Abstract
Although long-term repopulating hematopoietic stem cells (HSC) can self-renew and expand extensively in vivo, most efforts at expanding HSC in vitro have proved unsuccessful and have frequently resulted in compromised rather than improved HSC grafts. This has triggered the search for the optimal combination of cytokines for HSC expansion. Through such studies, c-kit ligand (KL), flt3 ligand (FL), thrombopoietin, and IL-11 have emerged as likely positive regulators of HSC self-renewal. In contrast, numerous studies have implicated a unique and potent negative regulatory role of IL-3, suggesting perhaps distinct regulation of HSC fate by different cytokines. However, the interpretations of these findings are complicated by the fact that different cytokines might target distinct subpopulations within the HSC compartment and by the lack of evidence for HSC undergoing self-renewal. Here, in the presence of KL+FL+megakaryocyte growth and development factor (MGDF), which recruits virtually all Lin−Sca-1+kit+ bone marrow cells into proliferation and promotes their self-renewal under serum-free conditions, IL-3 and IL-11 revealed an indistinguishable ability to further enhance proliferation. Surprisingly, and similar to IL-11, IL-3 supported KL+FL+MGDF-induced expansion of multilineage, long-term reconstituting activity in primary and secondary recipients. Furthermore, high-resolution cell division tracking demonstrated that all HSC underwent a minimum of 5 cell divisions, suggesting that long-term repopulating HSC are not compromised by IL-3 stimulation after multiple cell divisions. In striking contrast, the ex vivo expansion of murine HSC in fetal calf serum-containing medium resulted in extensive loss of reconstituting activity, an effect further facilitated by the presence of IL-3.
Introduction
In addition to their pluripotentiality, long-term repopulating hematopoietic stem cells (HSC) have a unique ability to undergo self-renewing cell divisions, as illustrated through their expansion in vivo during ontogeny and after transplantation.1-3
The self-renewing potential of HSC has stimulated extensive efforts at mimicking this process in vitro to develop clinical applications, such as retroviral-mediated stem cell gene therapy, and to expand the number of stem cells to improve the quality of stem cell transplants.3-7 It is clearly established that a minimum number of long-term repopulating HSC (LTRC) is required to ensure durable engraftment after transplantation,6 and LTRC may be the best short-term repopulating cells.8 9
Retroviral marking studies in mice have supported that LTRC can undergo self-renewal in vitro.10-13 However, with the exception of one recent study,14 the objective of expanding ex vivo the number of murine LTRC remains elusive.3 Rather, most previous studies have implicated that ex vivo culture of HSC results in compromised, or at best maintained, LTRC activity.3 15-19Thus, the mechanisms governing HSC self-renewal remain to be established.
As the number of cloned cytokines promoting the growth of candidate stem cells has increased, so has the focus on their potential role and their use in stem cell expansion.6,7,20,21 The lack of a net stem cell expansion under such conditions could be explained by an inability of the cytokines to promote HSC divisions, and thus the remaining stem cell activity could be derived from cytokine-unresponsive stem cells. In fact, findings from some studies have supported this.17,22 Conversely, the finding in one study of increased LTRC after ex vivo expansion14 would be difficult to reconcile unless the repopulating HSC had undergone cell divisions. In support of this, recent studies23 from our laboratory, in which murine HSC were successfully expanded or maintained in response to defined cytokines, demonstrated that most, if not all, the reconstituting activity was retained in cells that underwent multiple cell divisions. Significantly, these studies unequivocally demonstrated that combinations of early-acting cytokines efficiently promote LTRC to undergo self-renewing divisions.
The heterogeneous expression of various cytokine receptors on HSC could explain why some cytokines might be more efficient than others at promoting the recruitment of HSC into proliferation,24 a requisite for HSC expansion. More intriguingly, some studies have suggested that certain cytokines, in particular IL-3, may have a negative impact on in vitro stem cell expansion in mice16,19,25-27 and in humans.28,29 If this effect is mediated by direct action on HSC, it could implicate a novel negative regulation of stem cell self-renewal by IL-3, as previously proposed.16,27 Evidence for this would be of great importance because the role of cytokines in regulating the fate of HSC is controversial30,31 and because most data support a permissive rather than an instructive role of cytokines.32-35 However, an important limitation to the studies suggesting that IL-3 negatively affects long-term repopulating HSC is the lack of evidence for HSC self-renewal under the conditions used.22 In addition to potentially inducing commitment or maturation rather than self-renewal, there are multiple alternative mechanisms that may explain why IL-3 negatively affects stem cell expansion. One is inhibition of the number of LTRC recruited into proliferation. Although they usually promote growth, colony-stimulating factors have, under special circumstances, been shown to suppress growth.20 Another alternative mechanism concerns the unique effects on the cell cycle distribution of HSC because reconstituting stem cells have been proposed to reside predominantly in G0.3,17,36 A third alternative mechanism is that the negative effects of IL-3 may not be directly mediated on the HSC but may be mediated through effects on other cells in culture, resulting in the activation of negative regulators and pathways. In this regard, it is noteworthy that the ex vivo expansion studies that have yielded the most promising results have used purified target cells.14,15,19,37-40 41
To better dissect the mechanisms governing the negative influence of IL-3 on in vitro HSC expansion, we used a system in which we recently demonstrated that c-kit ligand (KL), flt3-ligand (FL), and megakaryocyte growth and development factor (MGDF) efficiently promote proliferation of murine Lin−Sca+kit+ (LSK) cells with sustained long-term repopulating ability.23 Significantly, this cytokine combination promoted the recruitment of virtually all LSK HSC into proliferation. Therefore, we investigated to what degree IL-3 would affect recruitment, total proliferation, and LTRC expansion of murine stem cell populations. For comparison we used IL-11, which has been implicated as positively affecting HSC expansion.2,14,19 42-44 Surprisingly, the presence of IL-3 in KL+FL+MGDF- stimulated serum-free (SF) cultures for as much as 10 days was associated with enhanced multilineage reconstituting activity, comparable to that obtained in the absence of IL-3 or in the presence of IL-11 and despite all LTRC undergoing multiple cell divisions. Thus, IL-3 does not (negatively) affect stem cell self-renewal under conditions that allow the expansion of murine LTRC activity.
Materials and methods
Hematopoietic growth factors
Recombinant human (rh) megakaryocyte growth and development factor (MGDF), recombinant human granulocyte colony-stimulating factor, and recombinant mouse granulocyte–macrophage colony-stimulating factor (GM-CSF) were generously provided by Amgen (Thousand Oaks, CA). Recombinant mouse mast cell growth factor (kit ligand; KL) and recombinant human fms-like tyrosine kinase-3 (flt3) ligand were kind gifts of Immunex (Seattle, WA). Recombinant human erythropoietin (EPO) was a generous gift of Boehringer Mannheim (Mannheim, Germany). rhIL-3 was from PeproTech (Rocky Hill, NJ). rhIL-6 and rhIL-11 were kind gifts of Genetics Institute (Cambridge, CA). Cytokines were used at predetermined optimal concentrations: 20 ng/mL for IL-3 and GM-CSF; 5 U/mL for EPO; 50 ng/mL for all other cytokines.
Purification of Lin−Sca-1+c-kit+ and Lin−Sca-1+c-kit+CD34−bone marrow cells
Isolation of Lin−Sca-1+c-kit+ (LSK) cells was performed as described previously.45-47 Briefly, lineage depleted (Lin−) bone marrow (BM) cells were isolated from 6- to 12-week-old C57Bl/6 (Ly5.2+) or congenic B6.SJL-PtprcaPepb/BoyJ (Ly5.1+) mice by incubating BM cells (at 250 × 106/mL) with a cocktail of purified lineage-specific rat antimouse antibodies (against B220, CD3, CD4, CD8, Mac-1, Gr-1, Ter-119; all from PharMingen, San Diego, CA). Lin− BM cells were subsequently enriched with sheep antirat IgG (Fc)-conjugated immunomagnetic beads (Dynal, Oslo, Norway). Lin− cells were incubated for 30 minutes on ice with a phycoerythrin-conjugated sheep antirat antibody (Southern Biotechnology, Birmingham, AL) and subsequently stained with rat antimouse Sca-1 fluorescein isothiocyanate (FITC) and c-kit allophycocyanine (APC) antibodies (or isotype-matched control antibodies; PharMingen). LSK cells were sorted on a FACSVantage (Becton Dickinson, San Jose, CA) for cells expressing Sca-1 and c-kit at high levels and lacking expression of lineage antigens. Reanalysis of LSK cells on a FACSCalibur (Becton Dickinson) showed reproducibly a purity of 96% to 99%. To purify LSKCD34− cells,48Lin− cells were incubated with CD34-FITC, Sca-1–PE, and c-kit–APC antibodies (or isotype-matched control antibodies; PharMingen). Cells were subsequently sorted for the dual expression of Sca-1 and c-kit and the lack of detectable expression of CD34 (lowest 5% to 7% of LSK cells).
Single-cell cultures
LSK cells or LSKCD34− cells (120 cells/group) were seeded in Terasaki plates (Nunc, Kamstrup, Denmark) at a density of 0.25 cell/well (LSKCD34−) or 1 cell/well (LSK) in 20 μL X-Vivo 15 (BioWhittaker, Walkersville, MD) supplemented with 1% detoxified bovine serum albumin (BSA; StemCell Technologies, Vancouver, Canada) as previously reported.47 Wells were scored for cell growth after 10 to 12 days of culture at 37°C. Because the statistical probability of a well (based on Poisson distribution) not receiving a cell is 37%, when plated at a density of 1 cell/well, the theoretical maximum number of expected clones was 76 for each group. In some experiments, each well was inspected within 12 hours of plating, and only wells containing 1 cell were included in the experiment, giving similar results.
Ex vivo expansion cultures
Ex vivo expansion cultures were performed using either serum-free (X-Vivo 15 with 1% BSA; BioWhittaker) or serum-containing medium (IMDM supplemented with 20% fetal calf serum [FCS]; BioWhittaker). Cell densities were never allowed to exceed 106 cells/mL. This was possible by seeding cells at low numbers (300 cells/mL) and adding prewarmed fresh medium and cytokines on day 7 of culture. At the time of harvest, cells were recovered, and each flask/well was washed twice with phosphate-buffered saline (PBS) without Ca/Mg (BioWhittaker) and cell dissociation buffer (Life Technologies, Gaithersburg, MD) to recover additional adherent cells. Cells were then washed and diluted in serum-free media together with competitor cells to make a final injection volume of 0.5 mL/mouse.
High-resolution cell division tracking of candidate murine stem cells
Staining and flow cytometric procedures for cell division tracking have been described previously.23 49 Briefly, 5- and 6-carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) from a 5 mmol/L stock in dimethyl sulfoxide was added to cells (at 5 × 106 cells/mL) in PBS to give a final concentration of 1 μmol/L. Cells were incubated at 37°C for 10 minutes, and the staining was stopped by adding a 10-fold excess of PBS with 10% to 20% FCS, after which cells were washed twice. To allow unbound dye to diffuse from cells, labeled cells were incubated for 12 to 15 hours at 37°C in serum-free media supplemented with KL (50 ng/mL) to promote stem cell survival rather than proliferation. Cells were subsequently stained with rat antimouse Sca-1 phycoerythrin and anti-c-kit APC and were sorted based on the dual expression of Sca-1 and c-kit from a 40- to 50-channel–wide sort gate set around the mean fluorescent channel for CFSE. Sorted cells were used in expansion cultures with indicated cytokines and, starting at 20 hours, were analyzed for “proliferation history” on a FACSCalibur (Becton Dickinson) at 12-hour intervals until 140 hours using identical instrument settings. As a control for undivided cells, a sample of freshly sorted cells was fixated in 2% paraformaldehyde (Sigma). We noted that some (though limited) CFSE diffusion appeared after the 12- to 15-hour preincubation (Bryder D, Jacobsen SEW, unpublished observation, 1998). However, because this diffusion of CFSE was limited and was never close to one cell division within a 12-hour interval, it was possible to determine accurately the number of cell divisions by overlaying histograms from each analysis time point.
In vivo reconstitution experiments
Animal experiments were conducted after approval was obtained from the local ethics committee. Ly5.1+ LSK or LSK CD34− cells were used as donor cells. Eight- to 12-week-old C57bl/6 mice (Ly5.2) were transplanted with freshly isolated HSC or with the expansion equivalent of the same number of HSC, together with Ly5.2+ competitor cells (150 000 or 200 000 unfractionated BM cells). This was done in part to allow the quantification of reconstitution activity and in part to ensure the survival of mice transplanted with potentially compromised grafts. Four to 8 hours before transplantation, each mouse received a lethal dose of irradiation of 950 cGy using a cesium Cs 137 source (Instrument AB Scanditronix; Husbyborg, Uppsala, Sweden). All mice were kept in individually ventilated cages throughout the experiments and given sterile food and autoclaved acidified water.
Peripheral blood was drawn from transplanted mice from the retro-orbital sinus and analyzed at 6 weeks and at 4 months after transplantation for donor (Ly5.1+) and for competitor/recipient-derived (Ly5.2+) reconstitution by flow cytometry. Lineage distribution was examined using antibodies against CD3ε (T cells), B220 (B cells), and a combination of Mac-1/Gr-1 (myeloid cells) along with antibodies against Ly5.1 and Ly5.2 (all from PharMingen).
For secondary transplantations, a half-femur equivalent (representing approximately 5% of the total BM) from primary recipients was injected into each lethally irradiated secondary recipient. Peripheral blood from secondary transplanted mice were analyzed 3 to 4 months after transplantations, as described above.
Statistics
The Mann-Whitney U test was used throughout to determine the statistical significance between the treatment groups.
Results
We recently demonstrated that ex vivo culture of LSK cells in serum-free medium in response to KL+FL+MGDF (KFM) efficiently promotes stem cell proliferation while sustaining long-term reconstituting activity.23 Preliminary studies in our laboratory suggested that IL-11, which, in contrast to IL-3, has been thought to affect stem cell expansion positively,2,14,19 42-44 had effects similar to those of IL-3 on the KFM-induced proliferation of LSK cells. If this is so, this could provide a unique system for determining how 2 cytokines with comparable effects on stem cell proliferation might have opposing effects on stem cell fate.
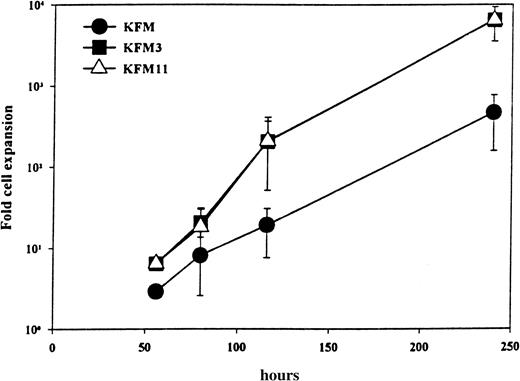
The kinetics and extent of cellular expansion of LSK cells incubated in KFM3 and KFM11 were indistinguishable (Figure1). Whereas KFM resulted in a 460-fold cellular expansion at 240 hours, as much as 6300- and 6400-fold cell expansion was observed with the addition of IL-3 and IL-11, respectively. The addition of IL-3 or IL-11 did not alter the number of emerging LSK clones (all combinations tested resulted in the recruitment of virtually all LSK cells), though both factors enhanced the average clone size (Table 1). Significantly, this finding would imply that any potential differences observed in the abilities of KFM, KFM3, and KFM11 to maintain or expand LTRC would probably not be explained by the recruitment of different stem cell populations into proliferation.
IL-3 and IL-11 display comparable ability to enhance KL+FL+MGDF-stimulated proliferation of Lin−Sca-1+c-kit+ cells.
LSK cells cultured in serum-free medium and indicated growth factors were evaluated for total cellular expansion after different periods of time. All data represent the mean (± SD) from 2 to 3 experiments.
IL-3 and IL-11 display comparable ability to enhance KL+FL+MGDF-stimulated proliferation of Lin−Sca-1+c-kit+ cells.
LSK cells cultured in serum-free medium and indicated growth factors were evaluated for total cellular expansion after different periods of time. All data represent the mean (± SD) from 2 to 3 experiments.
IL-3 and IL-11 enhance proliferation but do not affect the level of recruitment of Lin−Sca-1+c-kit+ cells into proliferation in response to KL + FL + MGDF
| . | Clone size . | Total . | ||
|---|---|---|---|---|
| 1 . | 2 . | 3 . | ||
| Medium | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| KFM | 12 (8) | 26 (6) | 39 (1) | 77 (13) |
| KFM3 | 9 (1) | 7 (1) | 63 (11) | 79 (11) |
| KFM11 | 10 (6) | 2 (3) | 62 (12) | 74 (9) |
| . | Clone size . | Total . | ||
|---|---|---|---|---|
| 1 . | 2 . | 3 . | ||
| Medium | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| KFM | 12 (8) | 26 (6) | 39 (1) | 77 (13) |
| KFM3 | 9 (1) | 7 (1) | 63 (11) | 79 (11) |
| KFM11 | 10 (6) | 2 (3) | 62 (12) | 74 (9) |
LSK cells were seeded at 1 cell/well in serum-free medium and cytokines as indicated. After 10 days of incubation, cultures were scored for clonal growth. Size criteria were: I, 2 to 49 cells; II, 50 cells to 10% well covered with cells; III, more than 10% well covered. Results are presented as mean (±SD) from two independent experiments.
Because HSC may undergo only a limited number of self-renewal divisions and have been demonstrated to undergo asymmetric and asynchronous cell divisions during in vitro culture,25,50 51 it may be possible to explain differences between cytokines in promoting ex vivo stem cell expansion by the distinctions in the number of induced stem cell divisions. To address this specifically, LSK cells were stained with CFSE to allow high-resolution tracking of the number of cell divisions undergone in response to KFM, KFM3, and KFM11 (Figure2). Both IL-3 and IL-11 increased the average number of KFM-stimulated cell divisions after 44 hours culture, demonstrating that each cytokine acts at an early stage to enhance the proliferative activity of LSK cells. Furthermore, CFSE profiles (performed at 12-hour intervals) demonstrated indistinguishable kinetics for KFM3- and KFM11-induced cell division. Of particular relevance for the subsequent LTRC ex vivo expansion experiments, virtually all LSK cells underwent at least one cell division by 68 hours in response to KFM3 and KFM11, whereas no cells underwent fewer than 5 divisions after 140 hours of culture (Figure 2). Thus, KFM3 and KFM11 reveal an identical and efficient pattern of promoting LSK recruitment and proliferation in vitro.
Effects of IL-3 and IL-11 on the “proliferation history” of Lin−Sca-1+c-kit+cells cultured in the presence of KL+FL+MGDF.
CFSE-stained LSK cells were cultured in serum-free medium in the presence of the indicated growth factors. At the indicated time points, cells were analyzed by flow cytometry to determine their proliferation history. In each histogram, the number of cell divisions is indicated. Results are from 1 of 3 representative experiments.
Effects of IL-3 and IL-11 on the “proliferation history” of Lin−Sca-1+c-kit+cells cultured in the presence of KL+FL+MGDF.
CFSE-stained LSK cells were cultured in serum-free medium in the presence of the indicated growth factors. At the indicated time points, cells were analyzed by flow cytometry to determine their proliferation history. In each histogram, the number of cell divisions is indicated. Results are from 1 of 3 representative experiments.
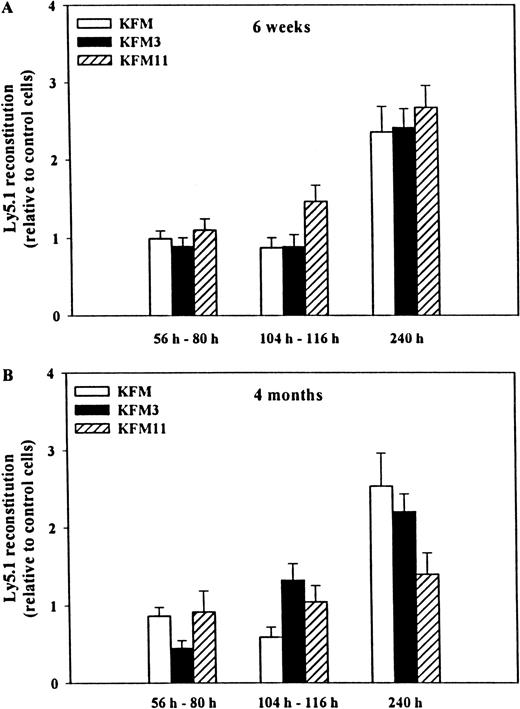
We next investigated the ability of KFM, in the absence or presence of IL-3 or IL-11, to expand the in vivo multilineage reconstituting activity of LSK cells. Because the extent or length of ex vivo culture could affect the level of HSC activity recovered,14 we specifically investigated reconstitution activity after 56 to 80 hours, 104 to 116 hours, and 240 hours of culture in serum-free medium. At each time point, the total cellular expansion was established (Figure1). Cells cultured in all 3 cytokine combinations for 56 to 80 hours and for 104 to 116 hours had similar and sustained multilineage reconstituting activity 6 weeks after transplantation (when compared to uncultured cells; Figure 3A). In contrast, LSK cells expanded for as much as 10 days had enhanced reconstituting activity (2.4- to 2.7-fold compared to fresh cells;P < .001 for all combinations), but there was no clear difference observed between the 3 cytokine combinations. After 4 months, a similar pattern of reconstituting activity was observed (Figure 3B). When compared to uncultured input cells, cells expanded in all 3 cytokine combinations for fewer than 116 hours showed sustained reconstituting activity, whereas 240 hours of expansion resulted in the following increases in reconstituting activity: KFM, 2.5-fold (P < .01); KFM3, 2.2-fold (P < .001); KFM11, 1.4-fold (not statistically significant). Of importance, transplanted cells from the various expansion conditions showed similar contributions to B, T, and myeloid blood cells as uncultured cells (Table 2). Unexpectedly, we did not observe any negative effects of IL-3 (or positive effects of IL-11) on either short- or long-term reconstituting activity or lineage contribution of KFM-expanded LSK cells. Rather, the extended culture of LSK cells exposed to IL-3, in which total cell numbers had been increased by as much as 6400-fold, showed enhanced reconstituting activity.
Effects of IL-3 and IL-11 on the in vivo reconstituting activity of KL+FL+MGDF-expanded Lin−Sca-1+c-kit+ cells.
Either immediately after isolation (day 0) or after cytokine-induced ex vivo expansion, 500 to 750 freshly isolated LSK Ly5.1+cells or their expansion equivalent were transplanted, together with 150 000 to 200 000 unfractionated Ly5.2+ BM cells, into lethally irradiated (Ly5.2+) recipients. Peripheral blood cells were analyzed by flow cytometry 6 weeks (A) or 4 months (B) after transplantation for donor (Ly5.1) and competitor/recipient (Ly5.2) origin. All data were derived from 4 experiments, with a total of 11 to 13 recipients per culture condition and time point, except for KFM at 240 hours, derived from 2 experiments with 7 recipients per culture condition and time point. Reconstitution levels (relative to the mean of that obtained with freshly isolated LSK cells within each individual experiment) are shown ± SEM. The mean reconstitution levels in mice transplanted with uncultured LSK cells were: 6 weeks, 50% (experiment 1), 35% (experiment 2), 17% (experiment 3), 40% (experiment 4); 4 months, 41% (experiment 1), 34% (experiment 2), 22% (experiment 3), and 40% (experiment 4). No differences in lineage distribution of donor-derived cells at 4 months were found between freshly isolated and cultured cells (see Table 2 for details).
Effects of IL-3 and IL-11 on the in vivo reconstituting activity of KL+FL+MGDF-expanded Lin−Sca-1+c-kit+ cells.
Either immediately after isolation (day 0) or after cytokine-induced ex vivo expansion, 500 to 750 freshly isolated LSK Ly5.1+cells or their expansion equivalent were transplanted, together with 150 000 to 200 000 unfractionated Ly5.2+ BM cells, into lethally irradiated (Ly5.2+) recipients. Peripheral blood cells were analyzed by flow cytometry 6 weeks (A) or 4 months (B) after transplantation for donor (Ly5.1) and competitor/recipient (Ly5.2) origin. All data were derived from 4 experiments, with a total of 11 to 13 recipients per culture condition and time point, except for KFM at 240 hours, derived from 2 experiments with 7 recipients per culture condition and time point. Reconstitution levels (relative to the mean of that obtained with freshly isolated LSK cells within each individual experiment) are shown ± SEM. The mean reconstitution levels in mice transplanted with uncultured LSK cells were: 6 weeks, 50% (experiment 1), 35% (experiment 2), 17% (experiment 3), 40% (experiment 4); 4 months, 41% (experiment 1), 34% (experiment 2), 22% (experiment 3), and 40% (experiment 4). No differences in lineage distribution of donor-derived cells at 4 months were found between freshly isolated and cultured cells (see Table 2 for details).
Ex vivo expansion of Lin−Sca-1+c-kit+ stem cells in the presence of IL-3 or IL-11 do not affect their ability for long-term reconstitution of lymphoid and myeloid cell lineages
| Ex vivo expansion . | Lineage reconstitution, % of Ly5.1+ cells . | ||
|---|---|---|---|
| B220+ . | CD3+ . | Gr-1+/Mac-1+ . | |
| None (control) | 53 ± 2 | 34 ± 3 | 14 ± 2 |
| KFM 116 h | 44 ± 10 | 47 ± 12 | 11 ± 5 |
| KFM3 116 h | 46 ± 9 | 34 ± 5 | 24 ± 10 |
| KFM11 116 h | 55 ± 3 | 33 ± 4 | 14 ± 4 |
| KFM 240 h | 56 ± 5 | 23 ± 1 | 21 ± 4 |
| KFM3 240 h | 52 ± 1 | 33 ± 2 | 15 ± 1 |
| KFM11 240 h | 56 ± 3 | 33 ± 3 | 10 ± 1 |
| Ex vivo expansion . | Lineage reconstitution, % of Ly5.1+ cells . | ||
|---|---|---|---|
| B220+ . | CD3+ . | Gr-1+/Mac-1+ . | |
| None (control) | 53 ± 2 | 34 ± 3 | 14 ± 2 |
| KFM 116 h | 44 ± 10 | 47 ± 12 | 11 ± 5 |
| KFM3 116 h | 46 ± 9 | 34 ± 5 | 24 ± 10 |
| KFM11 116 h | 55 ± 3 | 33 ± 4 | 14 ± 4 |
| KFM 240 h | 56 ± 5 | 23 ± 1 | 21 ± 4 |
| KFM3 240 h | 52 ± 1 | 33 ± 2 | 15 ± 1 |
| KFM11 240 h | 56 ± 3 | 33 ± 3 | 10 ± 1 |
Lethally irradiated C57bl/6 (Ly5.2+) mice were transplanted with 500 or 750 LSK cells freshly isolated (control) or the expansion equivalent of this cell number using various culture conditions, along with 150 000 or 200 000 unfractionated Ly5.2+ BM cells. Peripheral blood cells from transplanted mice were stained and analyzed by flow cytometry 4 months after transplantation for the presence of donor-derived (Ly5.1) cells along with lineage antibodies (B220, B cells; CD3, T cells; Gr-1/Mac-1, myeloid cells). Data are from the same experiments as in Figure 3 and are expressed as mean ± SEM.
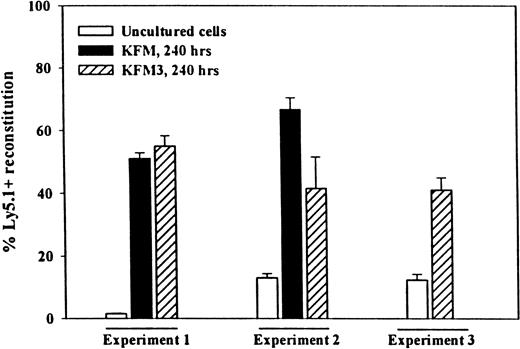
Because serial transplantations put enhanced proliferative pressure on HSC,52-54 the ability to reconstitute secondary recipients is considered a more stringent measure of true LTRC activity. Thus, to demonstrate unequivocally that IL-3 did not negatively affect the long-term reconstituting activity of LSK cells and that prolonged expansion culture did not exhaust the stem cell pool, BM cells from primary recipients of KFM and KFM3-expanded cells were transplanted into secondary recipients. After another 4 months, a much higher secondary reconstituting activity of KFM and KFM3-expanded cells was observed than was observed of the same number of uncultured input cells (Figure 4).
Lin−Sca-1+c-kit+cells expanded in KFM3 in serum-free medium for 10 days have enhanced ability to reconstitute lethally irradiated secondary recipients.
Primary recipients having received 500 fresh, or the expansion equivalent of 500, Ly5.1+ LSK cells cultured for 240 hours in serum-free medium in KFM (experiments 1 and 2) or KFM3 (experiments 1, 2, and 3) and 150 000 or 200 000 Ly5.2+ BM cells were sacrificed 4 months after transplantation. Lethally irradiated secondary recipients were transplanted with a half-femur equivalent from pooled primary recipients (4 recipients per group in each experiment). The mean reconstitution (of PB) in primary recipients of fresh LSK cells was 34%, 22%, and 40% in experiments 1, 2, and 3, respectively. The decrease in reconstituting activity in secondary recipients of BM from mice transplanted with fresh LSK cells (particularly in experiment 1) must be considered with the fact that only 5% of the total BM of primary recipients (half-femur equivalent) was transplanted into secondary recipients and that HSC lose reconstituting activity with serial transplantation.53 Reconstitution is shown from 3 experiments as the percentage of Ly5.1 cells in peripheral blood ± SEM.
Lin−Sca-1+c-kit+cells expanded in KFM3 in serum-free medium for 10 days have enhanced ability to reconstitute lethally irradiated secondary recipients.
Primary recipients having received 500 fresh, or the expansion equivalent of 500, Ly5.1+ LSK cells cultured for 240 hours in serum-free medium in KFM (experiments 1 and 2) or KFM3 (experiments 1, 2, and 3) and 150 000 or 200 000 Ly5.2+ BM cells were sacrificed 4 months after transplantation. Lethally irradiated secondary recipients were transplanted with a half-femur equivalent from pooled primary recipients (4 recipients per group in each experiment). The mean reconstitution (of PB) in primary recipients of fresh LSK cells was 34%, 22%, and 40% in experiments 1, 2, and 3, respectively. The decrease in reconstituting activity in secondary recipients of BM from mice transplanted with fresh LSK cells (particularly in experiment 1) must be considered with the fact that only 5% of the total BM of primary recipients (half-femur equivalent) was transplanted into secondary recipients and that HSC lose reconstituting activity with serial transplantation.53 Reconstitution is shown from 3 experiments as the percentage of Ly5.1 cells in peripheral blood ± SEM.
Because it has been suggested that LSKCD34+ and LSKCD34− cells represent functionally distinct subsets of murine BM HSC,48 55 with the CD34− subset having the most LTRC activity, we also performed a set of experiments to determine the effect on LSKCD34− LTRC of 10 days expansion in the presence of KFM3. Given that the cytokine responsiveness of CD34− stem cells had not been characterized in detail, we first performed single-cell experiments that revealed that LSKCD34−/lo cells were optimally recruited into proliferation by KFM3 (80% proliferated by 240 hours and to a degree similar to that of LSK cells; Table 1), though with delayed kinetics when compared to LSKCD34+cells (Bryder D, Jacobsen SEW, unpublished observations). To prove unequivocally that the reconstituting activity from cells cultured in KFM3 was derived from CD34− cells that had undergone division, cells were cultured for 240 hours at the single-cell level; 30 cells that proliferated were transplanted into lethally irradiated recipients (Table 3). Four months after transplantation, primary recipients of KFM3-expanded cells revealed higher multilineage reconstitution than mice transplanted with uncultured input cells. In secondary recipients, the enhanced long-term repopulating ability of KFM3-stimulated LSKCD34− cells that had undergone proliferation was even more evident (Table 3). Thus, at least a fraction of LSKCD34− HSC undergo multiple cell divisions in response to KFM3, resulting in enhanced rather than reduced reconstituting activity.
Lin−Sca-1+c-kit+CD34− stem cells expanded in KL + FL + MGDF + IL-3 at the single-cell level have enhanced long-term reconstituting ability
| . | Treatment . | Positive primary recipients . | % Ly5.1 cells . | |
|---|---|---|---|---|
| Primary . | Secondary . | |||
| Experiment 1 | No culture | 5/5 | 20.0 ± 11.8 | 1.1 ± 0.3 |
| 10-d culture | 5/5 | 40.1 ± 4.6 | 8.7 ± 3.0 | |
| Experiment 2 | No culture | 5/5 | 19.8 ± 3.8 | 0.7 ± 0.3 |
| 10-d culture | 5/5 | 41.7 ± 21.6 | 28.7 ± 11.8 | |
| . | Treatment . | Positive primary recipients . | % Ly5.1 cells . | |
|---|---|---|---|---|
| Primary . | Secondary . | |||
| Experiment 1 | No culture | 5/5 | 20.0 ± 11.8 | 1.1 ± 0.3 |
| 10-d culture | 5/5 | 40.1 ± 4.6 | 8.7 ± 3.0 | |
| Experiment 2 | No culture | 5/5 | 19.8 ± 3.8 | 0.7 ± 0.3 |
| 10-d culture | 5/5 | 41.7 ± 21.6 | 28.7 ± 11.8 | |
On day 0, 30 isolated LSKCD34− Ly5.1+cells were co-injected, together with 200 000 unfractionated Ly5.2+ bone marrow cells, into each lethally irradiated C57Bl/6 recipient. From the same isolations, cells were set in Terasaki plates at 0.25 cell/well using serum-free medium supplemented with KFM3; 12 to 16 h after seeding, wells were screened for the presence of 1 cell, and only wells containing 1 cell were included in experiments. On day 10, 30 wells containing cells that had proliferated were pooled and injected into each recipient, together with 200 000 unfractionated Ly5.2+ bone marrow cells. Reconstitution in peripheral blood was evaluated after 4 months. Data are shown as means (±SEM). Secondary transplantations were in experiment 1, performed by pooling cells from all primary recipients and injecting cells corresponding to a half-femur into 5 secondary recipients. In experiment 2, BM cells from each primary recipient was injected into 2 secondary recipients (total, 10 secondary recipients/group). In experiment 1, lineage analysis of donor-derived Ly5.1+cells revealed that 3 mice that received uncultured cells displayed only lymphoid reconstitution. In experiment 2 of the serially transplanted mice, 3 animals transplanted with BM from recipients of uncultured cells had too low reconstitution to allow meaningful lineage analysis. In all other mice, donor-cell reconstitution contributed to multilineage reconstitution.
Our findings of enhanced LTRC activity after extensive and prolonged HSC proliferation in the presence of IL-3 was a surprise in light of previous studies of murine and human stem cells that implicated IL-3 might have a potent negative effect on in vitro stem cell self-renewal.16,19,25,26,28,29 Although there were many possible explanations for this finding (addressed in more detail in the “Discussion”), most ex vivo expansion studies of murine stem cells showing a loss of reconstituting activity had been performed in FCS-containing medium.16-18,56 The only previous studies showing in vitro expansion of murine LTRC activity (in the absence of IL-3) were, as were our studies, performed under serum-free conditions.14 Although no differences in the recruitment of single LSK cells into proliferation were observed in serum-free and FCS-containing medium (S. E. W. Jacobsen and D. Bryder, unpublished observations) and the levels of total cell expansion were comparable (slightly higher in FCS-containing medium; Figure5A), the effect on the preservation of in vivo reconstituting activity was strikingly different. Whereas LSK cells that expanded for 116 or 240 hours in the presence of KFM3 or KFM11 under serum-free conditions demonstrated sustained or enhanced short-term (6 weeks; Figure 5B) or long-term (4 months; Figure 5C) reconstituting activity, cells cultured in FCS-containing medium always had severely reduced reconstituting activity, regardless of cytokine combination or duration of culture. However, at 240 hours, a clear tendency was observed toward more pronounced reduction in reconstitution activity in IL-3– than in IL-11–containing cultures (P < .01).
Ex vivo expansion of Lin−Sca-1+c-kit+ cells in FCS-containing cultures abrogates their ability for short- and long-term reconstitution of lethally irradiated recipients.
Five hundred freshly isolated LSK cells, or the expansion equivalent, subjected to indicated ex vivo expansion, were transplanted along with 150 000 or 200 000 unfractionated BM cells (Ly5.2+) into lethally irradiated (Ly5.2+) mice. (A) Total cellular expansion of LSK cells after 116 hours and 240 hours in serum-free and FCS-containing medium. After 6 weeks (B) and 4 months (C), peripheral blood cells from transplanted mice were analyzed for the presence of donor-derived (Ly5.1+) reconstitution. Data for 116-hour and 240-hour cultures are representative of 2 and 3 experiments, respectively. Data are presented as Ly5.1 reconstitution relative to the mean of that obtained with 500 freshly isolated LSK cells (mean, 17%-40% at 6 weeks and 22%-40% after 4 months). Numbers in parentheses show the total number of mice analyzed for each group of treatment.
Ex vivo expansion of Lin−Sca-1+c-kit+ cells in FCS-containing cultures abrogates their ability for short- and long-term reconstitution of lethally irradiated recipients.
Five hundred freshly isolated LSK cells, or the expansion equivalent, subjected to indicated ex vivo expansion, were transplanted along with 150 000 or 200 000 unfractionated BM cells (Ly5.2+) into lethally irradiated (Ly5.2+) mice. (A) Total cellular expansion of LSK cells after 116 hours and 240 hours in serum-free and FCS-containing medium. After 6 weeks (B) and 4 months (C), peripheral blood cells from transplanted mice were analyzed for the presence of donor-derived (Ly5.1+) reconstitution. Data for 116-hour and 240-hour cultures are representative of 2 and 3 experiments, respectively. Data are presented as Ly5.1 reconstitution relative to the mean of that obtained with 500 freshly isolated LSK cells (mean, 17%-40% at 6 weeks and 22%-40% after 4 months). Numbers in parentheses show the total number of mice analyzed for each group of treatment.
Because the serum-free and FCS-containing media differed not only with regard to the absence or presence of FCS (see “Materials and methods”), a final experiment was designed to address more specifically the effects of FCS. The fact that dramatically reduced LTRC activity of LSK cells was observed not only after culture in the standard (IMDM-based) FCS medium but also when FCS was added to the serum-free medium (Table 4) clearly supported a detrimental effect of the FCS on stem cell ex vivo expansion in the current studies.
FCS negatively affects the long-term reconstituting activity of Lin−Sca1+c−kit+ cells cultured under various conditions
| Cytokines . | Medium . | FCS . | BSA . | No.mice . | % Ly5.1+ cells in PB 4 months after transplantation . |
|---|---|---|---|---|---|
| Fresh cells | NA | NA | NA | 4 | 36.75 ± 18.37 |
| KFM3 | X-Vivo 15 | — | 1% | 5 | 64.02 ± 13.76 |
| KFM3 | IMDM | 20% | — | 3 | 0.74 ± 0.65 |
| KFM3 | X-Vivo 15 | 20% | 1% | 4 | 0.08 ± 0.01 |
| KFM3 | IMDM | 20% | 1% | 5 | 0.06 ± 0.03 |
| K6,11E34-150 | X-Vivo 15 | — | 1% | 5 | 60.2 ± 14.74 |
| K6,11E3 | IMDM | 20% | — | 3 | 0.57 ± 0.30 |
| Cytokines . | Medium . | FCS . | BSA . | No.mice . | % Ly5.1+ cells in PB 4 months after transplantation . |
|---|---|---|---|---|---|
| Fresh cells | NA | NA | NA | 4 | 36.75 ± 18.37 |
| KFM3 | X-Vivo 15 | — | 1% | 5 | 64.02 ± 13.76 |
| KFM3 | IMDM | 20% | — | 3 | 0.74 ± 0.65 |
| KFM3 | X-Vivo 15 | 20% | 1% | 4 | 0.08 ± 0.01 |
| KFM3 | IMDM | 20% | 1% | 5 | 0.06 ± 0.03 |
| K6,11E34-150 | X-Vivo 15 | — | 1% | 5 | 60.2 ± 14.74 |
| K6,11E3 | IMDM | 20% | — | 3 | 0.57 ± 0.30 |
LSK Ly5.1+ cells were cultured for 10 days in various media and cytokines. Five hundred uncultured LSK cells (control), or the expansion equivalent of 500 LSK cells, were injected into lethally irradiated C57Bl/6 recipients (Ly5.2+) together with 200 000 fresh Ly5.2+ BM cells. PB from transplanted mice was analyzed 4 months after transplantation for donor-derived hematopoiesis. Results are shown are means ± SEM. NA, not applicable.
K6,11E3 = mKL + hIL-6 + hIL-11 + hEPO + mIL-3.
Because we could not exclude that the negative effects of IL-3 on LTRC activity were dependent on the specific interacting cytokines, we also examined the effect of KL+IL6+IL11+EPO+IL-3 on LSK ex vivo expansion (Table 4). This specific cytokine combination has previously (in FCS-containing cultures) been demonstrated to have a severe negative effect on maintenance of LTRC.16 19 In striking contrast to the FCS-containing culture that reproduced this negative effect, the LTRC activity of cells exposed to the same cytokines in SF medium was enhanced in comparison with uncultured control cells (Table4).
Discussion
Although much progress has been made in recent years toward the development of better surrogate human stem cell assays,57-61 it remains unclear to what degree these assays detect true long-term repopulating HSC activity. Thus, clinical stem cell and gene-marking protocols will eventually have to address whether the recent success in expanding and retroviral-transducing candidate human HSC translates into true LTRC activity.37,40,41 62 In the meantime, complimentary studies in animal models that provide true assays for LTRC are paramount for a better understanding of the processes regulating HSC fate.
The HSC in mouse remain phenotypically and functionally the best characterized,3,48,63 and ample evidence suggest that human HSC are subjected to the same regulatory networks. This appears particularly evident with regard to the potential role of various cytokines in regulating HSC proliferation and differentiation.20,21 Thus, studies on murine HSC continue to govern efforts at using cytokines to promote the expansion of human HSC for use in stem cell transplantation and gene therapy.4-7 However, the success in expanding murine HSC with sustained long-term repopulating ability has been variable and limited and has more frequently resulted in compromised rather than enhanced LTRC activity.3 15-19
Although a number of variables can explain the lack of success in expanding LTRC, major focus has been directed to the ability of different cytokines (and combinations of these) to promote stem cell expansion.6,7 Whereas cytokines appear to support the differentiation of hematopoietic cells primarily through permissive rather than instructive mechanisms,32-35 their role in regulating stem cell fate (self-renewal, differentiation, or apoptosis) remains unclear.3,30,31 Some studies have suggested that IL-11, in combination with FL, KL, or both, can positively affect ex vivo LTRC maintenance and expansion. 2,14,19,42-44 Others have revealed a severe negative effect of IL-3.16,19,25-27These may imply distinct roles for these 2 cytokines in regulating stem cell fate. However, because HSC are also heterogeneous with regard to their cytokine receptor expression,24 it is difficult to exclude that differences observed between cytokine combinations might simply reflect heterogeneity in receptor expression and, thus, target cell populations. To overcome this limitation, we used a combination of KL, FL, and MGDF. We recently demonstrated23 that this combination efficiently recruits virtually all murine LSK HSC into proliferation while it maintains their long-term reconstituting activity. This, combined with the finding that IL-3 and IL-11 had a potent and indistinguishable ability to enhance the cycling of KFM-recruited LSK cells, allowed us to compare their effects on self-renewal divisions of the same population of HSC. Our finding that both IL-3 and IL-11 supported KFM-stimulated HSC self-renewal was surprising in light of previous studies suggesting opposing effects of these 2 cytokines in this process.16,19,25 As illustrated through the increase in total cellular expansion and the high-resolution cell division tracking, IL-3 clearly enhanced the cycling of LSK HSC without negatively affecting self-renewal after as much as 10 days of incubation. Although it remains possible that IL-3 may only negatively affect HSC self-renewal induced by certain cytokine combinations, this seems unlikely because IL-3 did not abrogate HSC self-renewal divisions induced in response to KL+IL-6+IL-11+EPO, for which a negative effect of IL-3 has been demonstrated.16,19 Furthermore, though IL-3 also has been demonstrated to regulate negatively the lymphoid potential of HSC,64 65 IL-3 (and IL-11) did not affect the relative levels of myeloid and lymphoid reconstitution by expanded stem cells.
Although these studies clearly demonstrate that IL-3 can support multiple self-renewing divisions of LTRC, they only provide clues as to why other studies have demonstrated that IL-3 can have a severe negative effect on ex vivo expansion of murine HSC.16,19,25-27 Common to all studies showing the suppression of murine LTRC activity by IL-3 is the presence of FCS in the expansion cultures, which, in the current study and in other recent studies in humans, proved detrimental for HSC expansion.23,66 Although the specific FCS (and concentration) used might vary in its ability to support HSC, as illustrated through a few studies demonstrating maintenance of LTRC activity,19 26 these studies clearly demonstrate that the presence of FCS can be detrimental to the preservation of HSC during ex vivo expansion.
The mechanism by which FCS abrogated stem cell maintenance was not addressed in the current study. However, because the level of recruitment and total cellular expansion of LSK cells in response to KFM3 and KFM11 were comparable in the serum-free and FCS-containing medium, the negative effect of FCS is unlikely to result from an effect on HSC proliferation. However, we have observed that FCS-containing cultures are more efficient at supporting the generation of fully differentiated myeloid (granulocyte-macrophage) cells (Bryder D, Jacobsen SEW, unpublished observations). In that regard, we did, in the presence of FCS, observe a facilitating effect of IL-3 on HSC loss in culture at 116 hours and at 240 hours. In contrast, in the presence of IL-11, the levels of HSC recovered slightly after 240 hours, which was similar to what we observed in serum-free media. Because the direct effects of IL-3 and IL-11 on proliferation of LTRC seem comparable, the possibility that the negative effects of IL-3 on HSC self-renewal might be mediated indirectly through effects on mature cells, generated in cultures, should be entertained.
Studies of primitive human progenitors have suggested that a negative effect of IL-3 may be dependent on relative cytokine concentrations.28 Although we cannot exclude such an effect on murine HSC, the levels of IL-3 and other cytokines used in the current study were comparable to those used in studies showing negative effects of IL-3.26
The proliferation history of LSK cells, as established through CFSE staining, clearly demonstrated that all LTRC had undergone multiple (at least 5) cell divisions after 140 hours of culture in KFM3 or KFM11, unequivocally demonstrating that LTRC can undergo multiple self-renewing divisions in vitro. This conclusion was also supported by the studies on single LSKCD34− cells, demonstrating an enhanced reconstituting activity of cells that had undergone proliferation. Several studies of candidate human CD34− HSC have suggested that these show little or no cytokine responsiveness,67,68 but the current and other studies demonstrate the extensive proliferation and expansion potential of highly purified LSKCD34− stem cells to early-acting cytokines.14
Although repopulating activity was enhanced in IL-3– and IL-11–containing cultures, specific quantification of expanded LTRC was not performed to demonstrate unequivocally an increase in stem cell numbers. However, the expanded HSC displayed enhanced long-term reconstituting activity, not only in primary but also in secondary lethally irradiated recipients, with lineage distribution indistinguishable from that of freshly isolated cells. Furthermore, the finding of mice lacking both myeloid and lymphoid reconstitution when transplanted with fresh LSKCD34-cells, but not expanded cells, supports an expansion in LTRC numbers.
That ex vivo expansion for 10 days was associated with enhanced reconstituting activity suggests that some of the self-renewing divisions must have been symmetric. However, the fact that a 2- to 3-fold expansion in LTRC activity was accompanied by at least 5 cell divisions by all LTRC and by as much as 400- to 6500-fold total cellular expansion implies either that most self-renewing divisions are asymmetric (resulting in the generation of only one new HSC) or that a high frequency of HSC, generated through symmetric cell divisions, undergoes apoptosis or is otherwise compromised in its ability to engraft.
One of the most intriguing findings in our studies was that the magnitude of HSC expansion was higher after 240 hours than at 116 hours, though all HSC appeared to have divided multiple times by 116 hours. The explanation for this remains unclear, but one possibility is that increased culture time enhances the likelihood of symmetric self-renewal divisions. Alternatively, our culture conditions might only support the preservation and expansion of a subset of HSC, resulting in loss of some HSC during the first days of culture.
A better understanding of the processes regulating stem cell fate will prove crucial for the development of a more efficient expansion of long-term repopulating stem cells. Regardless, the current study and that of Miller et al14 support the feasibility of prolonged expansion of HSC to promote retroviral-mediated gene transfer or to purge autologous stem cell transplants while maintaining the long-term repopulating ability of the graft.4-7
Supported by grants from ALF (Government Public Health Grant); Berta Kamprad Foundation; Crafoord Foundation; Georg Danielsson Foundation; Gunnar, Arvid and Elisabeth Nilsson Foundation; Harald and Greta Jeansson's Foundation; Thelma Zoega's Foundation; John and Augusta Persson Foundation; Medical Faculty, University of Lund; Swedish Medical Research Council (MFR); Svensson Siblings Foundation; O and E and Edla Johansson Foundation; Royal Physiographic Society in Lund; Swedish Foundation for Strategic Research; Swedish Cancer Society; Swedish Society of Pediatric Cancer; and Tobias Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
David Bryder, Stem Cell Laboratory, Institute of Laboratory Medicine, University Hospital of Lund, 221 85 Lund, Sweden; e-mail: David.Bryder@molmed.lu.se.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal