Abstract
Natural killer (NK) cells are deficient in patients with chronic myelogenous leukemia (CML), but the mechanisms responsible for the dysfunction are not completely understood. This study reports that CML cells effectively inhibit the baseline and interleukin-2 (IL-2)-induced NK cell cytotoxicity against a CML cell-derived line (K562). A sizable fraction of NK cells subsequently acquired features characteristic of programmed cell death/apoptosis. The CML cell-mediated inhibition of NK cells required triggering of reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-mediated formation of reactive oxygen species (ROS) and was prevented by catalase, a scavenger of ROS, and by histamine, acting via H2-receptor–mediated inhibition of ROS production in CML cells. In contrast, nonmalignant neutrophilic granulocytes inhibited NK cells via ROS production without the requirement of exogenous NADPH oxidase-triggering stimuli. We propose that paracrine production of ROS may contribute to the dysfunction of NK cells in CML and that histamine may serve as an autocrine inhibitor of ROS formation in leukemic granulocytes.
Introduction
Chronic myelogenous leukemia (CML) is a fatal myeloproliferative disease characterized by massive expansion of hematopoietic progenitor cells with the appearance of cells of the granulocyte (GR) lineage in the peripheral blood. Natural killer (NK) cells, a subset of lymphocytes endowed with spontaneous cytotoxicity against tumor cells,1,2 have been proposed to participate in surveillance of the malignant clone in CML.3 However, NK cells decrease in number and function during the course of CML; typically, the absolute number of NK cells is profoundly reduced in patients with late-stage disease as compared with healthy control subjects, and a reduction of NK cell inducibility and proliferation accompanies disease progression.3 The NK cell dysfunction is apparently unrelated to prior cytotoxic therapy and triggered by an as yet undefined inhibitory signal in the malignant microenvironment.4
Understanding the mechanisms of the NK cell dysfunction in CML could be useful in elucidating how this disease develops and in identifying therapeutic strategies. Earlier studies have revealed that cells of the monocyte/macrophage lineage effectively inhibit NK cell function; thus, monocytes suppress NK cell cytotoxicity, proliferation, and cytokine gene transcription5,6 and render NK cells resistant to activating cytokines such as interleukin-2 (IL-2) or interferon-α (IFN-α).5 Eventually, a sizable fraction of NK cells undergo fragmentation of nuclear DNA and die by apoptosis after cell-to-cell contact with monocytes.7 Reactive oxygen species (ROS), such as superoxide anion, hydrogen peroxide, and other reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-dependent oxygen metabolites, are the main mediators of the NK cell inhibition as suggested by the findings that (1) monocytes recovered from patients with a constitutively defective ability to generate ROS (chronic granulomatous disease) do not inhibit NK cells,5 and (2) catalase, a scavenger of hydrogen peroxide, and histamine, an inhibitor of NADPH oxidase-dependent formation of ROS, reverse the inhibition of NK cell function,5,6 and protect NK cells against monocyte-induced apoptosis.7
Malignant CML cells carry a functional membrane NADPH oxidase and are thus endowed with the capacity to produce ROS.8 We therefore explored whether ROS generated by CML cells alter functions of NK cells in vitro, and compared these properties of CML cells with those of nonmalignant neutrophilic granulocytes (GR). We report that CML cells profoundly inhibit the baseline and cytokine-induced cytotoxicity of NK cells and trigger significant apoptosis in NK cells. Histamine, a constituent of CML cells, and catalase almost completely rescue NK cell cytolytic activity and protect NK cells from CML cell-induced apoptosis. In contrast to nonmalignant GR, CML cells require an external stimulus to inhibit NK cells. It is hypothesized that paracrine production of ROS may contribute to the NK cell dysfunction in CML and that histamine may serve as an autocrine regulator of ROS production in leukemic granulocytes.
Materials and methods
Separation of lymphocytes, GR, and CML cells
Leukocytes were isolated from freshly prepared leukopacks obtained from healthy blood donors at the Blood Center, Sahlgren's Hospital, Göteborg, Sweden. The buffy coat (∼65 mL) was mixed with 92.5 mL Iscoves medium, 35 mL dextran (6%; Kabi Pharmacia, Stockholm, Sweden) and 7.5 mL acid-citrate-dextrose (Baxter, Deerfield, IL). After incubation for 15 minutes at room temperature, the supernatant was carefully layered onto Ficoll-Hypaque (Lymphoprep, Nyegaard, Norway). The mononuclear cells (MNC) were collected at the interface after centrifugation at 380g for 15 minutes, and washed twice in phosphate-buffered saline (PBS) followed by resuspension in Iscoves medium supplemented with 10% human AB+ serum (culture medium).
The MNC fraction was enriched for NK cells using counter-current centrifugal elutriation, as described in detail elsewhere.6 Briefly, the MNC were resuspended in elutriation buffer containing 0.5% bovine serum albumin (BSA) and 0.1% EDTA in buffered NaCl and fed into a Beckman J2-21 ultracentrifuge with a JE-6B rotor at 2100 rpm. A lymphocyte fraction enriched for NK cells (with CD3−/56+phenotype) and T cells (CD3+/56−) was obtained at flow rates of 15 to16 mL/min. This fraction did not contain monocytes (< 2%) and consisted of CD3−/56+NK cells (45-50%), CD3+/56−T cells (35-40%), CD3−/56−cells (5-10%), and CD3+/56+ cells (1-5%), as judged by flow cytometry.
To enrich GR, the red blood cell (RBC)/neutrophil pellet recovered after Ficoll-Hypaque separation was immediately mixed with 100 mL elutriation medium (ie, Krebs-Ringer phosphate buffer; KRG, pH 7.3; 120 nmol/L NaCl, 5 mmol/L KCl, 1.7 mmol/L KH2PO4, 8.3 mmol/L NaHPO4, and glucose 10 mmol/L, not supplemented with Ca++ to minimize spontaneous activation and adhesion of neutrophils to tubing). At a flow rate of 20 mL/min, the cells were fed into a Beckman ultracentrifuge with a JE-6B rotor spinning at 2150 rpm at 4°C. Under these conditions, GR remain in the elutriation chamber. After 25 minutes the flow rate was increased to 40 mL/min and neutrophilic GR were consistently recovered at a purity of more than 98%. Freshly isolated GR were thereafter resuspended in KRG buffer supplemented with Ca++ (1 mmol/L) and Mg++ (1.5 mmol/L), and kept on ice until used either in the cytotoxic or apoptosis assays or for measurement of ROS production.
The same elutriation procedure was used to enrich CML cells from a RBC/neutrophil pellet. The elutriated cell fraction contained cells with the morphology of mature neutrophilic granulocytes (95%), monocytes (1%), bands (1%), and eosinophils (3%). The CML cells were recovered from patients (n = 9; age 18-63) with newly diagnosed, untreated CML in chronic phase. Chromosome analysis of cells revealed 100% translocation 9:22 (Ph+) in all patients. Manual differential counts at the time of cell sampling showed a median of 0% (range, 0-0.5) blasts, 0.5% (0-1.5) promyelocytes, 4% (0-7.5) myelocytes, 1.5% (0-12) metamyelocytes, 4% (3-9) bands, and 65% (58-77) mature neutrophilic granulocytes.
Target cells and microcytotoxicity assay
K562 cells, an NK cell-sensitive cell line originating from a CML patient in blast crisis,9 were used as target cells. The K562 cells (5-10 × 106 cells/mL) were incubated with51Cr (Amersham, Stockholm, Sweden) at a concentration of 150 μCi/mL cell suspension for 2 hours. After centrifugation and resuspension in culture medium, 104target cells in 50-μL portions were added to effector cells in 96-well microplates (Nunc, Roskilde, Denmark).
The NK cell-enriched lymphocytes and target cells were incubated in sextuplicate in microplates in a total volume of 200 μL in the presence or absence of GR or CML cells. The compounds used were added at the onset of incubation with the exception of N-formyl-Met-Len-Phe (fMLF), which was added at 15 minutes. After incubation at 37°C for 16 hours, supernatant fluids were collected by a tissue collecting system (Amersham) and assayed for radioactivity in a γ-counter. Maximum 51Cr-release was determined in target cell cultures treated with Triton X-100. NK cell cytotoxicity was calculated as cell lysis percent according to the following formula: 100 x [(experimental release − spontaneous release)/(maximum release − spontaneous release)] = cell lysis %.
In accordance with earlier studies,5 6 more than 90% of the lymphocyte cytotoxicity against K562 cells was depleted by the removal of CD56+ NK cells (by use of anti-CD56-coated Dynabeads) from the effector lymphocyte preparation; in contrast, removal of CD3+ T cells (by use of anti-CD3-coated Dynabeads) did not significantly reduce cytotoxicity.
Detection of surface antigens
One million cells were incubated with appropriate fluorescein isothiocyanate (FITC)-and phycoerythrin (PE)-conjugated monoclonal antibodies (Becton & Dickinson, Stockholm, Sweden; 10 μL/106 cells) on ice for 30 minutes. The cells were washed twice in PBS and resuspended in 500 μL sterile filtrated PBS and analyzed by use of flow cytometry on a FACSort with a Lysys II software program (Becton & Dickinson). Lymphocytes were gated on the basis of forward and right angle scatter. The flow rate was adjusted to less than 200 cells/s and at the least 5 × 103 cells were analyzed for each sample.
Apoptosis in NK cells
Three methods were used to analyze the frequency of lymphocytes with apoptotic features after contact with GR or CML cells. Apoptotic morphology was detected by use of flow cytometry. A gate was set to comprise lymphocytes with reduced forward scatter and increased right angle scatter characteristic of apoptosis.7 Two FACS-based methods were used to confirm apoptosis in these cells, detection of extracellular annexin V (Annexin V-FITC Apoptosis Detection Kit I; PharMingen, San Diego, CA),10 and analysis of intracellular content of reduced intracellular glutathione (Cell Tracker, Eugene, OR).11 12
Chemiluminescence measurement
Chemiluminescence measurement was performed at 37° in a 6-channel Biolumat LB 9505 (Berthold Co, Wildbad, Germany) by using disposable 4-mL polypropylene tubes, as described.13 The reaction mixture contained 0.8 mL elutriated CML cells (5 × 106/mL) in a balanced salt solution (Krebs Ringer glucose) also containing horseradish peroxidase (HRP; 4 U) and luminol (20 μmol/L). The tubes were allowed to equilibrate for 5 minutes at 37° before fMLF (0.1 μmolL, final concentration) was added, and light emission was recorded (results given as cpm, and 7.2 × 107 cpm equals 1 nmole of superoxide anion, as measured with a cytochrome C-reduction technique).13
Compounds
Histamine dihydrochloride (Sigma Chemicals, St Louis, MO), the H2R antagonist ranitidine hydrochloride (Glaxo, Mölndal, Sweden), AH202399AA (a chemical control to ranitidine, kindly provided by Glaxo), catalase (Boehringer Mannheim, Mannheim, Germany), human recombinant IL-2 (EuroCetus, Amsterdam, The Netherlands), and fMLF (Sigma Chemical) were used. MNC treated with culture medium served as controls.
Results
Nonmalignant GR inhibit NK cell function
The effects of benign GR on NK cell function were determined by adding GR to lymphocytes enriched for NK cells in microplates for assay of cytotoxicity against CML cell-derived target cells (K562). The GR/effector cell ratios used were 1:1 to 1:10 (100 000 NK cell-enriched lymphocytes admixed with 100 000-10 000 GR).
Granulocytes effectively depressed NK cell-mediated cytotoxicity against K562 cells without the apparent requirement of additional exogenous stimuli. Figure 1 shows a representative experiment in which GR inhibited the spontaneous cytotoxicity of heterologous NK cells in a 16-hour 51Cr assay, and similar results were obtained using GR and NK cells recovered from 9 blood donors. In most experiments, GR/lymphocyte ratios of 1:2 or 1:4 were sufficient to significantly reduce cytotoxicity (Figure 1), and significant inhibition of NK cell cytotoxicity was observed also in 4-hour 51Cr assays (data not shown). Autologous and heterologous GR were equally effective in reducing NK cell cytotoxicity, and the degree of inhibition was similar after the removal of CD3+ T cells (by use of anti-CD3-coated Dynabeads) from the effector cell preparations (data not shown). We also explored whether GR affected the inducibility of NK cells by concomitantly treating mixtures of enriched NK cells and GR with IL-2, a prototypical NK cell activator.1 The presence of GR effectively reduced the NK cell activation induced by IL-2 (Table1).
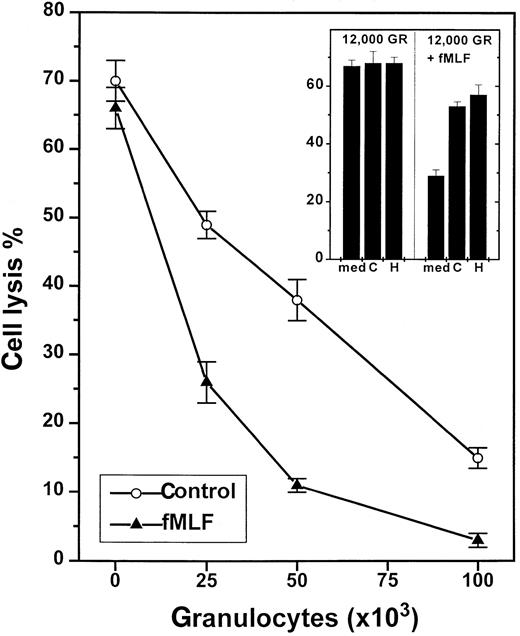
NADPH-oxidase-dependent inhibition of NK-cell cytotoxicity by nonmalignant GR.
One hundred thousand NK cell-enriched lymphocytes were admixed with GR and assayed for cytotoxicity against K562 cells (104cells/well) in a 16-hour assay. The cell cultures were treated with culture medium (control, ○) or fMLF (0.1 μmol/L, ▴), added at 15 minutes after the onset of incubation. Data are cell lysis percent ± SEM of sextuplicate determinations. The inset shows a corresponding experiment in which cells were treated with culture medium (med), histamine (H; 10 μmol/L), or catalase (C; 100 U/mL).
NADPH-oxidase-dependent inhibition of NK-cell cytotoxicity by nonmalignant GR.
One hundred thousand NK cell-enriched lymphocytes were admixed with GR and assayed for cytotoxicity against K562 cells (104cells/well) in a 16-hour assay. The cell cultures were treated with culture medium (control, ○) or fMLF (0.1 μmol/L, ▴), added at 15 minutes after the onset of incubation. Data are cell lysis percent ± SEM of sextuplicate determinations. The inset shows a corresponding experiment in which cells were treated with culture medium (med), histamine (H; 10 μmol/L), or catalase (C; 100 U/mL).
Inhibition of IL-2-induced NK cell cytotoxicity by GR and its reversal by histamine
| Experiment no. . | Cells . | Treatment* . | |||
|---|---|---|---|---|---|
| Medium . | Histamine . | IL-2 . | Histamine + IL-2 . | ||
| I | Lymph | 54 ± 3 | 53 ± 3 | 60 ± 3 | 66 ± 3 |
| Lymph + GR (80)† | 9 ± 1 | 23 ± 2 | 21 ± 3 | 48 ± 1 | |
| GR (80) | <3 | <3 | <3 | <3 | |
| II | Lymph | 48 ± 2 | 45 ± 3 | 62 ± 4 | 63 ± 2 |
| Lymph + GR (80) | 15 ± 2 | 30 ± 3 | 14 ± 3 | 47 ± 5 | |
| GR (80) | <3 | <3 | <3 | <3 | |
| Experiment no. . | Cells . | Treatment* . | |||
|---|---|---|---|---|---|
| Medium . | Histamine . | IL-2 . | Histamine + IL-2 . | ||
| I | Lymph | 54 ± 3 | 53 ± 3 | 60 ± 3 | 66 ± 3 |
| Lymph + GR (80)† | 9 ± 1 | 23 ± 2 | 21 ± 3 | 48 ± 1 | |
| GR (80) | <3 | <3 | <3 | <3 | |
| II | Lymph | 48 ± 2 | 45 ± 3 | 62 ± 4 | 63 ± 2 |
| Lymph + GR (80) | 15 ± 2 | 30 ± 3 | 14 ± 3 | 47 ± 5 | |
| GR (80) | <3 | <3 | <3 | <3 | |
Data are NK cell cytotoxicity against K 562 target cells (mean ± SEM of sextuplicate determinations). Results obtained using NK cell-enriched lymphocytes (lymph) and GR in two experiments are shown. Final histamine concentration was 10 μmol/L, IL-2 100 U/mL.
Numbers in parentheses refer to GR added × 103.
Role of ROS
To investigate the putative contribution by ROS for the observed GR-induced NK cell inhibition, we first added fMLF, a chemotactic tripeptide known to rapidly trigger NADPH-dependent ROS formation in GR.14 fMLF did not alter NK cell-mediated cytotoxicity in the absence of GR, but effectively reinforced the GR-induced inhibition of NK cells. A total of 7 experiments were performed in which fMLF was found to boost the GR-induced inhibition at GR/effector cell ratios of 1:8, 1:4, 1:2, and 1:1 (P < .01 for all ratios examined, Mann-Whitney U test). Figure 1 (inset) shows NK cell inhibition by a GR/effector cell ratio of 1:8. At this low density, unstimulated GR did not significantly affect NK cell cytotoxicity, whereas fMLF-treated GR reduced NK cell cytotoxicity by more than 50%.
Secondly, we introduced scavengers of ROS to corroborate the role of NADPH oxidase products for the GR-induced inhibition and to determine which species of ROS mediated the inhibitory signal. Superoxide dismutase, a scavenger of superoxide anion,5,14 did not reverse the spontaneous or fMLF-induced inhibition of NK cells, when used at concentrations sufficient to scavenge more than 99% of superoxide anion (200 U/mL; data not shown). Catalase, a scavenger of hydrogen peroxide,5,6 14 was found to significantly rescue NK cell function in the presence of suppressive GR (Figure2) with an ED50 of approximately 20 U/mL (Figure 2, inset), and to restore the NK cell response to IL-2. Catalase also prevented a major part of the fMLF-induced inhibition (Figure 1, inset).
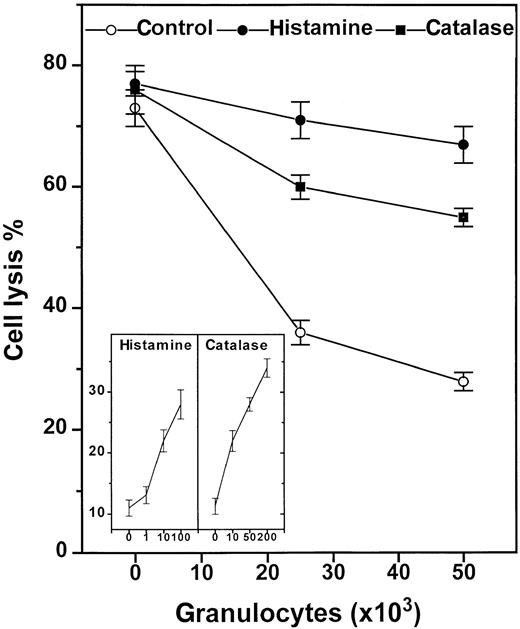
Histamine and catalase reverse GR-induced NK-cell inhibition.
Histamine (10 μmol/L, ●), catalase (100 U/mL, ▪), or culture medium (○) were added to 105 enriched NK cells alone or admixed with 2 × 104 or 5 × 104 GR in microplates. The compounds were added at the onset of a 16-hour assay. The inset shows a dose-response experiment using histamine (μmol/L) and catalase (U/mL) at an NK cell/GR ratio of 1:1. All data are cell lysis percent ± SEM of sextuplicate determinations.
Histamine and catalase reverse GR-induced NK-cell inhibition.
Histamine (10 μmol/L, ●), catalase (100 U/mL, ▪), or culture medium (○) were added to 105 enriched NK cells alone or admixed with 2 × 104 or 5 × 104 GR in microplates. The compounds were added at the onset of a 16-hour assay. The inset shows a dose-response experiment using histamine (μmol/L) and catalase (U/mL) at an NK cell/GR ratio of 1:1. All data are cell lysis percent ± SEM of sextuplicate determinations.
Granulocytes produce reactive nitrogen intermediates (NO).15 To study whether NO induction in GR contributed to the observed NK cell inhibition, we used a nitric oxide synthase inhibitor (N-nitro-l-arginine methyl ester [L-NAME]).15 This compound, used at final concentrations of 1 to 100 μmol/L, did not affect the GR-induced suppression of NK cells (data not shown).
Regulation by histamine via H2R
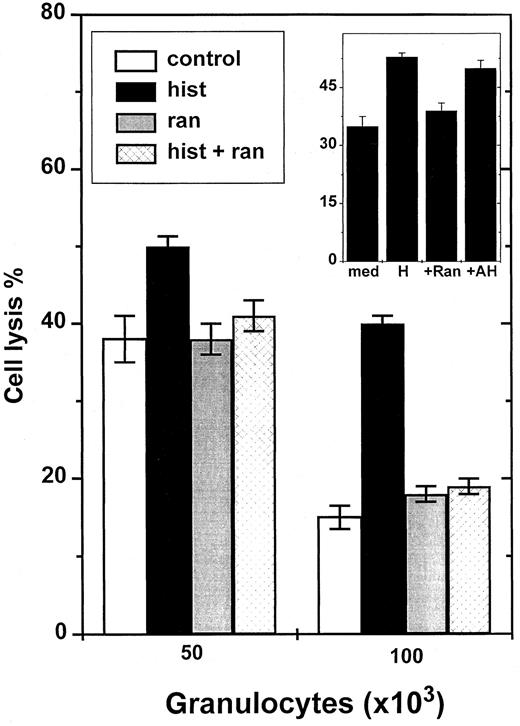
Histamine has earlier been shown to inhibit NADPH oxidase-dependent formation of ROS in neutrophilic GR and in other phagocytes.5 Histamine was found to protect NK cells from GR-induced inhibition with an efficacy similar to that of catalase (Figure 2), and significantly reversed the inhibition induced by fMLF-activated GR (Figure 1, inset). The effect of histamine was dose dependent with an ED50 of approximately 2 μmol/L (Figure2, inset). To determine which subtype of histamine receptors mediated the protective effect, we used ranitidine, an antagonist at H2R. Ranitidine, used at concentrations equimolar to histamine, completely blocked the histamine effect (Figure3). To exclude that nonspecific effects of ranitidine accounted for its blocking properties, we used AH202399AA, a ranitidine analogue in which a thioether group has been replaced by an ether, thereby reducing H2R antagonism more than 50-fold.5 12 This chemical control, used at concentrations equimolar to ranitidine, did not block the NK cell-protective effects of histamine (Figure 3, inset).
Ranitidine blocks histamine-induced reversal of GR-induced NK cell inhibition.
NK cells were added to microplate wells as described in Figure 1 and incubated with 5 × 104 or 105 GR. K562 cells were used as targets. The inset shows an experiment comparing effects of ranitidine and its inert chemical control, AH202399AA. All compounds were used at 10 μmol/L. Data are cell lysis percent ± SEM of sextuplicate determinations.
Ranitidine blocks histamine-induced reversal of GR-induced NK cell inhibition.
NK cells were added to microplate wells as described in Figure 1 and incubated with 5 × 104 or 105 GR. K562 cells were used as targets. The inset shows an experiment comparing effects of ranitidine and its inert chemical control, AH202399AA. All compounds were used at 10 μmol/L. Data are cell lysis percent ± SEM of sextuplicate determinations.
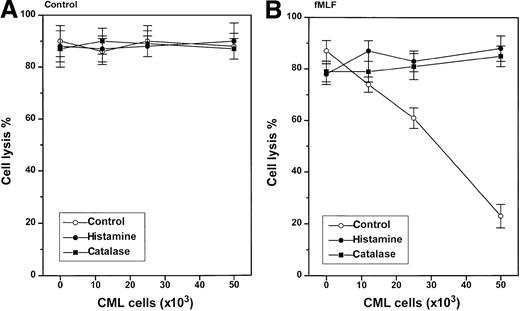
Stimulus-dependent inhibition of NK cell function by CML cells
In contrast to nonmalignant GR, CML cells did not constitutively inhibit NK cell function (Figure 4, left). However, CML cells induced to generate ROS by treatment with fMLF effectively inhibited the cytotoxicity of heterologous NK cells against K562 cells (Figure 4, right). The inhibition of NK cell cytotoxicity was observed at a CML cell/lymphocyte ratio of 1:1 using CML cells from all of the 9 patients examined, but the degree of inhibition was variable; fMLF-activated CML cells from some patients significantly suppressed NK cell cytotoxicity also at lower CML cell/lymphocyte ratios (data not shown). The inhibition of NK cell cytotoxicity was prevented by catalase and histamine (Figure 4, right). Ranitidine, used at concentrations equimolar to histamine, blocked the protective effect of histamine (data not shown). Figure5A summarizes the effect of GR or CML cells from 9 blood donors or 9 CML patients on NK cell function and its regulation by histamine. Figure 5B shows results obtained in parallel experiments in which NK cells were activated by IL-2.
Stimulus-dependent inhibition of NK-cells by CML cells: reversal by histamine and catalase.
One hundred thousand heterologous NK cell-enriched lymphocytes were admixed with 1.2 to 5 × 104 CML cells and assayed for cytotoxicity against K562 cells (104 cells/well) in a 16-hour assay. The cell cultures were treated with culture medium (control, ○), histamine (10 μmol/L, ●), or catalase (100 U/mL, ▪). The right part of the figure shows data obtained in cell cultures also treated with fMLF (0.1 μmol/L), added at 15 minutes after the onset of incubation. Data are cell lysis percent ± SEM of sextuplicate determinations.
Stimulus-dependent inhibition of NK-cells by CML cells: reversal by histamine and catalase.
One hundred thousand heterologous NK cell-enriched lymphocytes were admixed with 1.2 to 5 × 104 CML cells and assayed for cytotoxicity against K562 cells (104 cells/well) in a 16-hour assay. The cell cultures were treated with culture medium (control, ○), histamine (10 μmol/L, ●), or catalase (100 U/mL, ▪). The right part of the figure shows data obtained in cell cultures also treated with fMLF (0.1 μmol/L), added at 15 minutes after the onset of incubation. Data are cell lysis percent ± SEM of sextuplicate determinations.
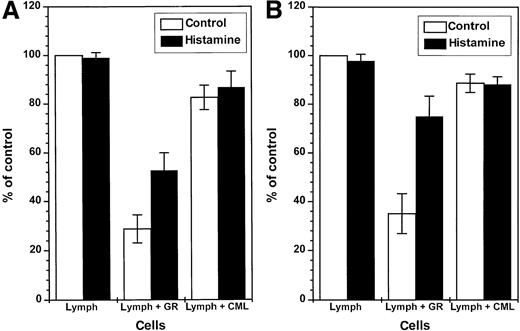
Constitutive inhibition of NK cells by GR: reversal by histamine.
One hundred thousand NK cell-enriched lymphocytes were admixed with 105 GR or 105 CML cells and assayed for cytotoxicity against K562 cells (104 cells/well) in a 16-hour assay. The cell cultures were treated with culture medium (■) or histamine (10 μmol/L, ▪). The results are expressed as the mean percent of baseline cytotoxicity against K 562 cells (control, part A), where the cytotoxicity of NK cell-enriched lymphocytes of each donor was set to 100% (mean baseline cytotoxicity of all donors: 49.2 cell lysis percent, range 32-74). Part B (IL-2) shows results obtained in parallel experiments in which cells were treated with IL-2 (100 U/mL, 16 hours), and the cytotoxicity of IL-2–activated lymphocytes of individual donors was set to 100% (mean IL-2–induced cytotoxicity: 59.2 cell lysis percent, range 38-85). Data are percent of control cytotoxicity (mean ± SEM) of NK cells incubated with GR from 9 healthy donors, or CML cells obtained from 9 CML patients in stable disease with 100% Ph+ cells. Statistics by Mann-WhitneyU test. Lymph (medium) versus lymph + GR (medium):P < .005 (control), P < .005 (IL-2); lymph + GR (medium) versus lymph + GR (histamine):P < .05 (control), P < .01 (IL-2); lymph + GR (medium) versus lymph + CML (medium):P < .005 (control), P < .005 (IL-2).
Constitutive inhibition of NK cells by GR: reversal by histamine.
One hundred thousand NK cell-enriched lymphocytes were admixed with 105 GR or 105 CML cells and assayed for cytotoxicity against K562 cells (104 cells/well) in a 16-hour assay. The cell cultures were treated with culture medium (■) or histamine (10 μmol/L, ▪). The results are expressed as the mean percent of baseline cytotoxicity against K 562 cells (control, part A), where the cytotoxicity of NK cell-enriched lymphocytes of each donor was set to 100% (mean baseline cytotoxicity of all donors: 49.2 cell lysis percent, range 32-74). Part B (IL-2) shows results obtained in parallel experiments in which cells were treated with IL-2 (100 U/mL, 16 hours), and the cytotoxicity of IL-2–activated lymphocytes of individual donors was set to 100% (mean IL-2–induced cytotoxicity: 59.2 cell lysis percent, range 38-85). Data are percent of control cytotoxicity (mean ± SEM) of NK cells incubated with GR from 9 healthy donors, or CML cells obtained from 9 CML patients in stable disease with 100% Ph+ cells. Statistics by Mann-WhitneyU test. Lymph (medium) versus lymph + GR (medium):P < .005 (control), P < .005 (IL-2); lymph + GR (medium) versus lymph + GR (histamine):P < .05 (control), P < .01 (IL-2); lymph + GR (medium) versus lymph + CML (medium):P < .005 (control), P < .005 (IL-2).
Because histamine is frequently a constituent of CML cells,17 we explored the possibility that release of histamine from CML cells in vitro could account for the lack of constitutive inhibition of NK cell cytotoxicity by CML cells. In these experiments, we added ranitidine, at 0.1 to 100 μmol/L, to mixtures of CML cells and NK cell-enriched lymphocytes, with the aim to antagonize extracellular histamine released from CML cells. Ranitidine did not alter NK cell-mediated cytotoxicity (against K562 target cells) in these cell mixtures (data not shown), indicating that release of histamine from CML cells was not a major mechanism responsible for the inability of resting CML cells to inhibit NK cells.
Histamine inhibits ROS formation in CML cells
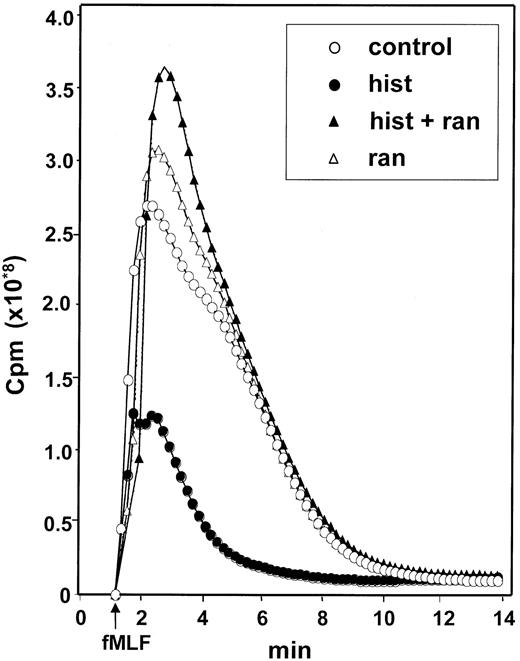
The finding that catalase prevented the CML cell-induced inhibition of NK cells suggested that ROS generated by CML cells significantly contributed to the inhibition. We therefore investigated whether histamine regulated ROS formation in CML cells using fMLF as the inducer. fMLF induced a prompt production of ROS in CML cells. This response was inhibited in a dose-dependent manner by histamine with an ED50 of approximately 2 μmol/L. The inhibitory effect of histamine was blocked by ranitidine but not by its chemical control, AH202399A, indicating that the effect of histamine was specifically transduced by H2-type receptors on CML cells (Figure6).
Histamine inhibits the generation of ROS in CML cells: reversal by ranitidine.
CML cells were treated with culture medium (control, ○), histamine (10 μmol/L, ●), ranitidine (10 μmol/L, ▵) and histamine + ranitidine (10 μmol/L + 10 μmol/L, ▴). Emission of chemiluminescence was recorded after addition of fMLF at t = 0. Abscissa, time of study (minute); ordinate, chemiluminescence in cpm. Similar results were obtained in experiments using CML cells from 4 patients with CML.
Histamine inhibits the generation of ROS in CML cells: reversal by ranitidine.
CML cells were treated with culture medium (control, ○), histamine (10 μmol/L, ●), ranitidine (10 μmol/L, ▵) and histamine + ranitidine (10 μmol/L + 10 μmol/L, ▴). Emission of chemiluminescence was recorded after addition of fMLF at t = 0. Abscissa, time of study (minute); ordinate, chemiluminescence in cpm. Similar results were obtained in experiments using CML cells from 4 patients with CML.
Benign and malignant GR induce apoptosis in NK cells
These results imply that GR, by producing ROS, are potent inhibitors of baseline and IL-2–induced NK cell cytotoxicity against a CML-derived target cell line, and that fMLF-activated CML cells share the NK cell-inhibitory properties of nonmalignant GR. In a series of experiments, we studied the fate of NK cells after contact with benign or malignant GR. We estimated the frequency of lymphocytes with morphologic features characteristic of apoptosis, that is, a reduced forward scatter and increased right angle scatter,7,12after overnight incubation with GR or CML cells. To confirm apoptosis in the gated lymphocytes, 2 additional cytometric methods were used—detection of extracellular annexin V and intracellular glutathione. These methods were chosen because they reflect early changes associated with apoptosis,10 11 and therefore allow a concomitant analysis of lymphocyte phenotype.
Apoptotic cells translocate the membrane phosphatidylserine from the inner leaflet of the plasma membrane to the outer, and phosphatidylserine is thereby exposed to the extracellular environment, and annexin V binds to externalized phosphatidylserine.10Simultaneous analyses of annexin V and morphology revealed a corresponding degree of annexin V staining and the appearance of lymphocytes with apoptotic morphology (data not shown). Oxidative-induced apoptotic cell death is also accompanied by depletion of intracellular stores of glutathione.11 12 The vast majority of lymphocytes with apoptotic morphology (> 95%) had intracellular glutathione levels, measured fluorimetrically as mean fluorescence intensity, which were 10- to 100-fold lower than those of lymphocytes with normal forward and side angle scatter.
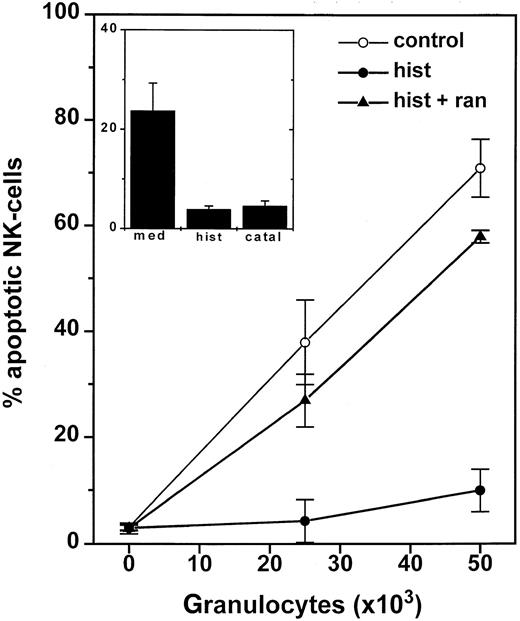
We observed an accumulation of CD56+ NK cells with apoptotic morphology and a parallel striking reduction of intracellular glutathione in NK cells after incubation with GR. These apoptotic features were barely detectable 4 hours after the onset of incubation with GR and reached a maximum at 16 to 24 hours. The GR-induced apoptotic morphology in NK cells was prevented by catalase and by histamine, and the effect of histamine was completely reversed by ranitidine (Figure 7). The protective effects of catalase and histamine were confirmed using annexin V staining and measurement of intracellular glutathione. Superoxide dismutase (200 U/mL) or L-NAME (1-100 μmol/L) did not prevent GR-induced apoptosis in NK cells (not shown).
GR-induced apoptosis in NK-cells.
One hundred thousand NK cell-enriched lymphocytes were admixed with 2.5 to 5 × 104 GR for 16 hours. The cell cultures were treated with culture medium (○), histamine (10 μmol/L, ●), or histamine + ranitidine (10 μmol/L + 10 μmol/L, ▴). After incubation, cells were labeled with anti-CD56 and assayed for morphologic apoptosis using flow cytometry. Data are the percentage of apoptotic NK cells among all gated lymphocytes and are the mean ± SEM of 5 blood donors. The inset shows the corresponding data for apoptotic T cells (CD3+/CD56−) and are the mean ± SEM of 5 donors.
GR-induced apoptosis in NK-cells.
One hundred thousand NK cell-enriched lymphocytes were admixed with 2.5 to 5 × 104 GR for 16 hours. The cell cultures were treated with culture medium (○), histamine (10 μmol/L, ●), or histamine + ranitidine (10 μmol/L + 10 μmol/L, ▴). After incubation, cells were labeled with anti-CD56 and assayed for morphologic apoptosis using flow cytometry. Data are the percentage of apoptotic NK cells among all gated lymphocytes and are the mean ± SEM of 5 blood donors. The inset shows the corresponding data for apoptotic T cells (CD3+/CD56−) and are the mean ± SEM of 5 donors.
We next examined whether CML cells induced apoptosis in NK cells in a fashion similar to GR. In contrast to GR, CML cells did not constitutively induce apoptosis in lymphocytes, but a sizable fraction of NK cells became apoptotic after overnight incubation with fMLF-activated CML cells. The CML cell-induced apoptosis in NK cells was prevented by histamine (Tables 2 and3). The protective effect of histamine was mimicked by catalase and completely reversed by ranitidine, but not by AH202399AA (data not shown).
CML cell-induced morphologic apoptosis in CD56+ NK cells: protection by histamine
| Cells . | CD56+ . | CD56− . | ||
|---|---|---|---|---|
| Medium . | Histamine . | Medium . | Histamine . | |
| Lymph + CML (50)* | 64.7 | 33.3 | 7.8 | 4.3 |
| Lymph + CML (25) | 30.7 | 16.3 | 1.6 | 1.6 |
| Lymph + CML (10) | 23.2 | 6.6 | 1.3 | 0.9 |
| Cells . | CD56+ . | CD56− . | ||
|---|---|---|---|---|
| Medium . | Histamine . | Medium . | Histamine . | |
| Lymph + CML (50)* | 64.7 | 33.3 | 7.8 | 4.3 |
| Lymph + CML (25) | 30.7 | 16.3 | 1.6 | 1.6 |
| Lymph + CML (10) | 23.2 | 6.6 | 1.3 | 0.9 |
Data are expressed as the percentage of NK cells (CD56+) and T cells (CD56−) with morphologic apoptotic features, as reflected by a reduced forward and side angle scatter. The population of gated CD56− lymphocytes consisted of >80% CD3+ T cells. Final histamine concentration was 10 μmol/L. Similar results were obtained in three separate experiments.
Numbers in parentheses refer to CML cells × 103.
CML cell-induced depletion of intracellular glutathione in CD56+ NK cells: protection by histamine
| Cells . | All lymphocytes . | CD56+ . | CD56− . | |||
|---|---|---|---|---|---|---|
| Medium . | Histamine . | Medium . | Histamine . | Medium . | Histamine . | |
| Lymph + CML (50)3-150 | 35.5 | 14.9 | 71.9 | 38.4 | 14.6 | 7.1 |
| Lymph + CML (25) | 14.5 | 4.4 | 35.6 | 19.2 | 3.7 | 2.7 |
| Lymph + CML (10) | 5.4 | 3.1 | 25.3 | 9.4 | 2.6 | 1.8 |
| Cells . | All lymphocytes . | CD56+ . | CD56− . | |||
|---|---|---|---|---|---|---|
| Medium . | Histamine . | Medium . | Histamine . | Medium . | Histamine . | |
| Lymph + CML (50)3-150 | 35.5 | 14.9 | 71.9 | 38.4 | 14.6 | 7.1 |
| Lymph + CML (25) | 14.5 | 4.4 | 35.6 | 19.2 | 3.7 | 2.7 |
| Lymph + CML (10) | 5.4 | 3.1 | 25.3 | 9.4 | 2.6 | 1.8 |
Data are expressed as the percentage of NK cells (CD56+) and T cells (CD56−) with morphologic apoptotic features, as reflected by reduced intracellular content of glutathione. The population of gated CD56− lymphocytes consisted of >80% CD3+ T cells. Results obtained using lymphocytes and fMLF-activated CML cells are shown. Final histamine concentration was 10 μmol/L. Similar results were obtained in three separate experiments.
Numbers in parentheses refer to CML cells × 103.
Lymphocyte subsets differ in sensitivity to GR or CML cell-induced apoptosis
We compared the degree of apoptosis in CD56+ NK cells and in other lymphocyte subsets. GR as well as CML cell-induced apoptosis was significantly more pronounced in CD56+ NK cells than in CD3+ T cells. Thus, at a GR density of 2.5 × 104/well, approximately 40% (mean of 5 donors; Figure 7) of all gated CD56+ NK cells had acquired apoptotic morphology, whereas less than 25% of gated T cells were apoptotic, and this difference attained statistical significance (P < .01, Student t test for pairs; Figure 7, inset). A similar difference in propensity to apoptosis was observed when GR were replaced by fMLF-activated CML cells (Tables 2and 3).
Discussion
In the first part of this study, we show that normal GR constitutively and effectively inhibit NK cell function, render NK cells resistant to activation by IL-2, and induce apoptosis in NK cells. These GR-triggered events were reduced or inhibited by a scavenger of hydrogen peroxide (catalase), but not by a scavenger of superoxide anion (superoxide dismutase) or a NO synthase inhibitor (L-NAME), suggesting that hydrogen peroxide, or NADPH oxidase-dependent ROS formed downstream of this compound, were the main mediators of the GR-induced suppression. Thus, GR share the NK cell-inhibitory properties previously reported for monocytes/macrophages,6 12 and use a similar inhibitory mechanism.
Several investigators have reported that GR inhibit the cytotoxicity of NK cells, but the mechanism responsible for the inhibition has been a matter of controversy. Shau and Golub reported that resting neutrophils inhibited lymphokine-activated killer cell-mediated cytotoxicity when added to IL-2–activated lymphocytes and target cells. The inhibition was resistant to scavengers of ROS such as superoxide dismutase or catalase.18 Lala and coworkers reported that long-term incubation (1-4 days) of NK cells with polymorphonuclear neutrophils resulted in the formation of NK cell-suppressive prostaglandins.19 In our hands, GR-derived ROS were the predominant suppressive mediators, although the contribution by other inhibitory factors could not be ruled out. For example, even optimal concentrations of catalase or histamine could not entirely eliminate the GR-induced suppression. In this regard, GR differ from monocytes/macrophages, because the inhibition of NK cells induced by these cells under similar experimental conditions is almost completely prevented by catalase and NADPH oxidase inhibitors.5 12
A second part of this study was devoted to effects of CML cells on NK cell function and viability. Our interest in this area was inspired by the numerous reports claiming that NK cells contribute in surveillance of the malignant clone in CML. Thus, CML cells are frequently susceptible to the lytic activity of NK cells; early studies demonstrated that allogeneic NK cells activated by IFN-α, an NK cell-activating cytokine, were cytotoxic for freshly recovered CML cells in vitro.3,20-22 These antileukemic properties of NK cells appear to be relevant in surveillance against CML cells in vivo; for example, patients with CML whose peripheral blood NK cells can mount lytic activity in vitro against malignant CML cells in response to IL-2 have a lower risk of relapse after allogeneic bone marrow transplantation than patients lacking such cytotoxicity,23 and a correlation between NK cell recovery after bone marrow transplantation and remission maintenance in CML has been demonstrated.24 A further indication of a role for NK cells in protection against malignant cells in CML is that NK cells are considered to contribute to the striking therapeutic benefit of IFN-α in CML. Pawelec and coworkers25 reported a steady increase in NK cell-mediated cytotoxicity in vitro in patients with CML during treatment with IFN-α, a phenomenon not observed for T-cell function, and Meseri and colleagues26 found a correlation between cytogenetic remission and the appearance of NK-like cytotoxicity in the peripheral blood of patients with CML treated with IFN-α.
In addition to the proposed role of NK cells in surveillance of malignant cells in CML, it seems well established that NK cells are deficient in number and function in this disease. Early studies showed that peripheral blood lymphocytes recovered from CML patients were less cytotoxic for conventional NK cell-sensitive target cells in vitro as compared with lymphocytes recovered from healthy donors,27or from patients with other chronic leukemias.28 In subsequent studies, it was shown that the absolute number of circulating NK cells and the cell cycle proliferation of NK cells of patients with CML are subnormal, and that a reduction of the number and inducibility of NK cells accompanies disease progression.4 29
Much effort has been devoted to the characterization and functional significance of the NK cell defect in CML, but the mechanism underlying the dysfunction has remained largely unknown.3 In brief, the results of this study reveal a complex interplay between CML cells and NK cells, which may be of relevance to the NK cell inhibition in CML. Thus, ROS produced by activated CML cells inhibited the baseline and cytokine-induced cytotoxicity of NK cells, and a significant fraction of NK cells from healthy blood donors became apoptotic after incubation with activated CML cells. These CML cell-triggered events—reduced cytotoxicity, reduced inducibility, and subsequent apoptosis—largely correspond with NK cells in CML patients, which are characterized by deficient cytolytic activity and deficient inducibility, paralleled by a reduction in NK cell number.4,29 30
However, our data also reveal that CML cells are less effective inhibitors of NK cells than normal GR. Thus, CML cells, but not GR, depended on an NADPH oxidase-triggering stimulus, fMLF, to inhibit NK cells. Whether CML cell-derived ROS inhibit NK cells in vivo and whether factors that trigger NADPH oxidase activity are required for such inhibition remains to be established. Two arguments may be put forward in support for the hypothesis that CML cell-derived ROS contribute to the NK cell dysfunction in this disease. First, in CML, the malignant cells are frequently present in high numbers in blood and in bone marrow and even a moderate production of ROS by these cells may have profound effects on adjacent cells. Second, recent data suggest that lymphocytes from CML patients may be subjected to oxidative inhibition. Buggins and coworkers demonstrated that NK cells and other lymphocytes in the peripheral blood of patients with CML at various stages of disease display a pronounced reduction of the expression of CD3ζ,31 a phenomenon associated with oxidative stress.6 32
We also report that histamine, acting via H2R, inhibited fMLF-induced ROS production by CML cells and, thereby, prevented the CML cell-induced inhibition of cytotoxicity and protected NK cells from apoptotic cell death. In earlier studies, H2-receptors transducing inhibition of ROS formation have been demonstrated on normal GR and on leukemic cell lines derived from phagocytes,15 but our report is the first to demonstrate functional histamine H2-receptors on freshly recovered CML cells. Whether the CML cell content of histamine significantly alters ROS synthesis in vivo is not known, but a role for histamine is suggested by the high serum levels of histamine and the occasional symptoms related to hypersecretion of gastric acid and pepsin in CML patients, suggesting that H2R can be targeted by CML cell-derived histamine in vivo.33
Our data suggest that histamine may serve as an autocrine inhibitor of ROS production in CML cells, thereby preventing functional inhibition and apoptosis in adjacent lymphocytes. However, if we accept that ROS production is a mechanism by which CML cells can escape NK cells or other lymphocytes with antineoplastic function, it seems reasonable to also assume that the production of ROS-inhibitory histamine by the CML cells is not sufficient to prevent inhibition of NK cells, because NK cells undoubtedly deteriorate in function and number during progression of the disease.3 4 This assumption is supported by our finding that CML cells did not constitutively release histamine in amounts sufficient to protect NK cells or T cells. Thus, a putative balance between ROS production and histamine in CML is probably shifted in favor of ROS production.
The frequency of apoptosis in lymphocytes after contact with activated CML cells or nonmalignant GR was considerably higher in CD56+ NK cells than in T cells. These findings are consonant with earlier studies demonstrating that NK cells are more prone than T cells to acquiring apoptotic features after exposure to hydrogen peroxide,7 12 and suggest that T cells are, at least in part, protected against oxidatively-induced apoptosis. The mechanisms underlying this difference in apoptotic propensity between NK cells and T cells should be the focus of further investigation.
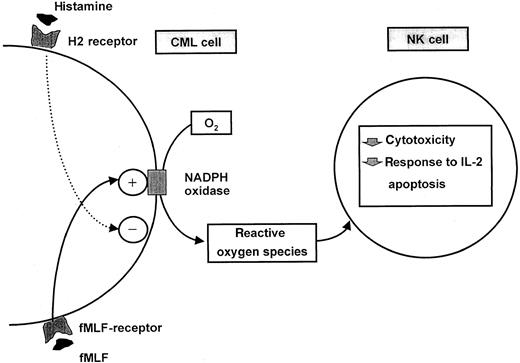
Our data are suggestive of novel mechanisms by which CML cells affect functions of NK cells. A balance may be at hand in CML, because CML cells are producers of NK cell-inhibitory ROS, and contain histamine, which can function as an autocrine inhibitor of ROS formation and, thereby, protect NK cells from CML cell-derived inhibition. These interactions are schematically depicted in Figure8.
Schematic description of an interaction between ROS-generating CML cells and NK-cells and its regulation by histamine.
Schematic description of an interaction between ROS-generating CML cells and NK-cells and its regulation by histamine.
Acknowledgments
We are indebted to Marie-Louise Landelius for expert technical assistance and to Bo Nilsson and Örjan Strannegård for critical review of this manuscript.
Supported by the Swedish Society Against Cancer (Cancerfonden), the Foundations of Jubileumskliniken at Sahlgren's University Hospital, the Assar Gabrielsson Foundation, the Faculty of Medicine, University of Göteborg, and Maxim Pharmaceuticals, Inc, San Diego, CA.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kristoffer Hellstrand, Department of Virology, Sahlgren's University Hospital, S-413 46 Göteborg, Sweden; e-mail: kristoffer.hellstrand@microbio.gu.se.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal