaestin, is induced. Although the enterocyte precursor absorbs, but does not transport iron, the differentiated enterocyte takes up iron from the duodenum at the apical membrane through the divalent metal transporter (DMT1) and transports it into the plasma. The basolateral iron transporter, Ferroportin1/Ireg1/MTP1 (F/I/M), has been shown to act independently as an iron exporter. The multicopper oxidase, hephaestin, is also required for efficient iron efflux from the enterocyte, but whether it acts through Ferroportin1/Ireg1/MTP1 or another pathway is not yet known.
Regulation of iron uptake into the body occurs at 2 interfaces of the intestinal epithelium: the apical and basolateral membranes. The apical membrane of the differentiated enterocyte, which faces the intestinal lumen, is specialized for transport of heme and ferrous iron into the cell. At least 3 pathways have been observed for this transport process. The most extensively characterized uptake pathway is via the divalent metal transporter, DMT1 (previously named Nramp2 and DCT1). The DMT1 sequence,8,9 function,10-17 and regulation 6,18-20 have been reviewed recently.21,22 DMT1 is a proton symporter that transports ferrous iron and other divalent metals from the intestinal lumen into the enterocyte.10-12 Its expression is regulated by body iron stores,11,19 but it may also be susceptible to regulation by dietary iron,23,24 or through a recently observed posttranslational mechanism.20 Iron can also be absorbed by the heme-iron uptake pathway that functions as a very efficient means of iron uptake, but the molecular mechanisms of transport into or across the intestinal epithelium have not yet been elucidated.25-27 Finally, a mucin-integrin-mobilferrin pathway has also been identified as a possible means of iron uptake.28
The basolateral membrane mediates the transfer of the iron transported into the intestinal epithelial cells to the rest of the body. Iron that is not exported into the plasma is lost with exfoliation of the intestinal epithelium.2,29,30 The proteins related to iron metabolism on the basolateral membrane of the precursor and mature enterocyte either sense body iron stores, or facilitate regulated iron transport into the plasma. These include the transferrin receptor–hereditary hemochromatosis protein (HFE) complex,31-33 the basolateral iron transporter,7,34,35 and the ceruloplasmin homologue, hephaestin.5 Although the transferrin receptor structure,36 function,37,38 and regulation39 have been well described, the remaining proteins have only recently been identified. Advances toward the understanding of their individual function and regulation are the subjects of this review.
Communication and regulation
The enterocyte receives signals from various tissues as to the relative repletion of iron stores. Although none have been clearly identified, several “regulators” for iron homeostasis have been hypothesized, based on the segregation of iron requirements within an organism. When the amount of iron found in body stores such as the liver, skeletal muscle, and blood drops below a critical level, the stores regulator increases iron uptake until the reserves are replete again.40-43 The stores regulator acts on a pathway that facilitates a slow accumulation of nonheme dietary iron (about 1 mg/d).44 It does not seem to significantly regulate heme-iron uptake.27,43,45 The stores regulator also has the important task of preventing iron overload after ensuring iron needs are met. It reconditions the intestinal epithelium such that iron absorption is reduced in the face of enlarged iron stores, and it reduces the average uptake of iron per day in adulthood when growth requirements decrease.46 Because this regulator must signal between the liver, muscle, and intestine, a soluble component is hypothesized. Serum ferritin,47,48transferrin,48,49 and the serum transferrin receptor50 51 have all been proposed as candidate molecules.
The erythropoietic regulator is a second hypothesized regulator of iron absorption that communicates the erythropoietic demand of the organism rather than directly reflecting iron stores. In support of this hypothesis, individuals with normal, or even increased, iron stores up-regulate iron absorption as marrow iron requirements increase.46,52 An increase in erythropoiesis alone is not enough to increase iron absorption. Rather, the imbalance between the rate of erythropoiesis of the marrow and its iron supply is thought to induce iron absorption.52-54 The absorptive pathway targeted by the erythropoietic regulator is probably distinct from the pathway targeted by the stores regulator, as evidenced by the rate of iron uptake. Anemic individuals can absorb between 20 and 40 mg of iron per day: an increase much greater than that which the stores regulator is capable of producing.46 Like the stores regulator, the erythropoietic regulator is hypothesized to be a soluble component of the plasma, as it must signal between the erythroid marrow and the intestine.55
Recent insights into the regulation of the machinery required for iron absorption have confirmed earlier hypotheses that hypoxia may play a role as an independent regulator that induces intestinal iron absorption.34,56 57 Whether this regulatory pathway is truly distinct from the one induced by the erythropoietic regulator is uncertain. The signaling pathways and molecular components involved in the up-regulation of iron absorption through any of these regulators remain to be determined.
The stores and erythropoietic regulators are humoral factors that maintain iron homeostasis for the entire organism. Other regulatory mechanisms are in place for the maintenance of iron homeostasis for a single cell. Briefly, iron regulatory elements (IREs) are stem loop structures in the 3′ or 5′ untranslated region of several key messenger RNA (mRNA)–encoding proteins of iron metabolism. Iron responsive proteins (IRPs) work in conjunction with these elements to sense and respond to changes in the amount of chelatable iron in the intracellular environment, or the “labile iron pool.” Through the interaction of IRPs with IREs, transferrin uptake increases by stabilizing the transferrin receptor mRNA, whereas ferritin storage of iron decreases by blocking translation of ferritin mRNA. These events result in an increase in the labile iron pool. Conversely, transferrin uptake decreases and ferritin levels increase when intracellular iron concentrations rise. The reciprocal regulation of these 2 proteins has been extensively reviewed for nonpolarized cells elsewhere.58 59
The labile iron pool of the enterocyte appears to regulate a subset of proteins involved in iron homeostasis. Transferrin receptor expression in duodenal crypts responds to increases or decreases in body iron stores.60-63 Similarly, duodenal ferritin levels decrease with the increased transfer of iron to the plasma.63 Much attention has been focused on the role of IRPs in the modulation of enterocyte-specific iron transport proteins, especially proteins with IRE containing messages such as DMT111,18 and the newly identified basolateral iron transporter, Ferroportin1/Ireg1/MTP1.7,34,35 DMT1 has an IRE in the 3′ untranslated region of its message, suggesting it would be degraded, like the transferrin receptor message, in the context of a high labile iron pool. Ferroportin1/Ireg1/MTP1 has an IRE in the 5′ untranslated region of its message, suggesting it would be translated more efficiently, like the ferritin message, in the context of a high labile iron pool. Experiments designed to test for the control of DMT1 and Ferroportin1/Ireg1/MTP1 by IRPs11,18,34,35 have suggested other regulatory mechanisms may be involved in addition to regulation through IRPs. Although the labile iron pool and IRPs influence iron transport across the enterocyte,64-66 other forms of regulation through the labile iron pool such as iron-regulated transcription,67,68 degradation,69,70 and intracellular trafficking events20 35 could also be involved.
The HFE–transferrin receptor complex
HFE-associated hereditary hemochromatosis is a disorder of enterocyte iron regulation characterized by increased dietary iron uptake and resulting in iron overload. Although an increase in transferrin saturation may be observed within the first 2 decades, the complications due to iron overload usually do not present until the fourth or fifth decade of life.71 The defect has been described as an increase in the iron regulatory “set point”42 because affected individuals experience a chronic increase in the rate of iron transfer from the enterocyte to the blood.72 The hereditary hemochromatosis defect has been attributed to a decrease in the amount of functional HFE protein.31 73-78
The first indication of how HFE might regulate iron metabolism came with the discovery that it associates with the transferrin receptor.32,33,79-82 The transferrin receptor is expressed at the cell surface and binds the serum iron transport protein, transferrin, with high affinity. Transferrin releases iron in endosomes following their acidification, after which the iron is transported across the endosomal membrane and targeted for use in iron containing enzymes, or for storage in ferritin.37,38 HFE binds transferrin receptor with an affinity close to that of transferrin, reducing the affinity of the transferrin receptor for transferrin and competing with transferrin binding.33,80,81 83
The close association of HFE with the transferrin-mediated iron uptake pathway84-86 and its location in endosomes81and on the basolateral side of enterocyte precursor cells87(Figure 2) implicates a role for HFE in sensing body iron stores. Transferrin48,49 and the serum transferrin receptor,50,51 2 proteins with which HFE may directly interact, have both been hypothesized as stores regulators. The mechanism by which HFE might facilitate the sensing of body iron stores remains unknown, however. Alternatively, the role of HFE in establishing the set point for iron transport may be independent of body iron stores. HFE-deficient mice are capable of changing the rate of dietary iron uptake with changes in body iron stores.77 88
Indirect evidence implies that the relative concentrations of HFE and transferrin receptor are important in iron loading in mice. An elegant study used the HFE and transferrin receptor knockout mice to determine the relative contributions of these 2 proteins to iron loading. The authors found that mice lacking HFE and one transferrin receptor allele experienced more iron loading than that of HFE knockout mice. The same trend was seen with the mice that have the human hemochromatosis C282Y HFE allele and only one transferrin receptor allele.89Because mice with only one copy of transferrin receptor cannot increase transferrin receptor levels to those of wild-type mice,90this result suggests that the ratio between HFE and transferrin receptor is critical for the maintenance of iron homeostasis. The actual HFE:transferrin receptor stoichiometry is not clear, nor are the relative amounts of each protein in intestinal precursor cells. Studies of HFE and transferrin receptor in transfected cells and in solution suggest the stoichiometry is 1:2, or one HFE per transferrin receptor dimer.80,81 However, the crystal structure indicates a 2:2 stoichiometry is also possible under very high concentrations of HFE (approximately 8 mmol/L).82 Although the iron-loading results of the compound HFE−/− and transferrin receptor+/− knockout mice are informative, they must be interpreted cautiously. The HFE knockout mice with only one transferrin receptor allele also have iron-deficient erythropoiesis. The compound knockout mice may take up more iron than the HFE knockout mice because of the response of the erythroid regulator rather than the stores regulator.89
Surprisingly, overexpression of HFE in cells grown in culture reduces iron uptake and lowers intracellular ferritin levels.81,84-86 This result was initially unexpected because the enterocytes of individuals with hereditary hemochromatosis, who possess little or no functional HFE, appear to have lower ferritin levels than healthy individuals.91 Although the effect on intracellular ferritin is a universal finding, the mechanism by which this might be accomplished is more controversial. Initial studies established that HFE does not reduce transferrin uptake at saturating transferrin concentrations or alter the cycling of the transferrin receptor.84 Other reports attributed differences in iron uptake to a reduction in transferrin uptake92 or a reduction in the rate of transferrin recycling to the cell surface.93
Consistent with its effect on ferritin and transferrin-receptor regulation,81 HFE has been shown to increase the RNA binding activity of IRPs.85,86 The involvement of IRPs is of great interest because of their seemingly universal control over cellular iron regulation, including pathways of iron uptake, storage, and utilization.39,58 IRPs are functional in individuals with hereditary hemochromatosis in that they can respond to fluctuations in iron levels.94,95 These results suggest that IRP RNA binding activity, though necessary, is not sufficient for the maintenance of iron homeostasis in an individual. Whether HFE has direct effects on IRP RNA binding activity as do protein kinase C and nitric oxide, or whether HFE increases IRP RNA binding activity indirectly through a reduction of the labile iron pool, remains to be seen.96-98
Ferroportin1/Ireg1/MTP1
An exciting addition to our understanding of iron metabolism has come with the cloning and characterization of a putative basolateral iron transporter. Several groups have used multiple systems by which to isolate and characterize the gene referred to asIreg134 or MTP135 in mice and weissherbst (weh) in zebrafish.7 The protein product is a putative multiple membrane-spanning transporter that has been shown to function as an iron exporter.7,34,35 Two of the aforementioned studies used aXenopus oocyte expression system to characterize iron export in oocytes expressing Ferroportin1/Ireg1/MTP1.7,34 Both groups coexpressed DMT1 to load oocytes with iron and then assayed for iron efflux in the presence of apotransferrin. One study also indicated that ceruloplasmin was necessary for iron efflux, whereas apotransferrin was not. These results are consistent with the ability of hypotransferrinemic mice to facilitate mucosal iron transfer, despite the lack of transferrin expression.34 99
The localization of Ferroportin1/Ireg1/MTP1 in cells and tissues is consistent with its proposed function of exporting iron from cells. In the duodenum, Ferroportin1/Ireg1/MTP1 localizes to mature enterocytes and is absent from the crypts7,34,35 (Figure 2). The protein is also found in the liver, predominantly in Kupffer cells where iron is scavenged from red blood cells, but some immunostaining also localizes to the hepatocytes.7,35 The specific intracellular localization of Ferroportin1/Ireg1/MTP1 in polarized cells is on the basolateral membranes of placental trophoblasts7 and MDCK cells overexpressing the transporter.34
Investigations aimed at understanding the regulation of this iron exporter have identified several potential levels of regulation. The Ferroportin1/Ireg1/MTP1 mRNA possesses an iron-responsive element in the 5′ untranslated region that binds IRPs.7,34,35 This stem loop structure is similar to other iron-responsive elements found at the 5′ end of mRNAs encoding ferritin, erythroid 5-aminolevulinate synthase, mitochondrial aconitase, and succinate dehydrogenase.34 The protein products of these messages are not translated under low iron conditions because of the binding of IRPs, suggesting Ferroportin1/Ireg1/MTP1 would be down-regulated under the condition of low intracellular iron.58 Although this stem loop structure confers iron-dependent regulation of luciferase in desferoxamine-treated COS7 cells35 and binds IRPs,34,35 the protein levels found in vivo under varying conditions of iron repletion are not entirely consistent with this type of regulation. Immunohistochemical staining for the iron exporter revealed strong reactivity in the Kupffer cells of iron-replete mice with weaker staining in iron-depleted mice, consistent with regulation through the IRPs. However, the reciprocal result was found in the duodenal epithelium; immunohistochemical staining was strong in iron-depleted mice and weaker in iron-replete mice.35
Other levels of regulation must also exist because the Ferroportin1/Ireg1/MTP1 message is increased in the duodenum of hypotransferrinemic mice.34 If translational control of protein synthesis was the only mode of regulation, message levels should not fluctuate. Up-regulation of the message was also observed under hypoxic conditions.34 A hypoxic response is in keeping with some studies that have observed a connection between the erythroid regulator and the mucosal oxygen supply in regulation of iron uptake.100,101 The rapid loading of hepatic iron stores in the hypotransferrinemic mice99 to levels above those of the HFE knockout mice77,78 implicates up-regulation of pathways that can increase iron uptake many times that of the stores regulator, such as that induced by the erythroid regulator.46 As hypotransferrinemic mice suffer severe anemia,49,99 either an erythropoietic or a hypoxic response may be the mechanism by which the Ferroportin1/Ireg1/MTP1 message is up-regulated in the hypotransferrinemic mice. In fact, transfusion of these mice with erythrocytes of wild-type littermates reduced transfer of iron across the basolateral membrane without changing iron uptake across the apical membrane.49 102 Although specific iron transporters were not addressed by these experiments, the data suggest that apical iron uptake may be unaffected by alleviation of anemia, but that transport of iron across the basolateral membrane was down-regulated with an increase in hematocrit. These results are consistent with what we now know concerning regulation of DMT1 and Ferroportin1/Ireg1/MTP1. Although DMT1 regulation would not be expected to change rapidly, Ferroportin1/Ireg1/MTP1 activity would be expected to diminish with an increase in hematocrit and the subsequent increase in oxygen delivery.
Recent findings argue for additional Ferroportin1/Ireg1/MTP1 regulation at the level of protein trafficking. Abboud and Haile35observed that this exporter has significant intracellular localization in enterocytes of iron-replete mice, whereas iron-depleted mice have more pronounced localization of Ferroportin1/Ireg1/MTP1 to the basolateral membrane.35 Similarly, an iron-dependent regulation of DMT1 subcellular localization has been observed in the duodenal epithelium. In this case, control and iron-loaded rats showed predominant staining of DMT1 in intracellular sites of the enterocyte, whereas iron-depleted rats showed predominant staining of DMT1 at the apical membrane.20
Hephaestin
Sex-linked anemia (sla) is a disorder that highlights the selectivity of the enterocyte basolateral iron transport machinery and emphasizes its role in the regulation of iron absorption. Sla mice suffer the paradox of iron-overloaded enterocytes and insufficient amounts of plasma iron for the production of red blood cells. Although dietary iron absorption by the enterocyte is relatively normal, efflux of iron through the basolateral membrane and into the plasma is inhibited.103,104 The iron that remains trapped within the sla enterocyte is hypothesized to be lost with exfoliation of these cells.30 Postnatal anemia is corrected as the mouse matures, but body iron stores remain depleted throughout the lifetime of the mouse, suggesting the total iron demand is never met.105 106
The sequence for hephaestin, the protein product of the gene mutated in the sla mouse,5 has significant homology to the serum protein, ceruloplasmin.107,108 Ceruloplasmin is a serum multicopper ferroxidase required for efficient recycling of iron between storage and donation sites in the liver, reticuloendothelial system, and the blood.109-111 The cloning of hephaestin emphasizes the role of copper in the transfer of iron from the enterocyte to the plasma. Copper is required for efficient iron transport in biologic systems from yeast to mammals and may even be considered to have a regulatory role because of the selectivity of iron transporters for ferrous or ferric iron forms.112 The 194 amino acid deletion in hephaestin that is responsible for the sla phenotype is expected to prevent oxidation of iron and transport across the basolateral membrane of the enterocyte.5 Whether the proposed oxidase activity of the hephaestin is necessary for the selectivity of a specific basolateral iron transporter, the selectivity of another iron transport protein, or to maintain a concentration gradient of ferrous iron across the basolateral membrane is not known.
Unlike ceruloplasmin, the C-terminal portion of the hephaestin sequence has a predicted membrane-spanning domain that would orient the multicopper ferroxidase activity on the cell surface or in the lumen of vesicles to act in concert with an iron exporter. In situ hybridization has localized hephaestin to the enterocytes of the villus, excluding the crypt cells,5 which confirms its role in the transport of iron through these cells (Figure 2). Immunostaining has localized hephaestin to perinuclear compartments,113 indicating that it may be part of a biosynthetic compartment like the trans-Golgi, or that it cycles, like transferrin receptor, between the basolateral membrane and endocytic compartments. The vesicular localization of hephaestin raises the possibility that iron derived from the cytoplasm could be loaded onto a specific carrier such as apotransferrin.114 115 Alternatively, the vesicular localization may prevent interaction of the iron targeted for export with undesired intracellular iron targets. Although hephaestin certainly plays a crucial role in iron efflux from the enterocyte, the actual transporter that shares the iron efflux pathway with hephaestin is not yet known. Future experiments that coexpress Ferroportin1/Ireg1/MTP1 and hephaestin in tissue culture orXenopus expression systems may identify complementary roles for these proteins.
The mutated gene products in both sla and hereditary hemochromatosis have been shown recently to modulate the same iron absorption pathway. HFE knockout mice bred to sla mice have higher hepatic iron levels than wild-type mice, but much lower than HFE knockout mice.89Although these experiments suggest that the additional iron transported in hereditary hemochromatosis utilizes the hephaestin pathway, HFE and hephaestin are not expected to physically interact. HFE is localized to epithelial cells located in the duodenal crypts, whereas hephaestin is localized to epithelial cells located on the duodenal villus. How HFE might modulate hephaestin is not known, but the iron set point established by HFE in the cells of the crypt may determine the activity of hephaestin in the enterocyte.
Model for iron homeostasis
On the basis of these recent developments in the identification of iron transport machinery, we propose a speculative, but testable, model for the maintenance of iron homeostasis (Figure3). Intestinal regulation of iron homeostasis begins in the crypt where the regulatory set point for iron uptake is established. Temporal and spatial separation of regulatory machinery is required for the appropriate regulation of what will be the final level of iron transport across the mature enterocyte. Because the HFE- and transferrin receptor–expressing cells of the intestinal crypt are not fully differentiated116,117 and do not express the DMT1 protein,6 20 they are not sensitive to iron levels in the lumen of the intestine. However, the body stores regulator and possibly the erythropoietic regulator can communicate the status of iron repletion and erythropoiesis through the plasma to these undifferentiated cells. By establishing the absorptive set point of the enterocyte in the undifferentiated state, the duodenum can isolate the signals received from the plasma without the confounding influence of the dietary iron pool derived from the lumen of the intestine, or the iron pool in transit across the epithelial layer.
Hereditary hemochromatosis exemplifies inappropriate regulation of the iron absorptive setpoint in the crypt.
Individuals with hereditary hemochromatosis (HH) have decreased levels of functional HFE. This contributes to a higher labile iron pool in the precursor cells of an individual with hereditary hemochromatosis than an individual with functional HFE (wild type). When the precursor cell of a hereditary hemochromatotic individual differentiates, the labile iron pool will initially be high. We speculate that this increase in the labile iron pool up-regulates machinery responsible for basolateral iron transfer such as Ferroportin1/Ireg1/MTP1 (F/I/M). The increased amount of basolateral iron efflux will lead to a depletion of the labile iron pool, which will increase levels of the apical iron transporter, DMT1. If Ferroportin1/Ireg1/MTP1 is a very stable protein, or its activity exceeds that of DMT1 in the individual with hereditary hemochromatosis, the iron efflux activity of that enterocyte will overcome the uptake activity of DMT1, keeping intracellular iron levels low. This model explains why individuals with hereditary hemochromatosis have higher levels of DMT1 and low ferritin levels in their duodenum and can be tested further by determining the activity of Ferroportin1/Ireg1/MTP1 in hereditary hemochromatotic individuals and controls as well as in HFE-deficient mice.
Hereditary hemochromatosis exemplifies inappropriate regulation of the iron absorptive setpoint in the crypt.
Individuals with hereditary hemochromatosis (HH) have decreased levels of functional HFE. This contributes to a higher labile iron pool in the precursor cells of an individual with hereditary hemochromatosis than an individual with functional HFE (wild type). When the precursor cell of a hereditary hemochromatotic individual differentiates, the labile iron pool will initially be high. We speculate that this increase in the labile iron pool up-regulates machinery responsible for basolateral iron transfer such as Ferroportin1/Ireg1/MTP1 (F/I/M). The increased amount of basolateral iron efflux will lead to a depletion of the labile iron pool, which will increase levels of the apical iron transporter, DMT1. If Ferroportin1/Ireg1/MTP1 is a very stable protein, or its activity exceeds that of DMT1 in the individual with hereditary hemochromatosis, the iron efflux activity of that enterocyte will overcome the uptake activity of DMT1, keeping intracellular iron levels low. This model explains why individuals with hereditary hemochromatosis have higher levels of DMT1 and low ferritin levels in their duodenum and can be tested further by determining the activity of Ferroportin1/Ireg1/MTP1 in hereditary hemochromatotic individuals and controls as well as in HFE-deficient mice.
HFE reduces the amount of iron absorbed from transferrin,84-86,92,93 stepping down the concentration of the labile iron pool (Figure 3). The set point established by the labile iron pool will determine the IRP RNA binding activity,58 iron-regulated transcriptional activity,67,68 and the modulation of iron-dependent posttranslational trafficking20,35 and degradation events.69 70 Thus, even before differentiation to an enterocyte, the set point for intestinal regulation of iron transport is determined by the labile iron pool of its precursor. The regulatory activities are set in place and will perform their respective functions when proteins specific to the differentiated enterocyte are expressed.
Differentiation occurs in a gradient along the crypt/villus axis, turning off the expression of proteins specific to the crypt and inducing the expression of enterocyte-specific proteins.1,3 4 With the initial synthesis of enterocyte-specific proteins, the set point of the labile iron pool that was established in the crypt determines the relative amounts of those proteins that are iron regulated. After differentiation induces expression of the iron transport machinery, changes in the labile iron pool are likely to occur. Whether iron-dependent regulation of these proteins will affect transport through the enterocyte will depend on the stability of the individual proteins. Iron-dependent transcriptional and translational regulation will not affect the activity of proteins with long half-lives, whereas iron-dependent degradation will.
The identification of IREs on the mRNA of DMT1 and Ferroportin1/Ireg1/MTP111,19,34,35 suggest they are iron regulated through IRPs and the labile iron pool. Regulation of these 2 proteins will establish the transport capacity of the fully differentiated enterocyte. Their balance is best exemplified by the hereditary hemochromatosis defect (Figure 3). In the enterocyte precursors of individuals with hereditary hemochromatosis, the concentration of the labile iron pool is increased compared with individuals with functional HFE. On differentiation of the hereditary hemochromatotic enterocyte, the increased labile iron pool increases the amount of Ferroportin1/Ireg1/MTP1 and the capacity for iron transport into the plasma, consistent with the hereditary hemochromatosis defect observed by McLaren and colleagues.72 At the same time, this increased regulatory iron pool reduces DMT1 protein levels.
The enterocytes of individuals with hereditary hemochromatosis have low ferritin levels91 and increased DMT1 message levels15,19 in the face of saturated body iron stores. Although the latter finding may seem to conflict with our model for regulation, we speculate that the levels of DMT1 and ferritin in the enterocytes of hereditary hemochromatotic individuals reflect the final, iron-depleted, steady state achieved by the increased activity of Ferroportin1/Ireg1/MTP1. If Ferroportin1/Ireg1/MTP1 transport surpasses that of DMT1 in individuals with hereditary hemochromatosis, intracellular iron stores from ferritin and the labile iron pool will be depleted, activating IRP RNA binding activity. The increased IRP RNA binding activity may lead to an increase in DMT1 protein, or the decrease in the labile iron pool may alter protein trafficking events and redistribute DMT1 to the apical membrane. Similar regulation leading to increased Ferroportin1/Ireg1/MTP1 protein activity in macrophages may explain why the Kupffer cells of hereditary hemochromatotic individuals are relatively free from iron deposits118,119 and have increased IRP RNA binding activity,95 whereas the rest of the liver is iron loaded. Experiments that quantitate relative DMT1 and Ferroportin1/Ireg1/MTP1 levels and activity in control and hereditary hemochromatosis samples and in HFE-deficient mouse models will reveal the suitability of this model.
Enterocytes may continue to be influenced after differentiation is complete by external signals such as the erythropoietic or hypoxic regulator that has been shown to up-regulate mucosal iron transfer in the hypotransferrinemic mouse.49,102 Up-regulation of Ferroportin1/Ireg1/MTP1 has been observed in the hypotransferrinemic mouse34 and may be the direct result of changes in transcriptional activity induced by either regulator. Because the iron loading of the hypotransferrinemic mouse is more severe than that of the HFE knockout mouse, the regulation of Ferroportin1/Ireg1/MTP1 is likely to differ between these 2 mouse models. A hierarchy of regulation through transcriptional, translational and degradation events, as well as the regulation of accessory proteins like hephaestin is likely to fine-tune the enterocyte iron export machinery that has been described. Experiments that test for differences in Ferroportin1/Ireg1/MTP1 activity in hypotransferrinemic and HFE-deficient mice may reveal differences in its regulation.
Finally, key to further understanding the details of iron homeostasis in the intestine will be the identification of the erythroid and body iron stores regulators and the mechanisms by which they regulate iron transporters, and possibly other iron-related proteins such as HFE and hephaestin.
Supported by National Institutes of Health (NIH) grant DK 54488. C.N.R. was supported in part by the Training Program in Molecular Hematology, T32-HL00781, NIH National Heart, Lung, and Blood Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Caroline A. Enns, Department of Cell and Developmental Biology, L215, Oregon Health Sciences University, Portland, OR 97201-3098; email: ennsca@ohsu.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal