Abstract
The BCR/ABL oncogene results from a balanced translocation between chromosomes 9 and 22 and is found in patients with chronic myeloid leukemia (CML) and in some patients with acute B-lymphoid leukemia. The Bcr/Abl fusion protein is a constitutively active tyrosine kinase that stimulates several intracellular signaling pathways, including activation of Ras through direct binding of the SH2-containing adapter protein Grb2 to Bcr tyrosine 177. A tyrosine-to-phenylalanine mutation (Y177F) at this site blocks the co-association of Bcr/Abl and Grb2 in vivo and impairs focus formation by Bcr/Abl in fibroblasts. However, the Bcr/Abl Y177F mutant can transform hematopoietic cell lines and primary bone marrow cells in vitro, so the importance of the Bcr/Abl–Grb2 interaction to myeloid and lymphoid leukemogenesis in vivo is unclear. We have recently demonstrated the efficient induction of CML-like myeloproliferative disease by BCR/ABL in a murine bone marrow transduction/transplantation model system. The Y177F mutation greatly attenuates the myeloproliferative disease induced by BCR/ABL, with mice developing B- and T-lymphoid leukemias of longer latency. In addition, the v-abl oncogene of Abelson murine leukemia virus, whose protein product lacks interaction with Grb2, is completely defective for the induction of CML-like disease. These results suggest that direct binding of Grb2 is required for the efficient induction of CML-like myeloproliferative disease by oncogenic Abl proteins.
The product of the t(9;22) Philadelphia chromosome translocation, the BCR/ABL oncogene, is found in virtually all patients with the myeloproliferative syndrome, chronic myeloid leukemia (CML), and in approximately 20% of patients with acute B-lymphoblastic leukemia.1 The Bcr/Abl fusion protein is a constitutively active tyrosine kinase that can transform fibroblasts,2factor-dependent hematopoietic cells,3 and primary bone marrow B-cell progenitors4 in vitro. Expression of Bcr/Abl stimulates a diverse array of cell signaling pathways,5including the activation of Ras, phosphatidylinositol 3-kinase, STATs, SAPK/JNK, and c-Myc. Studies with dominant negative mutants have demonstrated that Ras activation is required for the transformation of fibroblasts6 and hematopoietic cells6 7 by Bcr/Abl.
One documented mechanism of Ras activation by Bcr/Abl is through interaction with the Grb2/Sos protein complex.8,9 The SH2 domain of the Grb2 adapter protein binds directly to phosphorylated tyrosine 177 in the N-terminal Bcr portion of the Bcr/Abl fusion protein. A tyrosine-to-phenylalanine mutation (Y177F) at this site blocks co-association of Bcr/Abl and Grb2 in vivo, impairs Ras activation, and decreases focus formation by Bcr/Abl in fibroblasts.8 However, the Bcr/Abl Y177F mutant is still able to activate Ras and transform cytokine-dependent hematopoietic cell lines to become cytokine independent for survival and growth.10,11 Further, Bcr/Abl Y177F is capable of transforming primary bone marrow-derived B-lymphoid progenitors in vitro.11 These conflicting in vitro observations call into question the relevance of the Bcr/Abl–Grb2 interaction to myeloid and lymphoid leukemogenesis in vivo.
A complete understanding of the pathogenesis of human Philadelphia-positive leukemia requires expression of theBCR/ABL oncogene in the hematopoietic system. Currently, the only model in which BCR/ABL induces a form of leukemia similar to CML is the murine bone marrow transduction/transplantation system12 (for review see Van Etten13). Recently, we14 and others15,16 demonstrated the induction of a myeloproliferative disease closely resembling human CML in 100% of mice transplanted with marrow transduced with the p210BCR/ABL oncogene. That system14 allows a direct comparison of the leukemogenic activity of different forms and mutants of activated ABL oncogenes after the transduction of an identical pool of primary hematopoietic cells. In this study, we compared p210 BCR/ABL and the p210 BCR/ABL Y177F mutant for their ability to induce leukemia in the murine bone marrow transduction/transplantation assay. The p210 Y177F mutant was found to be defective for the induction of CML-like myeloproliferative disease in mice. In addition, we demonstrated that p160 v-Abl, a Gag/Abl fusion protein produced by Abelson murine leukemia virus, does not bind to Grb2 and completely lacks the ability to cause CML-like disease.
Materials and methods
Cell culture and transfection
293T cells were grown in Dulbecco modified Eagle's medium supplemented with 10% heat-inactivated fetal calf serum, penicillin/streptomycin, 2 mmol/L L-glutamine, and nonessential amino acids. For transient transfection, cDNA encoding the p210 form of Bcr/Abl, the p210 Y177F point mutant,8 the p160 form of v-Abl, or the p145 murine-type IV c-Abl were introduced into the expression vector pcDNA3 (Invitrogen, Carlsbad, CA). Abl proteins were expressed in 293T cells by modified calcium phosphate transfection as previously described.17
Far Western and immunoprecipitation Western blotting
Transfected 293T cells were lysed in RIPA buffer as previously described,18 and whole cell lysates were either fractionated directly by 5% to 20% gradient sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) or immunoprecipitated with polyclonal anti-Abl (anti-GEX4)19or anti-Grb2 (Santa Cruz Biotechnology, Santa Cruz, CA) antibodies before electrophoresis. After SDS-PAGE, proteins were electroblotted to nitrocellulose membranes. For Far Western analysis, GST-Grb2(SH2) fusion protein20 and parental GST protein were purified from Escherichia coli by affinity chromatography on glutathione agarose (Molecular Probes, Eugene, OR) and hybridized to membranes essentially as described.21 Bound GST proteins were detected by the hybridization of membranes with affinity-purified polyclonal rabbit–anti-GST antibodies22 and enhanced chemiluminescence (Amersham, Arlington Heights, IL). Immunoprecipitated proteins were detected by blotting with monoclonal anti-Abl (8E9; Pharmingen, San Diego, CA) or polyclonal anti-Grb2 antibodies and enhanced chemiluminescence.
Bone marrow transduction/transplantation
ABL oncogenes were introduced into the retroviral vector MSCVneo,23 and high-titer, helper-free retroviral stocks were prepared by transient transfection of 293T cells using thekat ecotropic packaging system.24 Viral stocks were titered by the transduction of NIH 3T3 cells with serial dilutions of stock, followed by the selection for neomycin resistance. Each viral stock had a titer of 3 to 5 × 106 neomycin-resistant colony-forming units per milliliter and lacked detectable replication-competent helper virus, as assessed by a sensitive provirus mobilization assay.25 Titers within this range gave an equivalent proviral copy number in transduced NIH 3T3 cells (approximately 2 proviral copies per diploid genome). Retroviral transduction of bone marrow, followed by transplantation into lethally irradiated syngeneic Balb/c recipient mice, was performed exactly as previously described.14 In all cases, donors were pretreated with 5-fluorouracil (200 mg/kg intravenously) 4 days before bone marrow harvest. In some experiments, genomic DNA was isolated from samples of transduced primary bone marrow just before transplantation. Southern blot analysis indicated that P210 Y177F-transduced bone marrow had a proviral copy number that was equal to or greater than that of P210 wild-type–transduced marrow (data not shown).
Analysis of diseased mice
Premoribund mice were killed by CO2 asphyxiation, and hematopoietic organs and tissues were harvested and analyzed by cytospin, histopathology, and Southern blotting of genomic DNA as previously described.14 Mast cell tumors were identified by basophilic Wright–Giemsa stain. For flow cytometric analysis, tumor cell populations were incubated with antibodies to murine CD90 (Thy1.2), CD8a, CD24, CD43, CD45RA (B220), and CD11b (Mac1), all from Pharmingen, stained with fluorescein isothiocyanate-conjugated donkey anti-rat IgG (Jackson Immunoresearch, West Grove, PA), and analyzed on a FACScan with CellQuest software (Becton Dickinson, Mountain View, CA).
Results
Bcr/Abl Y177F and v-Abl proteins do not bind Grb2
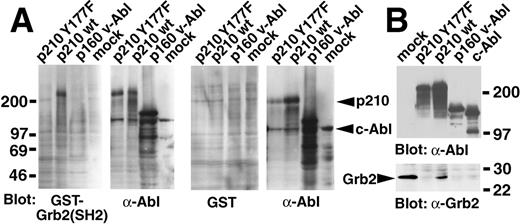
We detected a direct interaction between p210 Bcr/Abl and Grb2 by Far Western blotting, using the Grb2 SH2 domain as a probe (Figure1A), and we found the 2 proteins to co-immunoprecipitate in vivo (Figure 1B). Both interactions were completely abolished by the Y177F point mutation, in agreement with previous reports.8 9 In addition, no binding of Grb2 to the p160 form of v-Abl was detected in either assay (Figure 1).
Lack of association of Grb2 with the Bcr/Abl Y177F mutant and with v-Abl.
(A) Far Western blot. The indicated Abl proteins were expressed by transient transfection in 293T cells, whole cell extracts fractionated by SDS-PAGE and transferred to nitrocellulose filters, and hybridized with a GST-Grb2 (SH2) fusion protein (left pair) or GST alone (right pair). Bound GST protein was detected by anti-GST antibodies and enhanced chemiluminescence. Filters were subsequently stripped and rehybridized with anti-Abl antibody (right panel of each pair). Molecular weight standards are at left, and the positions of p210 Bcr/Abl and c-Abl proteins are indicated by arrowheads at the right. (B) Co-immunoprecipitation. The indicated Abl proteins were expressed by transient transfection of 293T cells, immunoprecipitated with anti-Grb2 (mock-transfected cells only, left lane) or anti-Abl antibodies (all other samples), fractionated by SDS-PAGE, transferred to nitrocellulose, and hybridized with anti-Abl (top panel) or anti-Grb2 (bottom panel) antibodies. Molecular weight standards are at the right, and the position of Grb2 protein is indicated by the arrowhead.
Lack of association of Grb2 with the Bcr/Abl Y177F mutant and with v-Abl.
(A) Far Western blot. The indicated Abl proteins were expressed by transient transfection in 293T cells, whole cell extracts fractionated by SDS-PAGE and transferred to nitrocellulose filters, and hybridized with a GST-Grb2 (SH2) fusion protein (left pair) or GST alone (right pair). Bound GST protein was detected by anti-GST antibodies and enhanced chemiluminescence. Filters were subsequently stripped and rehybridized with anti-Abl antibody (right panel of each pair). Molecular weight standards are at left, and the positions of p210 Bcr/Abl and c-Abl proteins are indicated by arrowheads at the right. (B) Co-immunoprecipitation. The indicated Abl proteins were expressed by transient transfection of 293T cells, immunoprecipitated with anti-Grb2 (mock-transfected cells only, left lane) or anti-Abl antibodies (all other samples), fractionated by SDS-PAGE, transferred to nitrocellulose, and hybridized with anti-Abl (top panel) or anti-Grb2 (bottom panel) antibodies. Molecular weight standards are at the right, and the position of Grb2 protein is indicated by the arrowhead.
p210 Y177F mutant preferentially induces B- and T-lymphoid leukemia in vivo
The leukemogenic activity of p210 BCR/ABL, p210 Y177F, and p160 v-abl were assessed in the mouse model system by retroviral transduction of bone marrow from 5-fluorouracil–treated donors. p210 BCR/ABL induced CML-like disease in all recipients within 3 to 4 weeks of transplantation (Figure2). These animals demonstrated distinctive pathologic features (Table 1), including high peripheral blood leukocyte counts (100-400 × 103/μL), massive splenomegaly (0.7-1.0 g), and infiltration of spleen, liver, and lungs by maturing neutrophils, as previously described.12 14
The p210 BCR/ABL Y177F and v-abloncogenes are defective for the induction of CML-like disease in mice.
Kaplan–Meier-style survival curve for recipients of bone marrow from 5-FU–treated donors transduced with the indicated ABLoncogene. The symbols in each curve designate individual mice (n = 15 for P210 WT, n = 10 for P210 Y177F, and n = 10 for v-abl). Solid black symbols, CML-like disease; open symbols, B-lymphoid leukemia; light gray symbols, macrophage disease; dark gray symbols, mast cell disease; hatched symbols, T-lymphoid leukemia. Animals diagnosed with 2 or more disease processes simultaneously, based on histopathologic and molecular analysis (see text), are indicated by multi-shaded symbols. The difference in survival between recipients of p210 WT-transduced marrow and either p210 Y177F- or v-abl–transduced marrow was highly significant (P < .0001, Mantel-Cox test). One recipient of v-abl-transduced marrow died 285 days after transplantation of nonleukemic causes. Asterisks indicate mice with increased peripheral blood neutrophils (see text).
The p210 BCR/ABL Y177F and v-abloncogenes are defective for the induction of CML-like disease in mice.
Kaplan–Meier-style survival curve for recipients of bone marrow from 5-FU–treated donors transduced with the indicated ABLoncogene. The symbols in each curve designate individual mice (n = 15 for P210 WT, n = 10 for P210 Y177F, and n = 10 for v-abl). Solid black symbols, CML-like disease; open symbols, B-lymphoid leukemia; light gray symbols, macrophage disease; dark gray symbols, mast cell disease; hatched symbols, T-lymphoid leukemia. Animals diagnosed with 2 or more disease processes simultaneously, based on histopathologic and molecular analysis (see text), are indicated by multi-shaded symbols. The difference in survival between recipients of p210 WT-transduced marrow and either p210 Y177F- or v-abl–transduced marrow was highly significant (P < .0001, Mantel-Cox test). One recipient of v-abl-transduced marrow died 285 days after transplantation of nonleukemic causes. Asterisks indicate mice with increased peripheral blood neutrophils (see text).
Characteristics of recipients of p210 BCR/ABLwild-type-transduced and Y177F-transduced bone marrow
| Mouse . | Latency* . | Diagnosis† . | PB WBC/ differential‡ . | Spleen Wt (g) . | Clinicopathologic features1-153 . | Clonality by Southern blot 1-155 . |
|---|---|---|---|---|---|---|
| p210 wild-type recipients1-154 | ||||||
| n = 15# | 21 ± 2 | CML | 323 000 ± 75 000 80% neutrophils | 1.1 ± 0.3 | Hepatosplenomegaly, normal LN, thymus | 9 ± 4 proviral clones common to neutrophils, ma, erythroid, and B-lymphoid cells |
| p210 Y177F recipients | ||||||
| 1 | 72 | B-ALL | ND | 0.48 | Enlarged inguinal LN, normal thymus, hindlimb paralysis | Monoclonal (spleen) |
| 2 | 80 | B-ALL | ND | 0.17 | Bloody pleural effusion (B220+), normal LN, thymus | Biclonal (LN) |
| 3 | 95 | B-ALL + T-ALL (+CML) | 101 000 80% neutrophils | 0.29 | Enlarged LN and thymus, bloody pleural effusion | Biclonal (spleen) 4 clones (pleural effusion) with 2 TCRβ rearrangements 1 unique clone (PB neutrophils) |
| 4 | 105 | T-ALL | 3200 50% neutrophils 20% lymphoblasts | 0.22 | Abdominal tumor with ascites (CD24+, B220−), bloody pleural effusion, enlarged thymus | Monoclonal (tumor, thymus), with clonal biallelic TCRβ gene rearrangements1-160 |
| 5 | 105 | T-ALL | 4700 50% lymphoblasts | 0.15 | Abdominal tumor, bloody pleural effusion, enlarged thymus | Biclonal (spleen), monoclonal (tumor) with clonal biallelic TCRβ rearrangement |
| 6 | 107 | T-ALL | 7400 50% lymphoblasts | 0.07 | Bloody pleural effusion, large thymic mass (Thy-1+, CD8+, B220−) | Biclonal (thymic mass) with 4 TCRβ gene rearrangements1-160 |
| 7 | 109 | T-ALL (+CML) | 356 000 80% neutrophils | 0.46 | Abdominal tumor with ascites, slightly enlarged thymus, splenic neutrophil infiltration | Monoclonal (tumor, thymus), 1 unique clone (PB neutrophils)1-164 |
| 8 | 113 | T-ALL (+CML) | 48 000 70% neutrophils 30% lymphoblasts | 0.20 | Abdominal tumor with ascites (Thy-1+, CD4/8+, B220−), enlarged thymus, pleural effusion | Monoclonal (tumor, thymus), 1 unique clone (PB neutrophils)1-164 |
| 9 | 113 | T-ALL | 10 800 70% neutrophils 20% sm. lymphoid | 0.13 | Abdominal tumor (Thy-1+, CD8+, B220−), bloody pleural effusion, enlarged thymus, paraspinous mass | 3 clones (tumor, thymus) |
| 10 | 155 | T-ALL (+CML) | 73 000 80% neutrophils | 0.18 | Abdominal tumor, pleural effusion (ma) | 4 clones (tumor) |
| Mouse . | Latency* . | Diagnosis† . | PB WBC/ differential‡ . | Spleen Wt (g) . | Clinicopathologic features1-153 . | Clonality by Southern blot 1-155 . |
|---|---|---|---|---|---|---|
| p210 wild-type recipients1-154 | ||||||
| n = 15# | 21 ± 2 | CML | 323 000 ± 75 000 80% neutrophils | 1.1 ± 0.3 | Hepatosplenomegaly, normal LN, thymus | 9 ± 4 proviral clones common to neutrophils, ma, erythroid, and B-lymphoid cells |
| p210 Y177F recipients | ||||||
| 1 | 72 | B-ALL | ND | 0.48 | Enlarged inguinal LN, normal thymus, hindlimb paralysis | Monoclonal (spleen) |
| 2 | 80 | B-ALL | ND | 0.17 | Bloody pleural effusion (B220+), normal LN, thymus | Biclonal (LN) |
| 3 | 95 | B-ALL + T-ALL (+CML) | 101 000 80% neutrophils | 0.29 | Enlarged LN and thymus, bloody pleural effusion | Biclonal (spleen) 4 clones (pleural effusion) with 2 TCRβ rearrangements 1 unique clone (PB neutrophils) |
| 4 | 105 | T-ALL | 3200 50% neutrophils 20% lymphoblasts | 0.22 | Abdominal tumor with ascites (CD24+, B220−), bloody pleural effusion, enlarged thymus | Monoclonal (tumor, thymus), with clonal biallelic TCRβ gene rearrangements1-160 |
| 5 | 105 | T-ALL | 4700 50% lymphoblasts | 0.15 | Abdominal tumor, bloody pleural effusion, enlarged thymus | Biclonal (spleen), monoclonal (tumor) with clonal biallelic TCRβ rearrangement |
| 6 | 107 | T-ALL | 7400 50% lymphoblasts | 0.07 | Bloody pleural effusion, large thymic mass (Thy-1+, CD8+, B220−) | Biclonal (thymic mass) with 4 TCRβ gene rearrangements1-160 |
| 7 | 109 | T-ALL (+CML) | 356 000 80% neutrophils | 0.46 | Abdominal tumor with ascites, slightly enlarged thymus, splenic neutrophil infiltration | Monoclonal (tumor, thymus), 1 unique clone (PB neutrophils)1-164 |
| 8 | 113 | T-ALL (+CML) | 48 000 70% neutrophils 30% lymphoblasts | 0.20 | Abdominal tumor with ascites (Thy-1+, CD4/8+, B220−), enlarged thymus, pleural effusion | Monoclonal (tumor, thymus), 1 unique clone (PB neutrophils)1-164 |
| 9 | 113 | T-ALL | 10 800 70% neutrophils 20% sm. lymphoid | 0.13 | Abdominal tumor (Thy-1+, CD8+, B220−), bloody pleural effusion, enlarged thymus, paraspinous mass | 3 clones (tumor, thymus) |
| 10 | 155 | T-ALL (+CML) | 73 000 80% neutrophils | 0.18 | Abdominal tumor, pleural effusion (ma) | 4 clones (tumor) |
Time in days to premorbidity or death after transplantation of transduced bone marrow.
Diagnosis based on histopathology, immunophenotype, and molecular analysis as discussed in the text: B-ALL, B-lymphoid leukemia; T-ALL, T-lymphoid leukemia; (+CML), indicates mouse with excess circulating neutrophils (see text).
Peripheral blood leukocyte count (per μL) and differential; ND, not determined.
Based on gross and microscopic pathology and flow cytometric analysis; LN, lymph node; ma, macrophage.
Analysis of proviral integration pattern by Southern blot.
Average ± standard error; data from reference 14.
#Four additional recipients of p210 WT-transduced marrow were analyzed in addition to mice previously reported (reference 14).
See Figure 3C.
See Figure 4A.
Mice transplanted with p210 Y177F-transduced bone marrow had strikingly different fates. The survival of these animals was extremely prolonged compared to recipients of wild-type p210-transduced marrow, ranging from 10 to 22 weeks (Figure 2). Further, the Y177F animals succumbed to distinct leukemias (Table 1). Several mice developed B-lymphoid leukemia/lymphoma within 10 to 14 weeks, characterized by peripheral lymphadenopathy, moderate splenomegaly, and a malignant hemorrhagic pleural effusion that appeared to be the cause of death. The tumor cells were positive for B220 (CD45R), CD43, 6C3/BP1, and CD24 cell surface antigens and demonstrated largely germline configuration of the immunoglobulin heavy chain locus (data not shown), consistent with a late pro-B cell phenotype.26 Southern blot analysis of genomic DNA from these tumors demonstrated monoclonal or oligoclonal proviral integration (data not shown). These B-lymphoid leukemias were identical to B-cell malignancies frequently observed in recipients of marrow from non-5-FU–treated donors transduced with wild-typeBCR/ABL.14
Most recipients of p210 Y177F-transduced marrow developed a novel form of T-cell leukemia/lymphoma (Figure 2, Table 1), not previously observed by us in primary recipients of BCR/ABL- transduced marrow. These T-cell lymphomas were characterized by a massive abdominal mesenteric tumor, an extremely enlarged thymus, or both (Figure 3A). Spleen weights (0.1-0.2 g) and peripheral white blood cell counts (5-10 × 103/μL) were only moderately elevated. Abdominal ascites was also observed in several animals. Tumor cells obtained from abdominal tumors and thymic masses expressed the T-lymphoid cell surface antigens Thy-1 (CD90), CD8, CD43, and CD24 (Figure 3B) but were negative for B-cell antigens B220 and 6C3/BP-1 and the myeloid antigen CD11b (Mac-1). DNA from these tumors contained 1 or 2 distinct proviral integrations (Figure 3C), confirming the presence of BCR/ABL in the malignant cells. In addition, these tumors exhibited germline configuration of the immunoglobulin heavy chain genes (data not shown) but clonal rearrangements of the T-cell receptor β locus (Figure 3C), verifying their T-lymphoid origin. Assay of tumor cell-conditioned medium for replication-competent retrovirus was negative (data not shown), excluding the possibility that the T-lymphoma was induced by Moloney murine leukemia virus contaminating the BCR/ABL virus stock.
The Bcr/Abl Y177F mutant preferentially induces T-lymphoid leukemia.
(A) Photomicrograph of section of abdominal tumor that developed 105 days after transplantation with p210 Y177F-transduced marrow (mouse 4, Table 1), hematoxylin–eosin stain, magnification 400×. Note the population of cells with large nuclei, prominent nucleoli, and moderate cytoplasm and the frequent mitotic and apoptotic figures (arrowheads). (B) Flow cytometric analysis of tumor cells from A, demonstrating uniform expression of Thy1.2, CD43, and CD24, variable expression of CD8, but lack of expression of the B-lymphoid marker B220 and myeloid marker Mac1. In each panel, expression of the indicated antigen is shown by the gray plot, whereas staining by an isotype control antibody is shown by the transparent plot. (C) Southern blot analysis of T-lymphoid tumor DNA. Left panel: genomic DNA from abdominal tumor (tum) and thymus (thy) from p210 Y177F mouse 4 demonstrates a single proviral integrant when hybridized with a radioactive probe from the retroviral neo gene, whereas thymus DNA from mouse 8 exhibits 2 different proviral clones. Control DNA (C) demonstrates the intensity of 1 proviral copy per diploid genome. Right panel: tumor and thymus DNA from both these mice show loss of the germline band of the T-cell receptor β chain locus (indicated by the GL arrowhead in the control sample) and clonal rearrangements of both alleles when hybridized with a TCRβ probe. Note the thymic lymphoma from mouse 8 shows 4 new bands, corresponding to distinct biallelic rearrangements in each of the 2 clones. Positions of DNA size markers (in kb) are indicated on the left.
The Bcr/Abl Y177F mutant preferentially induces T-lymphoid leukemia.
(A) Photomicrograph of section of abdominal tumor that developed 105 days after transplantation with p210 Y177F-transduced marrow (mouse 4, Table 1), hematoxylin–eosin stain, magnification 400×. Note the population of cells with large nuclei, prominent nucleoli, and moderate cytoplasm and the frequent mitotic and apoptotic figures (arrowheads). (B) Flow cytometric analysis of tumor cells from A, demonstrating uniform expression of Thy1.2, CD43, and CD24, variable expression of CD8, but lack of expression of the B-lymphoid marker B220 and myeloid marker Mac1. In each panel, expression of the indicated antigen is shown by the gray plot, whereas staining by an isotype control antibody is shown by the transparent plot. (C) Southern blot analysis of T-lymphoid tumor DNA. Left panel: genomic DNA from abdominal tumor (tum) and thymus (thy) from p210 Y177F mouse 4 demonstrates a single proviral integrant when hybridized with a radioactive probe from the retroviral neo gene, whereas thymus DNA from mouse 8 exhibits 2 different proviral clones. Control DNA (C) demonstrates the intensity of 1 proviral copy per diploid genome. Right panel: tumor and thymus DNA from both these mice show loss of the germline band of the T-cell receptor β chain locus (indicated by the GL arrowhead in the control sample) and clonal rearrangements of both alleles when hybridized with a TCRβ probe. Note the thymic lymphoma from mouse 8 shows 4 new bands, corresponding to distinct biallelic rearrangements in each of the 2 clones. Positions of DNA size markers (in kb) are indicated on the left.
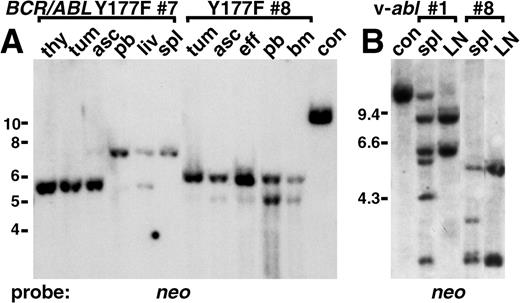
Although all recipients of p210 Y177F-transduced marrow died of lymphoid leukemia, a few animals had some features of CML-like disease (Figure 2, asterisks; Table 1), including moderately increased peripheral blood and bone marrow neutrophils and infiltration of maturing myeloid cells into the spleen and, in some cases, the lungs. In all such mice, analysis of provirus integration patterns showed a single proviral integrant in tissues containing the myeloid cells that was different from those found in the lymphoma cells (Figure4A). This indicates that the excess myeloid cells in these mice were derived from the transduction of a bone marrow target cell that was distinct from the target for induction of the T lymphoma.
Distinct target cells for different leukemias induced by p210 BCR/ABL Y177F and v-abl.
(A) Unique proviral integrants in myeloid cells from mice with T lymphoma and increased neutrophils. Genomic DNA from thymus (thy), abdominal tumor (tum), ascites (asc), spleen (spl), liver (liv), and peripheral blood (pb) of p210 Y177F mouse 7, and tumor, ascites, pleural effusion (eff), peripheral blood, and bone marrow (bm) of mouse 8 (Figure 2, Table 1) were analyzed by Southern blot for provirus integration pattern using a neo probe. In mouse 7 the thymus, tumor, and ascites were composed exclusively of malignant T-lymphoid cells, the liver was a mixture of T-lymphoma and myeloid cells, and the spleen and peripheral blood were exclusively maturing neutrophils (peripheral blood leukocyte count = 356 000/μL). A single provirus is present in the T-lymphoma cells with a distinct single proviral clone in neutrophils. In mouse 8, the tumor was composed exclusively of T-lymphoma cells, the ascites and pleural effusion were mostly lymphoma cells with a small amount of myeloid cells, the peripheral blood was 30% lymphoblasts and 70% neutrophils (peripheral blood leukocyte count = 48 000/μL), and the bone marrow was 50% lymphoblasts and 50% myeloid cells. Again, there is a single provirus in the T-lymphoma cells and a different single proviral clone in the myeloid cells. DNA from a control cell line (con) indicates 1 proviral copy per diploid genome. Positions of DNA size markers (in kb) are indicated on the left. (B) Unique proviral integrants in spleen from mice with v-abl–induced B-lymphoid leukemia and simultaneous macrophage or mast cell disease. Genomic DNA from spleen (spl) and lymph node (LN) from 2 representative mice (animals 1 and 8 in Figure 2, Table 2) with v-abl–induced B-lymphoid leukemia and coexisting macrophage and mast cell tumors (1) or mast cell tumors (8) were analyzed as in A. Lymph nodes from both mice were composed exclusively of malignant lymphoblasts, whereas spleen contained a mixture of lymphoblasts and infiltrating malignant macrophages or mast cells. B lymphoblasts from mouse 1 contained 2 proviral clones, and 4 additional integrants were present in spleen DNA. Similarly, the B-lymphoid disease was biclonal in mouse 8, with 2 additional integrants detected in spleen. DNA from a control cell line (con) indicates 1 proviral copy per diploid genome. Positions of DNA size markers (in kb) are indicated on the left.
Distinct target cells for different leukemias induced by p210 BCR/ABL Y177F and v-abl.
(A) Unique proviral integrants in myeloid cells from mice with T lymphoma and increased neutrophils. Genomic DNA from thymus (thy), abdominal tumor (tum), ascites (asc), spleen (spl), liver (liv), and peripheral blood (pb) of p210 Y177F mouse 7, and tumor, ascites, pleural effusion (eff), peripheral blood, and bone marrow (bm) of mouse 8 (Figure 2, Table 1) were analyzed by Southern blot for provirus integration pattern using a neo probe. In mouse 7 the thymus, tumor, and ascites were composed exclusively of malignant T-lymphoid cells, the liver was a mixture of T-lymphoma and myeloid cells, and the spleen and peripheral blood were exclusively maturing neutrophils (peripheral blood leukocyte count = 356 000/μL). A single provirus is present in the T-lymphoma cells with a distinct single proviral clone in neutrophils. In mouse 8, the tumor was composed exclusively of T-lymphoma cells, the ascites and pleural effusion were mostly lymphoma cells with a small amount of myeloid cells, the peripheral blood was 30% lymphoblasts and 70% neutrophils (peripheral blood leukocyte count = 48 000/μL), and the bone marrow was 50% lymphoblasts and 50% myeloid cells. Again, there is a single provirus in the T-lymphoma cells and a different single proviral clone in the myeloid cells. DNA from a control cell line (con) indicates 1 proviral copy per diploid genome. Positions of DNA size markers (in kb) are indicated on the left. (B) Unique proviral integrants in spleen from mice with v-abl–induced B-lymphoid leukemia and simultaneous macrophage or mast cell disease. Genomic DNA from spleen (spl) and lymph node (LN) from 2 representative mice (animals 1 and 8 in Figure 2, Table 2) with v-abl–induced B-lymphoid leukemia and coexisting macrophage and mast cell tumors (1) or mast cell tumors (8) were analyzed as in A. Lymph nodes from both mice were composed exclusively of malignant lymphoblasts, whereas spleen contained a mixture of lymphoblasts and infiltrating malignant macrophages or mast cells. B lymphoblasts from mouse 1 contained 2 proviral clones, and 4 additional integrants were present in spleen DNA. Similarly, the B-lymphoid disease was biclonal in mouse 8, with 2 additional integrants detected in spleen. DNA from a control cell line (con) indicates 1 proviral copy per diploid genome. Positions of DNA size markers (in kb) are indicated on the left.
p160 v-abl is completely defective for induction of CML-like disease in mice
Animals reconstituted with marrow transduced with v-abl also showed differences from the wild-type p210 BCR/ABL transplant recipients. Survival of these animals was prolonged, though not to the extent of p210 Y177F animals (Figure 2). None of the v-ablanimals developed CML-like disease; rather, these mice developed 3 distinct malignancies in varying combinations (Figure 2, Table2). Eight of 10 mice developed B-lymphoid leukemia that was identical to that observed in p210 Y177F mice. Six of 10 exhibited mast cell disease, characterized by tumors of malignant mast cells in liver, spleen, bone marrow, and occasionally lungs. The histopathology of mast cell disease was similar to that observed previously in recipients of v-abl–transduced bone marrow.27 Finally, 2 of 10 mice developed malignancies of monocytes/macrophages, with tumors predominantly occurring in the liver. As was B-lymphoma, this disease was observed previously in recipients of wild-type p210-transduced marrow when donors were not pretreated with 5-FU.14
Characteristics of recipients of p160 v-abl-transduced bone marrow
| Mouse* . | Latency† . | Diagnosis‡ . | PB WBC/ differential2-153 . | Spleen Wt (g) . | Clinicopathologic features2-155 . | Clonality by Southern blot2-154 . |
|---|---|---|---|---|---|---|
| 1 | 35 | B-ALL + mast | 3000# 50% neutrophils | 0.19 | Enlarged LNs, liver, spleen mast cell infiltration, BM replaced with lymphoblasts and mast cells, hindlimb paralysis | Biclonal (LN), 7 clones (spleen)2-160 |
| 2 | 40 | B-ALL | ND | 0.18 | Enlarged LNs | 3 clones (LN, spleen) |
| 3 | 41 | B-ALL | ND | ND | Enlarged LNs | ND |
| 4 | 50 | B-ALL + mast | 2200 80% lymphoblasts | 0.43 | Enlarged LNs, liver, spleen mast cell tumors | 3 clones (LN), 6 clones (liver) |
| 5 | 53 | B-ALL | 16 300 90% lymphoblasts | 0.25 | Enlarged LNs, bloody pleural effusion (B220+) | Biclonal (LN, effusion) |
| 6 | 54 | B-ALL + mast + ma | 5400 90% lymphoblasts | 0.44 | nl LN size but disrupted follicles, liver mast cell and ma tumors, BM replaced with mast/ma cells | Biclonal (spleen) |
| 7 | 62 | B-ALL + mast | 10 000 | 0.42 | Enlarged LNs, diffuse mast cell infiltration of liver, spleen, lungs | Biclonal (LN), 3 clones (spleen) |
| 8 | 63 | B-ALL + mast | ND | 0.34 | Enlarged LNs, paraspinous mass, extensive liver mast cell tumors | Biclonal (LN), 4 clones (spleen)2-160 |
| 9 | 81 | mast + ma | ND | 0.50 | Normal LNs, extensive liver, spleen mast and ma tumors | 4 clones (spleen) |
| Mouse* . | Latency† . | Diagnosis‡ . | PB WBC/ differential2-153 . | Spleen Wt (g) . | Clinicopathologic features2-155 . | Clonality by Southern blot2-154 . |
|---|---|---|---|---|---|---|
| 1 | 35 | B-ALL + mast | 3000# 50% neutrophils | 0.19 | Enlarged LNs, liver, spleen mast cell infiltration, BM replaced with lymphoblasts and mast cells, hindlimb paralysis | Biclonal (LN), 7 clones (spleen)2-160 |
| 2 | 40 | B-ALL | ND | 0.18 | Enlarged LNs | 3 clones (LN, spleen) |
| 3 | 41 | B-ALL | ND | ND | Enlarged LNs | ND |
| 4 | 50 | B-ALL + mast | 2200 80% lymphoblasts | 0.43 | Enlarged LNs, liver, spleen mast cell tumors | 3 clones (LN), 6 clones (liver) |
| 5 | 53 | B-ALL | 16 300 90% lymphoblasts | 0.25 | Enlarged LNs, bloody pleural effusion (B220+) | Biclonal (LN, effusion) |
| 6 | 54 | B-ALL + mast + ma | 5400 90% lymphoblasts | 0.44 | nl LN size but disrupted follicles, liver mast cell and ma tumors, BM replaced with mast/ma cells | Biclonal (spleen) |
| 7 | 62 | B-ALL + mast | 10 000 | 0.42 | Enlarged LNs, diffuse mast cell infiltration of liver, spleen, lungs | Biclonal (LN), 3 clones (spleen) |
| 8 | 63 | B-ALL + mast | ND | 0.34 | Enlarged LNs, paraspinous mass, extensive liver mast cell tumors | Biclonal (LN), 4 clones (spleen)2-160 |
| 9 | 81 | mast + ma | ND | 0.50 | Normal LNs, extensive liver, spleen mast and ma tumors | 4 clones (spleen) |
One recipient of v-abl-transduced marrow died at 285 days after transplantation without evidence of hematologic malignancy.
Time in days to premorbidity or death after transplantation of transduced bone marrow.
Diagnosis based on histopathology, immunophenotype, and molecular analysis as discussed in the text: B-ALL, B-lymphoid leukemia; mast, tumors of mast cells; ma, macrophage tumors (see text).
Peripheral blood leukocyte count (per μL) and differential; ND, not determined.
Based on gross and microscopic pathology and flow cytometric analysis; LN, lymph node; ma, macrophage; BM, bone marrow.
Analysis of proviral integration pattern by Southern blot.
#Estimated from blood smear.
See Figure 4B.
Several recipients of v-abl–transduced bone marrow developed 2 leukemias simultaneously, whereas 1 mouse had evidence of all 3 diseases (Figure 2). Identification of such mice was possible because of the unique clinicopathologic features of each form of leukemia. The distinct origin of the different leukemias was confirmed by the presence of unique proviral integrants in DNA from the various tumors (Figure 4B). Although some neutrophils were present in bone marrow and, to a lesser extent, in spleen of some v-abl mice, the total lack of increased peripheral blood neutrophils and of lung infiltration indicated that v-abl was completely defective for the induction of CML-like disease under these conditions.
Discussion
Previous work has demonstrated that mice transplanted with p210 BCR/ABL-transduced bone marrow from 5-FU–treated donors develop exclusively CML-like disease.14-16 In contrast, when bone marrow from non-5-FU–treated donors is used14 or when viral stocks of lower titer are used,12 recipients instead develop a mixture of CML-like disease, B-lymphoid leukemia, and monocyte/macrophage tumors. The time of development of these distinct hematologic malignancies differs, with CML-like disease occurring within 3 to 6 weeks of transplantation, B-lymphoid leukemia within 4 to 12 weeks, and macrophage disease within 8 to 20 weeks. Analysis of provirus integration patterns suggests that the bone marrow target cells for induction of the 3 diseases after retroviral transduction are distinct.12,14 The target cell for the CML-like disease exhibits a multilineage repopulating ability consistent with an early multipotential progenitor/stem cell, whereas the target cells for B-lymphoid leukemia and macrophage disease are lineage-restricted and resemble committed B-lymphoid and monocyte progenitors, respectively.14
In the current study, we found the p210 Y177F mutant to be severely compromised in its ability to induce CML-like disease in mice, and we found p160 v-abl to be completely defective. Instead, recipients of bone marrow transduced with these oncogenes developed fatal hematologic malignancies of other lineages, including B- and T-lymphoid leukemia, macrophage disease, and malignant mast cell disease. Similar results for p210 Y177F have been reported by others.28 Although the retroviral target cells for the T-lymphoid leukemia/lymphoma and mast cell tumors have not been characterized, it is plausible that they are committed T-lymphoid29 and mast cell27progenitors as well. Together, these observations suggest a model for understanding the patterns of leukemia induced by p210 Y177F and v-abl after the retroviral transduction of murine bone marrow. If a given form or mutant of an ABL oncogene is defective for the induction of CML-like disease in stem cells but is competent to induce leukemia on transduction of another target cell, transplanted animals will not develop CML-like disease early after transplantation but will succumb later to leukemia induced by the transduction of a sensitive target cell. Our results suggest that the p210 Y177F mutant is greatly deficient in its ability to induce the overproduction of maturing myeloid cells characteristic of the CML-like disease and that, rather than dying from vastly increased levels of neutrophils and the accompanying hepatic and pulmonary dysfunction, the mice survive and later develop lymphoid leukemia. The moderate increase in neutrophils observed in some p210 Y177F recipients suggests that this mutant has some residual capacity for the stimulation of myelopoiesis, and it demonstrates that this model system is sensitive enough to detect partial defects in leukemogenesis in different hematopoietic lineages. The appearance of a unique proviral clone in myeloid cells from these mice supports the hypothesis that the CML-like disease results from the transduction of a target cell distinct from that for the lymphoid leukemias. In related experiments, we have observed that the Bcr/Abl SH2 domain contributes to the efficient induction of CML-like disease in mice but is completely dispensable for the induction of B-lymphoid leukemia.30 Because mice transplanted with p210 SH2 mutant-transduced 5-FU–treated marrow die from B-lymphoid leukemia within 6 to 10 weeks after transplantation (data not shown) whereas most recipients of p210 Y177F-transduced marrow survive longer than this, we infer that the p210 Y177F mutant is also somewhat compromised in its ability to induce B-lymphoid leukemia after marrow transduction. In support of this, when marrow from non-5-FU–treated donors was transduced with p210 Y177F, no recipient developed CML-like disease or B-lymphoid leukemia by 12 weeks after transplantation (data not shown), whereas all recipients of non-5-FU marrow transduced with P210 wild-type died from CML or B-lymphoid leukemia within this period.14 A partial defect in B-lymphoid leukemogenesis by p210 Y177F may explain the conflicting reports of the ability of this mutant to transform bone marrow B-cell progenitors in vitro.8 11 Verification of this model of differential leukemogenesis will require purification and transduction of the individual target cells from bone marrow before transplantation.
Tyrosine 177 was initially identified as a potential Grb2 binding site because the immediate C-terminal amino acid sequence (Y177VNV) contained an asparagine residue at position +2, which is strongly preferred by the Grb2 SH2 domain.31,32The p210 BCR/ABL Y177F mutant lacks direct binding to Grb2 in vitro and fails to co-precipitate with Grb2 in vivo (Figure2).8,9 Because Grb2 is linked to the activation of Ras through binding of the Sos guanine nucleotide exchange protein33 and because both Grb234 and Ras6,7 contribute to Bcr/Abl transformation, it is likely that direct binding of Grb2 to pY177 in Bcr/Abl is required for the pathogenesis of CML. However, there are several other SH2-containing signaling proteins that also prefer asparagine at position +2 to the tyrosine, including the adapter protein 3BP2 and c-Abl itself.32,35 Establishing the identity of the critical effector molecule binding to phosphorylated Y177 in Bcr/Abl will require further study. Assuming direct binding of Grb2 is required for the induction of CML by Bcr/Abl, a mechanistic explanation of this requirement is lacking. Although the Bcr/Abl Y177F mutant does not induce transcription of a Ras-responsive reporter gene in fibroblasts,8 subsequent studies demonstrated that Bcr/Abl Y177F can still stimulate guanosine triphosphate loading of Ras in hematopoietic cells,10 possibly through the activation of pathways such as Shc.11 There are at least 2 models that might explain a requirement for the direct binding of Grb2 by Bcr/Abl for the induction of CML. Perhaps very high levels of Ras activation, mediated by the interaction of Grb2/Sos with Bcr/Abl, are necessary for the stimulation of myelopoiesis by Bcr/Abl. Alternatively, by analogy to Ras activation by transmembrane tyrosine kinases such as epithelial growth factor-receptor,36 it may be the stimulation of Ras in the immediate vicinity of Bcr/Abl that is critical for the pathogenesis of CML. These alternative models of Bcr/Abl signaling can now be experimentally tested.
We found that the product of the transforming gene of Abelson murine leukemia virus, v-Abl, lacked binding to Grb2 in vitro and in vivo and was completely unable to cause CML-like disease under conditions in which p210 Bcr/Abl induced this leukemia with 100% efficiency. These observations strengthen the correlation between binding of Grb2 and induction of CML-like disease by activated forms of Abl. In an early version of the marrow transduction/transplantation model system that used replication-competent helper virus to increase infection efficiency, it was reported that v-Abl did induce CML-like disease, with moderate elevations of neutrophils in peripheral blood and spleen.37 However, the neutrophils in such mice lack the v-abl provirus38 and probably represent a reactive process to cytokines produced by some Abl-induced tumors,39 not a true myeloproliferative disease. Others have described a chronic myeloproliferative disease induced by the transduction of bone marrow with v-abl,40 but careful analysis of the description and histopathology of the disease process suggests that these animals developed a mixture of B-lymphoid leukemia, mast cell tumors, and macrophage disease, similar to those observed in this study. We conclude that the v-Abl tyrosine kinase is unable to induce CML-like disease in this model system. In addition to failing to bind Grb2, the p160 Gag/Abl fusion protein differs from Bcr/Abl both in the nature of the polypeptide fused to Abl and in the site of fusion within Abl, and it lacks the Abl SH3 domain. The SH3 domain of p210 Bcr/Abl is not required for the induction of CML-like disease in mice,41 but, because Bcr motifs other than the Grb2 binding site42 may contribute to Bcr/Abl transformation, further experiments will be necessary to determine whether lack of Grb2 binding is the sole explanation for the inability of v-Abl to induce CML- like disease.
Although other oncogenic tyrosine kinases such as the Tel–Jak2 fusion protein43 induce a myeloproliferative-like disease after the transduction of murine bone marrow, it is clearly not the case that any activated tyrosine kinase will induce CML-like disease in mice. This suggests that the activation of specific signaling pathways, such as that mediated by the Grb2-Bcr/Abl interaction, is necessary for the pathogenesis of CML-like myeloproliferative disease. Careful comparison of the signaling induced in hematopoietic cells by different tyrosine kinases should help identify these mechanisms. This study also validates the interaction between Grb2 and tyrosine 177 of Bcr as a target for rational drug design. Small molecules that specifically disrupt binding of the Grb2 SH2 domain to tyrosine-phosphorylated ligands may have clinical usefulness in the treatment of chronic-phase CML. In conclusion, our study provides strong support for the use of animal models to study human leukemia. In this instance, the bone marrow transduction/transplantation model system has resolved an important unanswered question about the pathogenesis of CML. Our results also illustrate the complexity of analyzing leukemogenesis in vivo. Careful and creative application of this and other animal models should continue to provide novel insights into the molecular pathophysiology of human leukemia.
Acknowledgments
We thank Dr Ann-Marie Pendergast for providing the Bcr/Abl Y177F mutant, Dr Wojciech Swat for the gift of the TCR probe, Dr Jim Griffin for the GST-Grb2(SH2) construct, and Dr Warren Pear for helpful discussions and for communicating data before publication.
Supported in part by National Institutes of Health grants CA09595 (R.P.M.) and CA57593 (R.A.V.E.).
R.A.V.E. is a Scholar of the Leukemia Society of America and the Carl and Margaret Walter Scholar in Blood Research at Harvard Medical School.
Reprints:Richard A. Van Etten, Center for Blood Research, Department of Genetics, Harvard Medical School, 200 Longwood Avenue, Boston MA 02115; e-mail: vanetten@cbr.med.harvard.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal