Abstract
The chemokine receptors CCR5 and CXCR4 are coreceptors for the human immunodeficiency virus (HIV) and determine the cell tropism of different HIV strains. Previous studies on their regulation were performed under conditions of unspecific T-lymphocyte stimulation and provided conflicting results. To mimick physiologic conditions, highly purified primary Staphylococcus enterotoxin B (SEB)-reactive CD4 T lymphocytes were stimulated in the presence of autologous antigen-presenting cells and the kinetics of CCR5 and CXCR4 surface expression and HIV replication were studied. Both chemokine receptors were transiently up-regulated with maximal expression at day 3 after stimulation. The stimulated T cells were equally susceptible to productive infection with R5-and X4-tropic virus strains. Thus, antigenic stimulation of T cells promotes efficient replication of both, T cell-tropic and macrophage-tropic HIV.
Introduction
Chemokines are small chemotactic active cytokines. Together with their receptors, they orchestrate the distribution of effector cells of the immune system. With the finding that the coreceptors for the human immunodeficiency virus (HIV) belong to the chemokine receptor family, these molecules came into the center of interest of cytokine research. The 2 main coreceptors of HIV are CCR5 and CXCR4, and they determine the tropism of the virus. Macrophage-tropic or nonsyncytium-inducing (NSI) viruses utilize CCR5 (now referred to as R5-tropic HIV), whereas T-lymphotropic or SI viruses utilize CXCR4 (X4-tropic HIV). CCR5 has been shown to be of great importance for horizontal HIV transmission. A homozygous 32-base pair (bp) deletion in the CCR gene (ΔCCR5) was significantly more frequent in a large cohort of seronegative individuals when compared with HIV-infected persons, suggesting a protective effect against infection.1 In contrast, deletions in CXCR4 have not been observed in humans. Studies with knockout mice indicated that these are not compatible with normal fetal development.2
Because chemokine receptors are crucial to HIV entry, it is essential to determine their distribution and to study their regulation. The main target cells for HIV in vivo are CD4+ T lymphocytes.3 Various in vitro studies have suggested that CXCR4 and CCR5 are differentially expressed and regulated on these cells. For example, CXCR4 is mainly expressed on naive CD4+T cells (CD26low, CD45RA+, CD45RO−) and CCR5 mainly on effector/memory T cells (CD26high, CD45RAlow, CD45ROhigh).4 The stimulation of peripheral blood mononuclear cells (PBMCs) with PHA, interleukin-2 (IL-2), or immobilized antibodies to CD3 leads to a rapid up-regulation of CXCR4. In contrast, CCR5 expression decreases slightly up to day 3 and shows a significant up-regulation only on IL-2 stimulation at later times.4 The activation of CD4+ T lymphocytes by immobilized CD3 and CD28 antibodies induces an R5 HIV-resistant state. This is in part mediated by the CD28-dependent down-regulation of CCR5 transcription. In addition, the induced synthesis of the chemokines RANTES (regulated on activation, normal T cell expressed and secreted), MIP-1α and MIP-1β,5,6 leads to an internalization of surface CCR5.7 These results contrast previous observations made in vivo, where antigen-induced T-cell activation promotes efficient HIV and simian immunodeficiency virus (SIV) replication.8-11 Experiments of Loetscher and colleagues12 are compatible with these latter observations. They have shown that CCR5 is highly expressed on activated CD4 T cells in vivo, ie, on the Th1 cells of rheumatoid joints.12
The conditions of T-cell stimulation seem to influence the expression pattern of CCR5 and CXCR4 on CD4 T cells and may effect the propagation of HIV strains with different chemokine receptor usage. However, so far it is unknown as to what extent and magnitude these parameters may be affected by conditions of physiologic stimulation. This was addressed in a stimulation system of primary CD4 T lymphocytes and autologous antigen-presenting cells in vitro.
Material and methods
Cells and reagents
Nickel sulfate (NiSO46H2O) andStaphylococcus enterotoxin B (SEB) were purchased from Sigma, Deisenhofen, Germany; Staphylococcus enterotoxin E (SEE) from Toxin Technology, FL. IL-2 and 3H-thymidine were obtained from Amersham, Braunschweig. Nickel-specific T-cell clones were generated and stimulated as described elsewhere.13Blood from healthy blood donors was obtained from the local blood bank as buffy coats and used for the isolation of primary T cells and monocytes. Primary CD4 T lymphocytes and monocytes were isolated from blood and grown in RPMI 1640 supplemented with 5% heat-inactivated, pooled human AB serum.
Purification and positive selection of SEB reactive CD4+ T cells
PBMCs were isolated from buffy coats by Ficoll-Hypaque (Pharmacia, Freiburg, Germany) density gradient centrifugation. Monocytes were separated by plastic adherence. CD4+ cells were enriched from the remaining cells in suspension by depletion of CD19- and CD8-positive cells with the respective antibody-coated Dynabeads (Dynal, Hamburg, Germany). Positive selection of Vβ3-, Vβ14-, and Vβ17-positive CD4 T cells was performed with monoclonal Vβ-specific antibodies (Coulter, Hamburg, Germany) and the Dynal RAM CELLection Kit (Dynal). Cells were recovered from the beads by DNase digestion. The purity was determined by flow cytometry using antimouse phycoerythrin (Sigma) as a secondary antibody and was always more than 97%.
Stimulation of purified Vβ3-, Vβ14- and Vβ17, and CD4+ T cells with SEB
Monocytes were grown overnight and used as autologous antigen-presenting cells. Briefly, monocytes were detached from the plastic surface by incubating in phosphate-buffered saline/ethylenediamine tetraacetic acid (PBS/EDTA) (3 mmol/L) for 10 minutes at 37°C. Cells were washed, seeded at a density of 1 × 106 cells/mL in 96 flat-bottomed well plates, and incubated at 37°C overnight. Subsequently, cells were irradiated with 60 Gy and Vβ3-, Vβ14-, and Vβ17-positive CD4+ T cells were added in a ratio of 1:1. The cells were cultured with and without addition of SEE, SEB, and SEB plus IL-2, respectively.
Human immunodeficiency virus infection
R4-tropic HIV-1Lai was propagated on Molt-4 clone 8 cells, the R5-tropic HIV-1BaL on primary macrophages. HIV-containing supernatants were used for the infection of PBMCs, corresponding to 200 cpm of reverse transcriptase activity per 106 cells. Virus production was monitored for up to 3 weeks by testing culture supernatants for HIV p24 twice a week with a specific enzyme-linked immunosorbent assay (ELISA) (Dupont, Köln, Germany).
Flow cytometry
Cells were harvested, washed, and fixed in 2% paraformaldehyde. Thereafter, they were washed and immunostained with anti–CD4-PE-Cy5 (Coulter), anti-CCR5 (Number MAB180F; R&D, Wiesbaden, Germany), anti-CXCR4 (Pharmingen, Hamburg, Germany), or anti–HLA-DR (Dako, Hamburg, Germany). Phenotypic analysis was performed on a FACScan using CellQuest software version 3.1, both from Becton Dickinson, Heidelberg, Germany.
Proliferation assays
Proliferation of primary CD4 T cells was assayed by3H-thymidine incorporation from days 4 to 5 and 7 to 8 in duplicate. Cultures were pulsed with 0.037 Mbq (1 μCi) per well 3H-thymidine for 16 hours. Cells were harvested on GF/A filters, and thymidine uptake was determined using a Berthold-Inotech Trace-96 (Berthold, Wildbad, Germany).
Results and discussion
Research on the effect of anti-CD3 T-cell stimulation on CCR5 expression and HIV replication has produced conflicting results. Rabin and colleagues14 have documented a moderate increase of CCR5 on memory CD4 T cells after stimulation with soluble anti-CD3 antibody. Carroll and colleagues15 have shown that immobilized anti-CD3 and anti-CD28 reduce CCR5 surface expression and lead to an HIV-resistant phenotype. The latter conditions might resemble the physiologic way of immune cell activation. In our hands, however, the stimulation with immobilized anti-CD3 and anti-CD28 results in the up-regulation of CCR5 and expansion of R5-tropic HIV (data not shown). This is concordant with the use of plastic-coated anti-CD3 and anti-CD28 to recover HIV from the PBMCs of patients under highly active antiretroviral therapy.16 Although these discrepancies might be due to the clonotype of the antibodies used or their accessibility for T-cell ligands, it is uncertain what would happen during the stimulation of antigen-specific CD4 T cells by professional antigen-presenting cells.17
To elucidate the chemokine receptor expression under such conditions, initial experiments were performed with 2 nickel (Ni2+)-specific CD4+ T-cell clones and autologous Epstein-Barr virus (EBV)-immortalized B cells as antigen-presenting cells (APCs). Besides their specificity for Ni2+, they bear a Vβ17 T-cell receptor (TCR) and could therefore be stimulated with SEB.18 On the basis of the cytokine-expression pattern, the investigated clones had a Th2 and Th0 phenotype, respectively. In accord with previous data, CXCR4 was expressed and CCR5 was hardly detectable without stimulation.12 However, on stimulation with Ni2+ or SEB, CCR5 and CXCR4 expression were up-regulated in comparable quantities (data not shown).
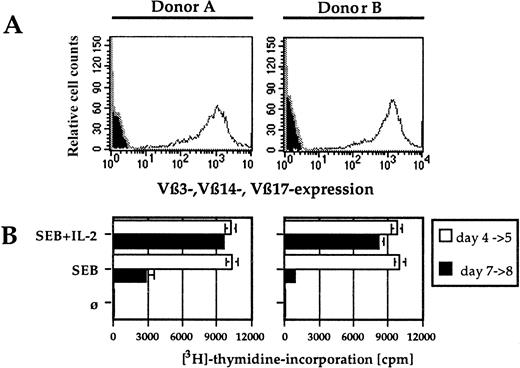
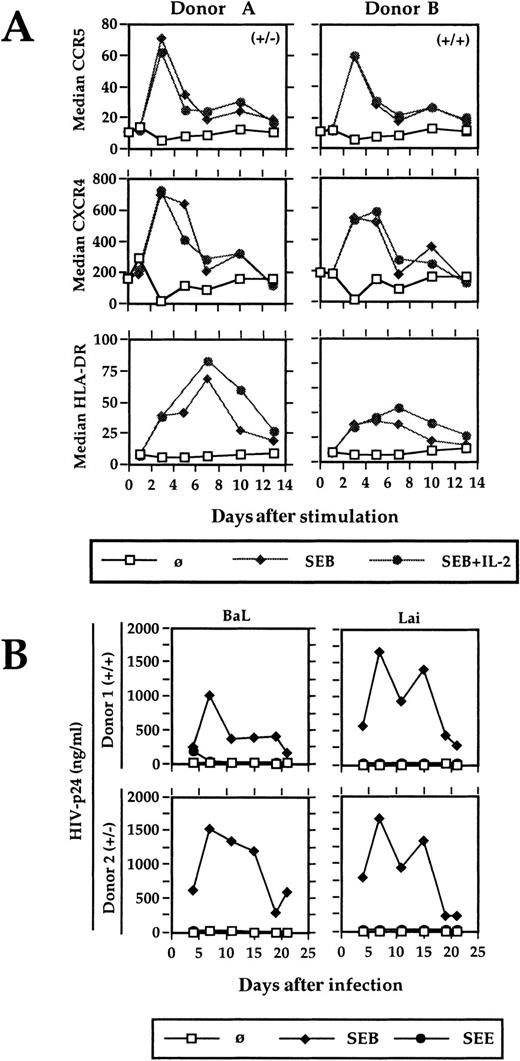
Stimulation of highly purified antigen-specific primary CD4 T lymphocytes leads to a transient up-regulation of CCR5 and CXCR4. To mimick most closely the conditions of physiologic primary T-cell stimulation and to analyze the effect on HIV coreceptor expression, an experimental system was established that contains highly purified antigen-specific T cells, autologous cultured monocytes as APCs, and a superantigen as the specific antigen. No exogenous IL-2 was added, thus the T cells were solely dependent on the cytokine produced on stimulation. SEB-reactive, Vβ3-, Vβ14-, and Vβ17-expressing primary CD4 T cells were isolated via antibody-coated magnetic beads from the PBMCs of healthy blood donors. The purity was always greater then 97% (Figure 1A). Autologous monocytes were enriched by plastic adherence and cultured overnight. During this time, they increase their stimulatory capacity significantly and can even be used to generate primary immune responses in vitro.19 Before using them as APCs, they were irradiated to avoid any background replication of HIV.20SEB-reactive T cells were either stimulated with SEB and IL-2 or with SEB alone. The proliferative response was measured in a standard proliferation assay. Chemokine receptor expression and the activation markers CD69 and HLA-DR were determined by flow cytometry. CCR5 and CXCR4 expression were significantly increased on CD4 T-cell stimulation (Figure 2A). The stimulation was antigen-dependent and Vβ-specific, because (1) the positively selected CD4 T cells per se neither proliferated nor expressed the activation markers CD69 and HLA-DR, (2) the SEB reactive Vβ3-, Vβ14-, and Vβ17-expressing T cells responded to SEB (Figure 1B, Figure 2A) but not to SEE, which reacts with Vβ5 or Vβ8 (Figure 2B and data not shown), and (3) SEB did not activate non–SEB-reactive Vβ5- and Vβ8-expressing CD4 T cells (data not shown). The presence of exogenous IL-2 failed to show any influence on the kinetics or the degree of CCR5 and CXCR4 up-regulation, but prolongated the proliferative response (Figure 1B, Figure 2A).
Vβ3, Vβ14, and Vβ17 expression on positively selected primary human CD4 T cells and their proliferation after antigenic stimulation.
(A) Cells were positively selected with Vβ-specific mouse antibodies, stained with a PE-labeled antimouse antibody, and analyzed on a FACSsort. The filled histogram shows a staining of nonselected cells with the secondary antibody alone. (B) The proliferative response of primary CD4+ T cells was determined at day 4 and 7 in duplicates. Cells were incubated over night with 0.037 MBq (1 μCi)3H-thymidine and harvested on glass fiber filters. These filters were measured with a Trace 96.
Vβ3, Vβ14, and Vβ17 expression on positively selected primary human CD4 T cells and their proliferation after antigenic stimulation.
(A) Cells were positively selected with Vβ-specific mouse antibodies, stained with a PE-labeled antimouse antibody, and analyzed on a FACSsort. The filled histogram shows a staining of nonselected cells with the secondary antibody alone. (B) The proliferative response of primary CD4+ T cells was determined at day 4 and 7 in duplicates. Cells were incubated over night with 0.037 MBq (1 μCi)3H-thymidine and harvested on glass fiber filters. These filters were measured with a Trace 96.
Kinetic of CCR5, CXCR4, and HLA-DR expression and HIV expansion after antigenic stimulation.
(A) Positively selected Vβ3-, Vβ14-, and Vβ17-bearing CD4 T cells were stimulated with SEB (20 ng/mL) in the presence of autologous APCs and the surface expression of CCR5, CXCR4, and HLA-DR was analyzed by flow cytometry. Two representative results from 8 different blood donors are shown. (B) The SEB-reactive CD4+ T cells were stimulated and infected with HIV-1Lai and HIV-1BaL. HIV-p24 was determined in the cell-free culture supernatant by ELISA. (+/−) and (+/+) characterizes the genotype of CCR5 of the respective blood donors A and B as determined by PCR. (−) represents the 32-bp deletion of one allele. A comparable HIV expansion was observed with T cells from 4 different blood donors.
Kinetic of CCR5, CXCR4, and HLA-DR expression and HIV expansion after antigenic stimulation.
(A) Positively selected Vβ3-, Vβ14-, and Vβ17-bearing CD4 T cells were stimulated with SEB (20 ng/mL) in the presence of autologous APCs and the surface expression of CCR5, CXCR4, and HLA-DR was analyzed by flow cytometry. Two representative results from 8 different blood donors are shown. (B) The SEB-reactive CD4+ T cells were stimulated and infected with HIV-1Lai and HIV-1BaL. HIV-p24 was determined in the cell-free culture supernatant by ELISA. (+/−) and (+/+) characterizes the genotype of CCR5 of the respective blood donors A and B as determined by PCR. (−) represents the 32-bp deletion of one allele. A comparable HIV expansion was observed with T cells from 4 different blood donors.
Maximal expression of CCR5 and CXCR4 was observed at day 3 after antigen-specific CD4 T-cell stimulation. This coincides with the time when naive T cells leave the lymphoid organs after antigenic stimulation in vivo.21 Using in vitro differentiated Th1 and Th2 cells, a switch of chemokine receptor expression was observed on TCR stimulation and was associated with an altered homing behavior.22 Likewise, especially the up-regulation of CCR5 might help T-cell migration into sites of inflammation where the CCR5 ligands RANTES, MIP-1α, and MIP-1β are abundant. However, because about 1% of white individuals lack functional CCR5 expression and seem not to be especially susceptible to infectious diseases, other chemokine receptors might compensate for a CCR5 defect.
The physiologic stimulation of antigen-specific CD4 T lymphocytes promotes the productive HIV infection with R5- and X4-tropic viruses (Figure 2B). To determine whether the degree of chemokine receptor up-regulation was sufficient to support HIV replication, highly purified SEB-specific Vβ3, 14, and 17 CD4 T cells were stimulated with SEB as above and infected with R4-tropic HIV-1Lai and R5-tropic HIV-1BaL. These virus strains were chosen because of their strict coreceptor usage, as evidenced by their capacity to induce only syncytia in U87-CXCR4– and U87-CCR5–expressing indicator cell lines, respectively (data not shown). Virus replication was monitored by measuring HIV-p24 in the cell culture supernatant. Only SEB stimulation supported virus growth, whereas SEE as a control antigen did not.
The stimulation of primary CD4 T cells leads to a significant up-regulation of CCR5 and CXCR4 and a HIV-permissive phenotype of the cells. This provides evidence that antigenic stimulation per se cannot be the reason for the preference of R5 tropic viruses in HIV transmission. Rather, the dominant expression of CCR5, ie, in the genital tract or on immature dendritic cells might be a more important factor.23 24 Overall, the results strengthen the important role of antigen for efficient HIV replication. As bacterial superantigens are very common antigens, these antigens might contribute significantly to the HIV load in vivo.
Acknowledgments
We thank Prof Nikolaus Mueller-Lantzsch for continuous support and Christa Püttmann and Doris Wild for expert technical assistance.
Supported by the BMBF and SFB399.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Andreas Meyerhans, Abteilung Virologie, Institut für Medizinische Mikrobiologie und Hygiene, Universität des Saarlandes, 66424 Homburg, Germany; e-mail:andreas.meyerhans@med-rz.uni-sb.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal