Abstract
Deficiency of lysosomal acid β-glucosidase induces glycolipid storage in the macrophages of Gaucher disease but the pathways of multisystem tissue injury and destruction are unknown. To investigate the cognate molecular pathology of this inflammatory disorder, genes that were differentially expressed in spleen samples from a patient with Gaucher disease (Gaucher spleen) were isolated. Of 64 complementary DNA (cDNA) fragments sequenced from an enriched Gaucher cDNA library, 5 encode lysosomal proteins (cathepsins B, K, and S, α-fucosidase, and acid lipase), 10 encode other known proteins, and 2 represent novel sequences from human macrophage cell lines. Transcript abundance of the cathepsins, novel genes, pulmonary and activation-regulated chemokine (PARC), and NMB, a putative tumor suppressor gene, was greatly increased. Immunoblotting showed increased mature forms of all 3 cathepsins found in samples of Gaucher spleens. Immunofluorescence microscopy showed strong cathepsin B and K reactions in sinusoidal endothelium and Gaucher cells. The respective means, plus or minus SD, of cathepsin B, K, and S activities were 183 ± 35, 97 ± 39, and 91 ± 45 nmol/min/mg protein in 4 Gaucher spleens, and 26 ± 4, 10.5 ± 2, and 4.0 ± 2.1 nmol/min/mg protein in 3 control spleens. Plasma cathepsin B, K, and S activities were also elevated in Gaucher disease plasma (P < .001), but compared with control plasma samples, neither cathepsin B nor K activities were significantly elevated in 8 patients with nonglycosphingolipid lysosomal storage diseases or in 9 patients with other glycosphingolipidoses, which suggests disease specificity. All 3 cathepsin activities were increased 2-fold to 3-fold in Gaucher sera compared with control sera. In all 6 patients treated by enzyme replacement for 16-22 months, serum cathepsin activities decreased significantly (P < .01). Longitudinal studies confirmed the progressive reduction of proteinase activities during imiglucerase therapy but in 3 Gaucher patients with mild disease not so treated, serum cathepsin activities remained constant or increased during follow-up. Enhanced expression of cysteine proteinases may promote tissue destruction. Moreover, the first identification of aberrant cathepsin K expression in hematopoietic tissue other than osteoclasts implicates this protease in the breakdown of the matrix that characterizes lytic bone lesions in Gaucher disease.
Introduction
Lysosomal storage diseases result from inborn defects of acid hydrolases or membrane transporters that lead to the pathologic accumulation of complex cellular macromolecules. Gaucher disease, a multisystem disorder affecting macrophages, identified in the Online Mendelian Inheritance in Man (OMIM) website as OMIM 23080, 23091, and 23100, has become the prototype of the glycosphingolipidoses, an important group of lysosomal disorders (www.ncbi.nlm.nih.gov/omim). The condition is caused by inherited deficiency of the acid β-glucosidase, glucocerebrosidase (Enzyme Commission [EC] 3.2.1.45), which is widely distributed in the lysosomes.1,2 The principal storage material in Gaucher disease is N-acyl-sphingosyl-1-O-β-D-glucoside, but other minor components, including glucosyl sphingosine, also accumulate. These glycolipids are metabolic intermediates derived from the cellular turnover of membrane gangliosides and globosides.3
In the nonneuronopathic (type I) form of Gaucher disease, partial deficiency of glucocerebrosidase is associated with the accumulation of glycolipids in the mononuclear phagocyte system, especially the liver, bone marrow, and spleen. Here the stored material originates from the turnover of exogenous lipids derived from the breakdown of blood cells by macrophages; indeed the pathognomonic Gaucher cell that characterizes this disorder is of the macrophage lineage.4Severe deficiency of glucocerebrosidase caused by disabling mutations is additionally associated with neurologic manifestations: failure to degrade endogenous glycosphingolipids present in brain tissue leads to the neuronopathic (type II and III) disease variants.
Although the Gaucher cell is a striking histologic feature of Gaucher disease, the relationship between the lysosomal storage of glycosphingolipid and the protean manifestations of this disorder are unexplained. The disease is accompanied by weight loss, fatigue, increased metabolic rate, pingueculae, and a sustained acute inflammatory reaction with polyclonal or monoclonal B-cell proliferative responses. These manifestations accompany massive enlargement of the spleen and liver; bone infarction crises; and osteolytic lesions as well as tissue injury in the liver, lung, bone marrow, and brain stem.3,5 Although the liver and spleen are the main sites for the accumulation of glycolipid in the body and may increase to a mass more than 4-fold and 80-fold greater than normal, respectively, the pathologic lipid accounts for less than 2% of the additional tissue weight.6,7 Thus the connection between the macrophage abnormality and the complex inflammatory phenotype that characterizes this disease remains obscure. It seems likely that the accumulated glycolipid activates macrophages to induce an inflammatory response. Hence, factors released by Gaucher cells may provide a mechanistic link between the lysosomal storage and the clinical manifestations of established Gaucher disease.8-11
To understand better the pathogenesis of Gaucher disease, we examined the molecular characteristics of the disorder by studying the gene expression profile of the Gaucher cell within its tissue context. We used a newly described subtractive procedure based on the polymerase chain reaction (PCR) to identify genes whose transcription products are increased in Gaucher disease tissue.12 The method overcomes the problem of differences in the distribution of gene transcripts in the 2 populations by incorporating a hybridization step that normalizes complementary DNA (cDNA) abundance within each population. We report the identification of multiple genes, including a chemokine and others associated with the inflammatory response whose expression is enhanced in Gaucher disease; 5 lysosome protein-specific cDNAs were also identified. Here we focus upon overexpression of the lysosomal cysteine proteases, cathepsins B, K and S, which are now known to participate in tissue modeling, antigen presentation, and, in the case of cathepsin K, bone matrix destruction.
Patients, materials, and methods
Patients
Samples of serum were collected from 12 patients with type I Gaucher disease who were attending a specialist center; the serum was taken with patient consent during the course of routine clinical monitoring. Gaucher disease was diagnosed on the basis of histologic and enzymatic criteria.3 Serum samples were also collected from 26 healthy control subjects, none of whom had a history of arthritis or Gaucher disease. The mean age of the patients with Gaucher disease was 48 years (range, 28-71 years). Of these 12 patients, 5 were men and 7 were women; 2 of the women and 3 of the men had undergone prior splenectomy. The age range of the control subjects, 12 men and 14 women, was 19-60 years.
Venous blood was collected from each subject and allowed to clot. It was centrifuged at 2000g for 10 minutes to separate the serum, and aliquots were transferred to polypropylene Eppendorf tubes and frozen at −70°C before assay. Plasma samples obtained from heparinized blood were also collected at the time of diagnosis from 11 patients with enzymatically confirmed Gaucher disease, 8 patients with nonglycolipid storage disorders, 9 patients with glycosphingolipidoses other than Gaucher disease, and 21 healthy control subjects. The experimental protocol was reviewed and approved by the Cambridge Research Ethics Committee of the East Anglian Health Authority at Addenbrooke's Hospital, Cambridge, England. Radiological lesions, indicating skeletal complications of Gaucher disease, were evident in 5 patients.
Tissue samples
Fresh splenic tissue was diced into 1-cm cubes and frozen in liquid nitrogen within a few minutes of removal; care was taken to use sections of tissue that were neither infarcted nor necrotic, as confirmed by histologic examination. Tissue integrity and polypeptide content were monitored in these samples using protein polyacrylamide gel electrophoresis (PAGE). Therapeutic splenectomy was carried out in these patients if they had intractable cytopenia, hypersplenism, or marked pressure symptoms. In 2 of 4 patients, enzyme replacement therapy in the form of human placental mannose-terminated glucocerebrosidase (Ceredase-alglucerase) had been administered at a dose of 40 U/kg/mo for 6 months without hematologic improvement. Macroscopic inspection of splenectomy specimens showed a large single area of recent infarction and necrosis. The material used for a subsequent study was sampled from tissue remote from these areas of disease. Control spleen samples were obtained from 3 individuals: a 32-year-old man with T-cell lymphoma and associated hypersplenism; a 52-year-old man suffering from idiopathic thrombocytopenic purpura (ITP) refractory to corticosteroids; and a 14-year-old male suffering from a recessive variant of hereditary spherocytosis.
Reagents and laboratory materials
We obtained the following materials: nitrocellulose filters (Schleicher and Schuell); α–32P-dCTP (cytidine 5′-triphosphate) (Amersham International, Little Chalfont, England) with a specific activity of 11.1 × 1013 Bq/mmol [3000 Ci/mmol]; RNA markers (Promega Company, Charbonnieres, France); prestained molecular weight protein markers and precast sodium dodecyl sulfate (SDS)-PAGE (Bio-Rad Laboratories, Hercules, CA); fluorogenic substrates N-carbobenzyloxy (CBZ)–phenylalanine–arginine–7-amido-4-methylcoumarin (AMC) (CBZ-F-R-AMC) and CBZ-R-R-AMC (Sigma Chemical, Poole, England); and antibodies to cathepsin B (Serotec, Oxford, England). All other biochemicals were of the highest purity commercially available for molecular biology use. The proteins were stained with Coomassie R250 blue. Rabbit antiserum to human cathepsin K was prepared as reported by Sukhova et al.45
Isolation of RNA
Total RNA was isolated from approximately 100 mg frozen splenic tissue using the Trizol reagent (Gibco BRL Life Technologies, Ergany, France) according to the manufacturer's instructions. Poly (A+) RNA was purified by the use of oligo d(T) spin columns (R & D Systems Europe, Abingdon, England).
Subtractive hybridization
A subtracted cDNA library of spleen cells from a patient with Gaucher disease (Gaucher spleen) was constructed using a minor modification of the PCR Select cDNA subtraction system (Clontech Laboratories, Heidelberg, Germany) using RNA prepared from the ITP control and a single Gaucher spleen sample.12 A double-stranded cDNA population was obtained from the Gaucher spleen, and a reference population of cDNAs was obtained from the control spleen by reverse transcription using oligo d(T) primers and amplification of DNA polymerase I. These were digested withRsaI, a restriction endonuclease. Pools of the Gaucher spleen cDNA in separated tubes were ligated to separate adaptor molecules overnight at 16°C. The sequences of these oligonucleotides and of the oligonucleotide primers subsequently employed in PCR-based amplification reactions are set out at the end of this section (see below). We mixed 20 ng ligated Gaucher spleen cDNA and 600 ng nonligated control spleen cDNA and the samples were denatured by heating to 98°C for 90 seconds. These samples were allowed to anneal for 10 hours at 68°C. After this first hybridization, the 2 samples were combined, and a fresh portion of approximately 150 ng of pooled heat-denatured control spleen cDNA was added. These samples were allowed to hybridize for a further 16 hours at 68°C.
Amplification of subtracted cDNA pools by PCR
For each subtraction, 2 amplifications by PCR were carried out according to the manufacturer's protocol, with modifications as set out below. The primary PCR contained 1 μL diluted subtractive cDNA population, 1 μL of a 5 μmol/L solution of primary primer P1, and 20 μL PCR master mix prepared using the cDNA PCR or reagent (Clontech). Amplification was carried out in the PCR with the following parameters: denaturation at 75°C for 7 minutes was followed by 30 cycles at 94°C for 30 seconds and 30 cycles at 72°C for 2.5 minutes. The amplified products were diluted 10-fold in deionized water. We used 1 μL of this product as a template for a secondary amplification in which the PCR was carried out for 15 cycles using the same conditions as described in the primary amplification. The only exception was that the oligonucleotide primer P1 was replaced with the following internally orientated and nested PCR oligonucleotide primers: adaptor 1, 5′-CTAATACGACTCACTATAGGGCTCGAGCGGCCGCCCGGGCAGGT-3′; adaptor 2, 5′-CTAATACGACTCACTATAGGGCAGCGTGGTCGCGGCCGAGGT-3′; PCR primer 1, 5′-CTAATACGACTCACTATAGGGC-3′; nested PCR primer 1, 5′-TCGAGCGGCCGCCCGGGCAGGT-3′; and nested PCR primer 2, 5′-AGCGTGGTCGCGGCCAGAGGT-3′.
Cloning and analysis of subtracted cDNA populations
The products of the secondary amplification reactions were ligated into the pT-Adv vector (Clontech). Approximately 150 ng PCR-amplified cDNA were ligated into 50 ng of the vector and the ligation mixture was introduced into competent cells (TOP 10 F′, Clontech) by heat shock at 42°C for 30 seconds. The bacteria were plated onto 20 × 22-cm agar plates containing 100 μg/mL ampicillin, 100 μmol isopropyl-β-D-thiogalactopyranoside, and 50 μg/mL X-Gal bromo-4-chloro-3 indoyl-D-β-galactopyranoside. The plates were incubated at 37°C until small colonies became visible; they were then further incubated at 4°C until blue-white staining could be distinguished clearly. A total of 100 individual colonies were picked and inoculated into 1.3 mL TB broth containing 100 μg/mL ampicillin. The bacteria were grown overnight in a rotary shaker at 325 rpm. Plasmid DNA was prepared using a spin minute prep kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. Purified plasmid DNA inserts were sequenced by single-pass fluorescent dye terminator cycle sequencing13 using an applied 373 Automated Sequencer (Applied Biosystems Inc, Weiterstadt, Germany). The edited DNA sequences were searched against the nucleic acid databases using the Basic Local Alignment Search Tool (BLAST) algorithm and repeat masker (www.ncbi.nlm.nih.gov/BLAST/).
Northern blot analysis
Aliquots of 4 μg poly (A+) RNA prepared from additional Gaucher and control spleen samples and RNA markers were resolved by electrophoresis in a 1.4% wt/vol formaldehyde agarose gel14 in 10 times SSC (side scatter criteria) buffer comprising 0.3 mol/L sodium chloride (NaCl) and 0.03 mol/L sodium citrate (pH 7.0). The RNA samples were transferred overnight onto a Hybond N+ membrane (Amersham) or nitrocellulose (Schleicher and Schuell). The filters were hybridized at 65°C for 3 hours with cDNA probes generated by digesting 2 μg plasmid with EcoRI for 2 hours at 37°C. The inserts (approximately 1 μg) were purified using the Prepagene procedure (Boehringer Mannheim, Mannheim, Germany) according to the manufacturer's protocol. We radio-labeled cDNA probes with 11.1×1013 Bq (3000 Ci/mmol) α–32P-dCTP using random primers (Strategene, France) for 2 hours at 37°C.15 The unincorporated label was removed before hybridization using columns of Sephadex G50 fine (Pharmacia Biotech, Lund, Sweden) 5% wt/vol in 10 mmol/L Tris HCl (tris[hydroxymethyl] aminomethane hydrochloride) (pH 7.5), 1 mmol/L EDTA (ethylenediamine tetraacetic acid), 50 mmol/L NaCl, and 0.05% wt/vol Triton-X 100. The filters were prehybridized and then hybridized to a radioactive probe at 65°C for 3 hours using rapid high buffer (Amersham). Washing was done with 2 times SSC and 0.1% wt/vol SDS for 20 minutes, followed by 1 times SSC and 0.1% SDS at 65°C for 30 minutes, and finally 0.1 times SSC and 0.1% SDS at 65°C for 45 minutes. Washed filters were then exposed to autoradiography film (Kodak X-Omat; Kodak, Rochester, NY) at −80°C for 1-400 hours, depending on the intensity of the signal. The relative amounts of RNA in Gaucher and control spleen tissue were quantified by scanner laser densitometry (LKB Bromma, Sweden) of the autoradiograms. All Northern blotting studies were conducted with RNA samples isolated from at least 2 independent Gaucher and control tissue specimens with similar results.
Western immunoblot analysis
Tissue preparation.
Samples containing approximately 50 mg freshly thawed Gaucher spleen tissue and control spleen tissue were homogenized in 500 μL of a lysis buffer (0.02 mol/L sodium acetate and 1 mmol/L EDTA [pH 5.5]) with a Dounce homogenizer. The samples were then rendered 0.05% wt/vol in the detergent Brij-35 and clarified by gentle mixing at 4°C.
Immunoblotting.
For electrophoresis, 10 μg protein from each extract was denatured in solubilizing buffer containing 62.5 mmol/L Tris HCl, 2% wt/vol SDS, 100 mmol/L dithiothreitol, and 10% wt/vol glycerol containing 0.025% bromophenol blue (pH 6.8). The protein standards (Bio-Rad) were run in parallel. The samples were electrophoresed in 12% SDS-PAGE16 and transferred onto a polyvinylidene difluoride (PDVF) membrane17 electrophoretically at 100 V for 90 minutes in 25 mmol/L Tris, 190 mmol/L glycine, and 20% wt/vol methanol (pH 8.3). The membrane was then incubated for 2 hours in a blocking solution containing 5% wt/vol dried milk, 5% wt/vol bovine serum albumin (BSA), 0.1% wt/vol Triton X-100, and 0.1% sodium deoxycholate.
This was followed by a sequential incubation with anticathepsin B (dilution, 1:500) followed by biotin-conjugated antisheep (dilution, 1:1000) or antirabbit immunoglobulin G (IgG) (Sigma) and avidin-alkaline phosphatase (dilution, 1:500) (Sigma). Each incubation was carried out for 2 hours at room temperature, and the membrane was washed 3 times with phosphate-buffered saline (PBS) and 0.2% wt/vol Tween 20 between each change of reagent wash. The membrane was incubated with 0.1 mol/L ethanolamine HCl (pH 9.6) for 10 minutes and then with substrate solution comprising 100 μg/mL 5-bromo-chloro-indolyl-phosphate, 100 μg/mL nitroblue tetrazolium, 1 mmol/L magnesium dichloride (MgCl2), and 0.1 mol/L ethanolamine (pH 9.6). The reaction was terminated by rinsing the membrane with distilled water. The same procedure was used for the detection of cathepsin K and S immunoreactive polypeptides in the tissue extracts except that the primary antibodies were rabbit anticathepsin K (dilution, 1:10) and anticathepsin S (dilution, 1:50), respectively. The secondary antibody used for both was goat antirabbit IgG alkaline phosphatase (dilution, 1:5000) (Sigma).
Immunohistochemistry
Frozen specimens of spleen were sectioned at 4 μm, placed on 0.1% poly-L-lysine–coated slides, fixed in 100% acetone at −20°C for 15 minutes, and then fixed in acetone at room temperature for 15 minutes. Nonspecific binding was blocked using normal goat serum in PBS (dilution, 1:10) containing 0.1% wt/vol BSA. The cathepsin B protein was detected by using cathepsin B immune-specific antibody (dilution, 1:500) prepared in the same buffer and by incubating at room temperature for 60 minutes. Negative controls using either no primary antibody or preimmune serum (dilution, 1:500) were used to identify nonspecific staining. Immunoreactive polypeptides were detected using swine antisheep fluoroscein-conjugated secondary antibody (dilution, 1:10) and incubating in the dark at room temperature for 1 hour. Cathepsin K protein was detected in a similar manner using cathepsin K antibody (dilution, 1:100) and goat antirabbit fluoroscein-conjugated secondary antibody (Sigma) (dilution, 1:50). The slides were mounted using Cytofluor (ChemLab, Canterbury, England) in PBS and glycerol, and they were examined using an ultraviolet (UV) fluorescence microscope (Nikon, Japan).
Measurement of cathepsin activities
Activities of cathepsins B, K, and S were measured by the method of Barrett18 with modifications. The fluorogenic substrates used were CBZ-R-R-AMC (pH 5.5) to measure cathepsin B and CBZ-F-R-AMC (neutral pH) to measure cathepsins K and S. We diluted 50 μL plasma, serum, or tissue extracts to 250 μL using 0.1% Brij-35 and preincubated the extracts with 250 μL incubation buffer containing 352 mmol/L KH2PO4, 48 mmol/L Na2PO4, and 4 mmol/L disodium EDTA (pH 6.0), containing 10 mmol/L cysteine as an activator, at 37°C for 5 minutes. The assays were started by the introduction of 250 μL substrate (concentration, 0.02 mmol/L; final concentration, 6.66 mmol/L). After incubation at 37°C for 15 minutes, the reaction was stopped by the addition of 1 mL 100 mmol/L sodium chloroacetate in a buffer containing 30 mmol/L sodium acetate and 70 mmol/L acetic acid (pH 4.3). Fluorescence emission at 460 nm was determined using a Perkin Elmer luminescence spectrophotometer L530 (Perkin Elmer, Courbevoie, France) after excitation at 380 nm.
Cathepsin K activity was measured similarly, except we used a buffer with 100 mmol/L sodium acetate (pH 5.5) containing 20 mmol/L cysteine and 5 mmol/L EDTA, and the substrate was CBZ-F-R-AMC.19The same substrate was used to measure cathepsin S activity, except that the acetate buffer was adjusted to pH 7 with TRIS base. A fluorescence calibration curve was obtained with solutions of known concentrations of the released product, AMC. One unit of enzyme activity represents the amount of enzyme sufficient to hydrolyze 1 nmol substrate per minute at 37°C. The activity was expressed according to the protein concentration determined by the method of Bradford using a Coomassie reagent (Bio-Rad) and crystalline BSA as a standard and as described by the supplier.
Statistical analysis
All numerical data are expressed as the arithmetic means with standard deviations, unless otherwise stated. Statistical significance of the mean differences was examined by the Student t test and Lord's range test for small numbers, where applicable.20
Results
Differential gene expression in Gaucher spleen
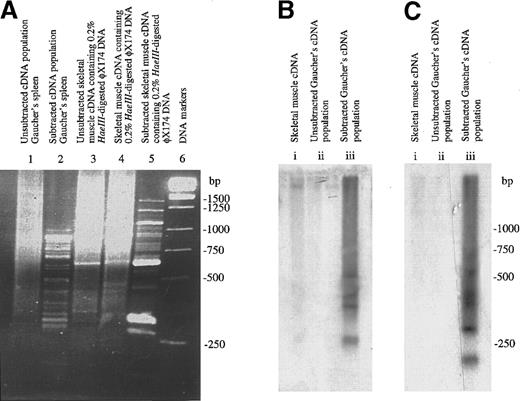
The RsaI–digested cDNA species differentially expressed in Gaucher spleen and obtained by suppression subtractive hybridization followed by amplification in the PCR was resolved by electrophoresis in agarose gels and visualized by ethidium bromide staining under UV light (Figure 1A). As shown in the subtracted population (lane 2), at least 30 visible cDNA fragments were present in this population obtained from the diseased spleen. This contrasts strikingly with the cDNA population not subject to selection (lane 1). Also depicted are the results of model subtractive hybridization experiments conducted with human muscle cDNA populations to which HaeIII–digested φ-X 174 DNA fragments had been added. As shown in the figure, this confirms the ability of the procedure to identify dominant DNA species present in the tester population of amplified skeletal muscle cDNA fragments (lanes 4 and 5).
Enrichment of genes differentially expressed in Gaucher tissue.
(A) Suppression subtraction hybridization. The figure shows ethidium bromide–stained cDNA fragments resolved by electrophoresis in a 2% wt/vol agarose gel. (B) Southern blot analysis of subtracted (enriched) and unsubtracted Gaucher spleen cDNA library hybridized with32P-labeled human tartrate-resistant Acp 5 cDNA. The principal hybridization species correspond to the predictedRsaI fragments of 423 and 590 bp.23 (C) Southern blot of subtracted (enriched) and unsubtracted Gaucher spleen cDNA library hybridized with 32P-labeled human chitotriosidase cDNA. The principal hybridizing species correspond to the predicted RsaI fragments of 123, 423, and 488 bp.21
Enrichment of genes differentially expressed in Gaucher tissue.
(A) Suppression subtraction hybridization. The figure shows ethidium bromide–stained cDNA fragments resolved by electrophoresis in a 2% wt/vol agarose gel. (B) Southern blot analysis of subtracted (enriched) and unsubtracted Gaucher spleen cDNA library hybridized with32P-labeled human tartrate-resistant Acp 5 cDNA. The principal hybridization species correspond to the predictedRsaI fragments of 423 and 590 bp.23 (C) Southern blot of subtracted (enriched) and unsubtracted Gaucher spleen cDNA library hybridized with 32P-labeled human chitotriosidase cDNA. The principal hybridizing species correspond to the predicted RsaI fragments of 123, 423, and 488 bp.21
To determine whether the procedure would permit the identification of genes already known to be differentially expressed in Gaucher tissue, the cDNA fragments were transferred to nylon filters by Southern blotting and hybridized with cDNA oligonucleotide probes for human chitotriosidase11,21,22 and type 5 acid phosphatase (Acp 5),23 whose expression is known to be enhanced in Gaucher tissue. As depicted also in Figure 1B,C, strong hybridization signals were obtained in the subtracted population of cDNAs with both gene probes; in the unsubtracted population, there was no signal obtained after prolonged film exposure, thus confirming the ability of the subtractive procedure to enrich for the products of genes known to be differentially expressed in Gaucher tissue.
Products of the cDNA subtraction procedure
In all, 64 cDNA fragments obtained from the PCR-subtracted cDNA population were sequenced. The sequences of 5 known genes encoding lysosomal proteins were identified, with an additional 10 cDNAs derived from known genes; 2 cDNA sequences representing hitherto uncharacterized human genes were also obtained. The sequences of the genes encoding lysosomal proteins showed an absolute sequence identity with human α-fucosidase, lysosomal acid lipase, and the human cathepsins B, K, and S.24-30 The cognate polypeptides derived from the 10 other known sequences showed identity with mucin core glycoprotein (MCG 24),31 the NMB protein previously identified in malignant melanoma,32 and the heparin sulfate proteoglycan (HSPG).33 In addition, 2 cDNA sequences with identity to human immunoglobulin-κ protein JC-κ of B-cell progenitors,34 and an IgG-κ immunoglobulin gene fragment and a cDNA sequence with identity to human β2-microglobulin were also identified. In the population of known genes, a further sequence of interest was obtained by encoding a recently identified protein in the human C-C chemokine class, PARC, that shows closest homology to the human macrophage inflammatory protein-1–α (MIP-1–α).35
Three cDNA fragments encoding presumptive housekeeping genes were also identified in the selected population; these encode mitochondrial cytochrome oxidase and a ribosomal protein. The 2 novel cDNA fragments identified in the cDNA population obtained from Gaucher spleen show complete identity with expressed sequence tags. One is known to map to human chromosome 13, and both were derived from macrophage cell lines.36
Studies of mRNA abundance in Gaucher tissue
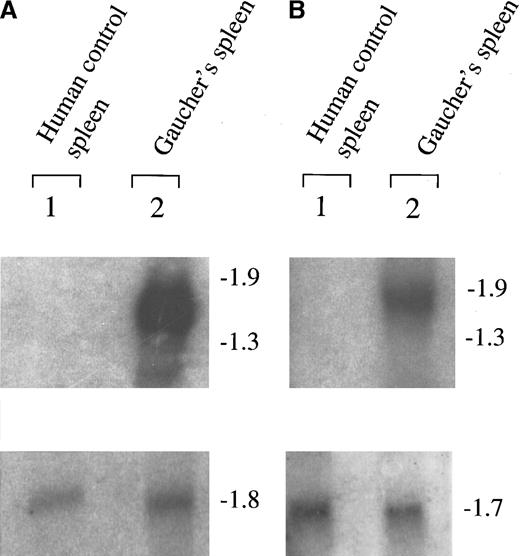
To determine whether the cDNA sequences identified within the subtracted population were up-regulated in Gaucher spleen, we carried out Northern blotting studies to examine the abundance and size of the expressed transcripts (Figures 2 and3). These studies were conducted with RNA obtained from Gaucher spleen samples and control spleen samples other than those used in the original cDNA selection procedure. To control for mRNA transfer and quality on the filter tracks, Northern hybridization was carried out with radio-labeled mouse β-actin cDNA or human glyceraldehyde phosphate dehydrogenase cDNA to provide control signals that compensate for loading. Greatly increased signal intensities of transcripts hybridizing to the chitotriosidase and human tartrate-resistant Acp 5 of 1.7-kilobase (kb) and 1.5-kb probes, respectively, were found as expected (Figure 2).
Northern blot analysis showing up-regulation of tartrate-resistant Acp 5 and chitotriosidase in Gaucher spleen.
(A) The up-regulation of tartrate-resistant Acp 5 and (B) chitotriosidase in Gaucher spleen are depicted. The filters were also later hybridized to radio-labeled β-actin, shown in panel A, and glyceraldehyde phosphate dehydrogenase control cDNA probes, shown in panel B. The figure is a radioautograph and shows hybridization to samples of RNA obtained from control and Gaucher disease tissue independent from that used in the generation of the Gaucher-specific cDNA library by suppression subtraction hybridization.
Northern blot analysis showing up-regulation of tartrate-resistant Acp 5 and chitotriosidase in Gaucher spleen.
(A) The up-regulation of tartrate-resistant Acp 5 and (B) chitotriosidase in Gaucher spleen are depicted. The filters were also later hybridized to radio-labeled β-actin, shown in panel A, and glyceraldehyde phosphate dehydrogenase control cDNA probes, shown in panel B. The figure is a radioautograph and shows hybridization to samples of RNA obtained from control and Gaucher disease tissue independent from that used in the generation of the Gaucher-specific cDNA library by suppression subtraction hybridization.
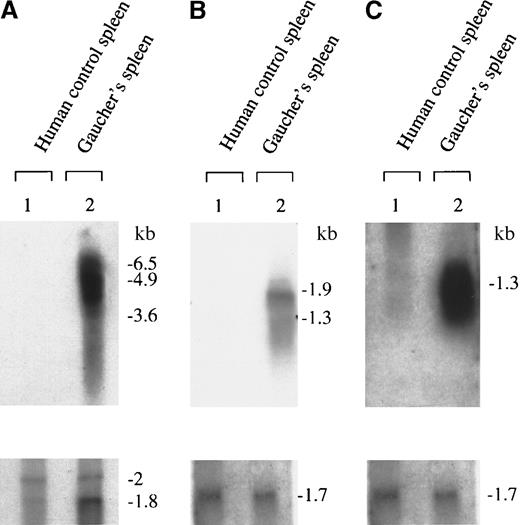
Northern blot analysis showing up-regulation of cathepsin B, K, and S transcripts in Gaucher spleen.
Up-regulation of cathepsins B, K, and S is shown in panels A, B, and C, respectively. After washing, the filters were rehybridized to (A) mouse β-actin or (B and C) human glyceraldehyde phosphate dehydrogenase cDNA probes that serve as loading controls, as shown. The figure is a radioautograph and depicts signals obtained from spleen samples independent of those used to generate the Gaucher-specific cDNA library.
Northern blot analysis showing up-regulation of cathepsin B, K, and S transcripts in Gaucher spleen.
Up-regulation of cathepsins B, K, and S is shown in panels A, B, and C, respectively. After washing, the filters were rehybridized to (A) mouse β-actin or (B and C) human glyceraldehyde phosphate dehydrogenase cDNA probes that serve as loading controls, as shown. The figure is a radioautograph and depicts signals obtained from spleen samples independent of those used to generate the Gaucher-specific cDNA library.
In Figure 3, representative Northern blots demonstrate up-regulated signals for transcripts hybridizing to the human cathepsin B, K, and S cDNA probes compared with the signals obtained with loading control cDNAs. The apparent size of the transcripts for cathepsin B, at 5 kb and 4 kb, was unexpected because the transcript size in most tissues has been reported to be 2.2 kb.37 However, multiple large transcripts of cathepsin B have been reported in human osteoclastoma and melanoma tissues.38-40 The hybridization signals for cathepsin K and human cathepsin S, at 1.8 kb and 1.3 kb, respectively, correspond to those previously reported as the dominant transcripts in human tissues.27-29 Northern blotting experiments also confirmed striking increases in the expression of the human chemokine PARC as well as the novel geneNMB and chromosome 13–related expressed sequence tag (EST) fragment transcripts of sizes corresponding to those previously reported for these expressed human genes, as described above. The relative abundance of these transcripts in Gaucher spleen RNA was compared with control spleen RNA. Steady-state enhanced expression of these genes was estimated to be increased at least 10-fold; in the case of cathepsins B and K, there was no signal detected on prolonged exposure of control spleen RNA samples to the hybridization probe, whereas intense signals were demonstrated in Gaucher spleen samples.
Tissue distribution of differentially expressed genes
To determine the range of gene expression for genes not yet studied, Northern blots using RNA samples obtained from a range of human tissues were hybridized to 32P-labeled cDNA probes carried out for the PARC human chemokine, the NMB protein, and human EST mapping to chromosome 13 (not shown). Selective expression of the PARC chemokine was noted principally in tissues containing antigen-presenting cells including macrophages, ie, spleen, thymus, intestine, blood leukocytes, and lung. The distribution of expression of the other genes was widespread in human tissues (not shown).
Detection of altered protein expression in Gaucher disease
Immunoblotting for cathepsin antigens in spleen.
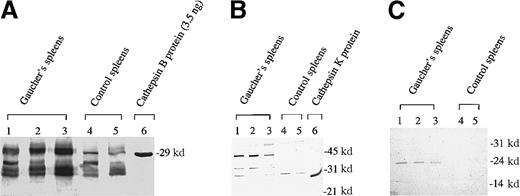
To determine the molecular forms of cathepsin B, K, and S polypeptides in splenic tissue and to estimate changes in protein abundance, samples of spleen homogenate from Gaucher disease patients and controls were subjected to SDS-PAGE under denaturing conditions, and the polypeptides were immobilized by transfer to PVDF membranes for specific antibody-binding studies (Figure 4). Electrophoretic protein analysis showed few reproducible differences in the principal polypeptides stained between the disease and control samples; this procedure confirmed the uniform integrity of the tissue samples used for analysis in this study. A single polypeptide, approximately 24 kd, was found to be reproducibly increased in all 4 Gaucher spleen samples compared with 3 controls (not shown). Peptide sequencing of this protein, which was isolated from the dried gel, confirmed the identity of this species as the human L-chain ferritin molecule. However, immunoblotting experiments conducted with polyclonal antisera specific to human cathepsins revealed marked differences in Gaucher tissue. Immune staining for cathepsins B, K, and S (Figure 4) was greatly enhanced in the samples of Gaucher spleen. Cathepsin B demonstrated an additional isoform in the Gaucher spleen (MW, approximately 30 kd) compared to the 3 immunoreactive species present in control spleens (MW, approximately 29 and 20-21 kd), respectively.
Western blot analysis of immunoreactive cathepsin B, K, and S polypeptides in human spleen homogenates.
Immunostaining of cathespins B, K, and S are shown in panels A, B, and C, respectively. We electrophoresed 3 independent Gaucher spleen samples and 2 control spleen samples with 12% wt/vol polyacrylamide gels as described in “Materials and methods.”
Western blot analysis of immunoreactive cathepsin B, K, and S polypeptides in human spleen homogenates.
Immunostaining of cathespins B, K, and S are shown in panels A, B, and C, respectively. We electrophoresed 3 independent Gaucher spleen samples and 2 control spleen samples with 12% wt/vol polyacrylamide gels as described in “Materials and methods.”
The abundance of immunoreactive cathepsin B in whole spleen homogenate was determined by enzyme-linked immunoabsorbent assay (ELISA), which confirmed the increased abundance of immunoreactive cathepsin B. In 2 control spleens the mean concentration was 0.17 ng/mg protein compared with 3.8 ng/mg protein in the 3 Gaucher spleen extracts (Table1). Immunoblotting also showed increased immunoreactive mature cathepsin K (MW, approximately 29 kd) and more procathepsin K (MW, approximately 38 kd) than in control tissue samples. Immunoreactive cathepsin S was also enhanced in Gaucher spleen, but it occurred as a single polypeptide species (MW, approximately 24 kd); this species was barely detectable in control spleen extracts.
Acid hydrolases in human spleen
| . | Control spleens (n = 3) . | Gaucher's spleens (n = 4) . |
|---|---|---|
| Acp 5 | 2.6 ± 0.2 | 24 ± 13 |
| Chitotriosidase | 1.2 ± 0.7 × 103 | 3.8 ± 2.0 × 107 |
| Cathepsin | ||
| B | 26 ± 4.1 | 183 ± 35 |
| K | 10 ± 2.0 | 97 ± 39 |
| S | 4.0 ± 2.1 | 91 ± 45 |
| Immunoreactive cathepsin B | 0.16, 0.18* | 3.8 ± 0.9† |
| . | Control spleens (n = 3) . | Gaucher's spleens (n = 4) . |
|---|---|---|
| Acp 5 | 2.6 ± 0.2 | 24 ± 13 |
| Chitotriosidase | 1.2 ± 0.7 × 103 | 3.8 ± 2.0 × 107 |
| Cathepsin | ||
| B | 26 ± 4.1 | 183 ± 35 |
| K | 10 ± 2.0 | 97 ± 39 |
| S | 4.0 ± 2.1 | 91 ± 45 |
| Immunoreactive cathepsin B | 0.16, 0.18* | 3.8 ± 0.9† |
Measurements are given as nmol/min/mg protein (mean plus or minus SD), except for immunoreactive cathepsin B, which is measured as ng/mg protein. For Gaucher spleen, P < .01.
Indicates n = 2.
Indicates n = 3.
Immunohistochemical studies.
Having demonstrated enhanced expression of cathepsin RNA transcripts and polypeptides in Gaucher spleen, the cellular localization of the enhanced expression of antigen was examined by immunofluorescence microscopy using human isozyme-specific cathepsin antisera. To orientate these studies, sections of Gaucher spleen were stained with antihuman CD68 monoclonal antibody that recognizes antigens expressed on human macrophages (Figure5). Immunofluorescence microscopy with cathepsin B-specific antiserum showed marked staining in the sinusoidal endothelium of Gaucher spleens as well as localized staining within some Gaucher cells, as identified by their characteristic morphology and strong staining with anti-CD68. In contrast, cathepsin K antiserum reacted strongly and specifically with Gaucher cells, which demonstrated punctate staining; cathepsin K antigen was also stained in perisinusoidal lymphocytes and dendritic cells.
Immunofluorescence microscopy of human spleen sections.
(A and B) Paraffin sections of Gaucher spleen used in the subtraction procedure stained with CD68 monoclonal antibody detected by an immunoperoxidase method to show pathologic macrophages (panel A, original magnification × 50; panel B, original magnification × 400). (C and E) Cathepsin B immunofluoresence in sections of 2 different independent Gaucher spleens (original magnification × 400). (G and H) Nonimmune control fluorescence in Gaucher spleens (original magnification × 400). (D and F) Cathepsin K immunofluorescence in 2 Gaucher spleen sections (panel D, original magnification × 100; panel F, original magnification × 400).
Immunofluorescence microscopy of human spleen sections.
(A and B) Paraffin sections of Gaucher spleen used in the subtraction procedure stained with CD68 monoclonal antibody detected by an immunoperoxidase method to show pathologic macrophages (panel A, original magnification × 50; panel B, original magnification × 400). (C and E) Cathepsin B immunofluoresence in sections of 2 different independent Gaucher spleens (original magnification × 400). (G and H) Nonimmune control fluorescence in Gaucher spleens (original magnification × 400). (D and F) Cathepsin K immunofluorescence in 2 Gaucher spleen sections (panel D, original magnification × 100; panel F, original magnification × 400).
Acid hydrolase activities in Gaucher spleen extracts
Because increased expression of known and unexpected acid hydrolases was identified at the level of messenger RNA (mRNA) and protein antigen, studies were undertaken to determine their activities as well as the activities of known marker enzymes elevated in Gaucher disease (tartrate-resistant Acp 5) and chitotriosidase (Table 1). As expected,11 the mean activity of tartrate-resistant Acp 5 was increased approximately 10-fold in Gaucher spleen samples, and the mean activity of chitotriosidase was enhanced more than 30 000-fold in the Gaucher spleen extracts. The specific activities of cathepsins B, K, and S were determined by fluorimetric assays in Gaucher and control spleen extracts (Table 1). It is evident from the table that the specific activities of cathepsins B, K, and S were increased 7-fold to 23-fold in extracts of Gaucher spleen.
Cathepsin activities in human blood
Given the greatly increased enzymatic activities of cathepsins and known acid hydrolase marker enzymes in Gaucher spleen, it was important to determine whether or not these proteins were secreted into the plasma for enzymatic or immunochemical detection. Specific fluorimetric assays for cathepsins B, K, and S were conducted in plasma samples obtained from healthy subjects and in samples obtained from untreated Gaucher disease patients obtained at the time of diagnosis. As depicted in Table 2, the activities of all 3 cysteine proteinases were significantly enhanced in plasma obtained from patients with Gaucher disease.
Cathepsin activities in human plasma
| . | Cathepsin B . | Cathepsin K . | Cathepsin S . |
|---|---|---|---|
| Gaucher's disease (n = 11) | 2.12 ± 0.63* | 5.11 ± 1.39* | 5.63 ± 1.75* |
| Nonglycolipid storage diseases (n = 8) | 0.95 ± 0.12 (NS) | 2.16 ± 0.47 (NS) | 2.35 ± 0.90† |
| Glycosphingolipidoses (n = 9) | 1.97 ± 0.32 (NS) | 2.93 ± 1.67 (NS) | 4.25 ± 2.99† |
| Control subjects (n = 21) | 1.17 ± 0.41 | 1.81 ± 0.46 | 1.32 ± 0.39 |
| . | Cathepsin B . | Cathepsin K . | Cathepsin S . |
|---|---|---|---|
| Gaucher's disease (n = 11) | 2.12 ± 0.63* | 5.11 ± 1.39* | 5.63 ± 1.75* |
| Nonglycolipid storage diseases (n = 8) | 0.95 ± 0.12 (NS) | 2.16 ± 0.47 (NS) | 2.35 ± 0.90† |
| Glycosphingolipidoses (n = 9) | 1.97 ± 0.32 (NS) | 2.93 ± 1.67 (NS) | 4.25 ± 2.99† |
| Control subjects (n = 21) | 1.17 ± 0.41 | 1.81 ± 0.46 | 1.32 ± 0.39 |
Measurements are given as nmol/min/mL (mean plus or minus SD). NS indicates not significant (P ≥ .05).
Indicates P < .001.
Indicates P < .01.
Cathepsin activities in other lysosomal storage diseases
To determine the specificity of the elevated blood cathepsin activities, we assayed the following for cathepsin B, K, and S activities: samples of plasma from control subjects; patients with nonglycolipid lysosomal storage diseases (mucopolysaccharidoses, glycoproteinoses, and Batten's disease); and glycosphingolipidoses other than Gaucher disease (Niemann-Pick diseases A and C, GM1 and GM2 gangliosidoses, Fabry's disease, and Krabbe's disease) as well as 11 additional untreated Gaucher disease patients at the time of diagnosis. Gaucher disease was associated with significantly increased plasma activities of all 3 proteinases, unlike the other lysosomal diseases (Table 2). Cathepsin S activities were significantly elevated in the glycolipid and nonglycolipid disorders; although the glycolipid disorders showed a trend toward greater activity of cathepsins B and K, unlike Gaucher disease, this did not reach significance.
To confirm whether cathepsin activities were sufficiently stable for measurement in archival samples of sera as well as plasma, cathepsin B, K, and S assays were carried out with serum that was obtained from 26 healthy control subjects and 12 additional untreated adult patients with Gaucher disease and stored at the time of diagnostic evaluation. These assays were done after one freeze and thaw cycle and confirmed that cathepsin activities are significantly elevated in Gaucher disease: the determinations showed 1.86 ± 1.05, 3.59 ± 1.52, and 3.93 ± 1.27 nmol/min/mL for cathepsins B, K, and S, respectively, in Gaucher sera compared with 0.64 ± 0.07, 1.36 ± 0.87, and 1.97 ± 1.05 nmol/min/mL for cathepsins B, K, and S, respectively, in control sera (P < .001). To establish whether removal of the spleen, the organ in which overexpression of cathepsins had been determined, would also remove the source of excess serum cathepsin activity, sera from Gaucher disease patients treated by splenectomy and those with intact spleens were compared. In the 7 patients with intact spleens, the activities were 1.73 ± 1.07, 3.76 ± 1.93, and 3.72 ± 0.79 nmol/min/mL serum for cathepsins B, K, and S, respectively. In the 5 patients previously treated by a splenectomy and before receiving enzyme therapy, the corresponding activities were 2.06 ± 1.11, 3.35 ± 0.81, and 4.21 ± 1.82 nmol/min/mL serum, respectively. These values do not differ significantly.
Serum cathepsin activities in response to enzyme replacement therapy
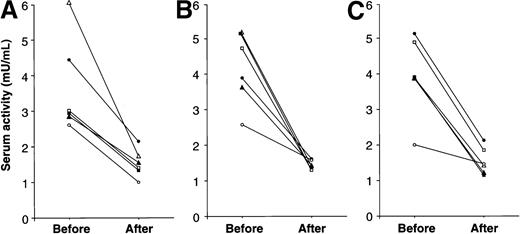
To investigate the disease-related specificity of our findings, serum cathepsin activities were determined in stored sera obtained from 6 patients with Gaucher disease before and after introduction of enzyme replacement therapy. All 6 patients had received treatment with either alglucerase or imiglucerase for 18-22 months and had demonstrated clinical and objective improvements in disease activity as indicated by improvement in blood counts; visceral volumes; symptoms; and other surrogate markers of disease such as serum chitotriosidase, angiotensin-converting enzyme, or tartrate-resistant Acp 5 activities. As depicted in Figure 6, the activities of all cathepsins decreased in response to enzyme therapy for Gaucher disease in all 6 patients examined.
Serum cathepsin activities in patients with Gaucher disease before and after enzyme replacement therapy.
Serum samples from 6 patients with type I Gaucher disease were assayed retrospectively before and 16-22 months after institution of enzyme therapy with imiglucerase and when the principal manifestations of the disease had regressed. The values for each patient were determined without prior knowledge of treatment status and are depicted by individual symbols. Cathepsins B, K, and S are shown in panels A, B, and C, respectively.
Serum cathepsin activities in patients with Gaucher disease before and after enzyme replacement therapy.
Serum samples from 6 patients with type I Gaucher disease were assayed retrospectively before and 16-22 months after institution of enzyme therapy with imiglucerase and when the principal manifestations of the disease had regressed. The values for each patient were determined without prior knowledge of treatment status and are depicted by individual symbols. Cathepsins B, K, and S are shown in panels A, B, and C, respectively.
In 3 patients, serial stored serum samples were available during the institution of enzyme therapy and could be compared retrospectively with serial samples obtained during the course of follow-up from 3 patients with mild symptoms of Gaucher disease who did not receive enzyme therapy during several months of monitoring. The sequential data demonstrated that although cathepsin K activities decreased progressively during treatment in patients who received enzyme therapy, serum activities of cathepsin K remained unchanged or increased slowly in the 3 mildly affected patients who did not receive enzyme therapy (not shown). Enzyme therapy, which has a beneficial effect specifically for Gaucher disease, also reduces blood cathepsin activities. These results moreover suggest that cathepsin release into the bloodstream may be correlated with disease severity and treatment responses.
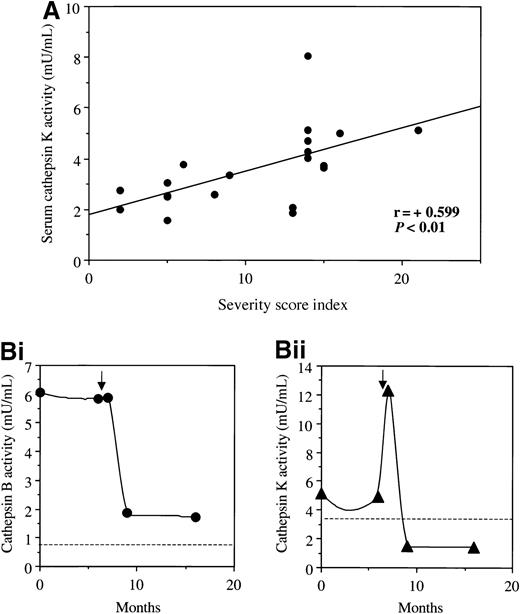
To determine further if serum cathepsin activities correlated with disease severity, activities were examined in relation to the clinical severity score index derived for 20 type I patients with Gaucher disease.41 Serum B, K, and S activities were significantly correlated with the severity score index (r = + 0.442, 0.599, and 0.472, respectively, P < .01). The relation between cathepsin K and this clinical index is depicted in Figure7A. Further information about the relation between serum cathepsin activity and clinical disease was provided by retrospective enzyme assays in serial samples from a 52-year-old man with Gaucher disease complicated by severe osteoporosis. The samples were collected over a period of 16 months since he had started 30 IU/kg/mo enzyme therapy. During the period of follow-up, an episode of radiologically confirmed bone necrosis of the right femur developed. As shown in Figure 7B, there was greatly increased activity of serum cathepsin B before the onset of this bone crisis and a striking increase in cathepsin K activity immediately after its onset. Similar behavior was noted for cathepsin S (not shown in figure). These findings strongly suggest that active cathepsins are released from Gaucher tissue into the blood and that they reflect (and may predict) disease activity at different stages of its evolution in the bone marrow.
Cathepsin activities and disease manifestations.
(A) The relation between clinical severity score index calculated according to Zimran et al41 and serum cathepsin K activity in 20 patients with untreated type I Gaucher disease; similar data were obtained for measurements of cathepsins B and S (see text). (B) Serial measurements of cathepsins B (i) and K (ii) in a 52-year-old man with osteoporosis due to Gaucher disease (N370S/84GG genotype), who was previously treated by splenectomy. The horizontal axis shows the time after starting 30 IU/kg/mo imiglucerase. At 6 months the patient experienced an episode of avascular necrosis of the femoral head, shown by the arrow. Cathepsin activities were determined retrospectively in stored sera routinely collected at clinical evaluations. The dotted lines represent the 95% upper confidence limits of serum cathepsin activities in 26 healthy control subjects.
Cathepsin activities and disease manifestations.
(A) The relation between clinical severity score index calculated according to Zimran et al41 and serum cathepsin K activity in 20 patients with untreated type I Gaucher disease; similar data were obtained for measurements of cathepsins B and S (see text). (B) Serial measurements of cathepsins B (i) and K (ii) in a 52-year-old man with osteoporosis due to Gaucher disease (N370S/84GG genotype), who was previously treated by splenectomy. The horizontal axis shows the time after starting 30 IU/kg/mo imiglucerase. At 6 months the patient experienced an episode of avascular necrosis of the femoral head, shown by the arrow. Cathepsin activities were determined retrospectively in stored sera routinely collected at clinical evaluations. The dotted lines represent the 95% upper confidence limits of serum cathepsin activities in 26 healthy control subjects.
The availability of ELISA for human immunoreactive cathepsin B rendered it possible to investigate the presence of cathepsin B protein in serum samples obtained from patients with Gaucher disease. Serum from 11 control subjects contained 1.8 ± 1.7 ng (mean plus or minus SD) of immunoreactive cathepsin B per mL, and the serum from 10 untreated Gaucher disease patients contained 11.5 ± 6.5 ng/mL, respectively (P < .01). The relative increase (22-fold) in cathepsin B immunoreactivity in the spleen is greater than the relative increase (7-fold) in measured enzymatic activity compatible with the presence of more cathepsin B precursors with lower specific activity. In the serum, cathepsin B immunoreactivity was increased 6.4-fold but the absolute specific activity of cathepsin B in the serum was reduced by about 500-fold, which indicated that unlike the Gaucher spleen cathepsin B, most of the immunoreactive serum cathepsin B was catalytically inactive. This suggests that the cathepsin B is partially inactivated in serum by forming complexes with one or more circulating proteinase inhibitors such as α2-macroglobulin.
Discussion
Validation of the suppression subtractive hybridization method
The suppression subtractive hybridization procedure is based on the suppression of the PCR, which allows a normalization step to be carried out between the 2 populations of cDNA under study. This step is designed to overcome the wide differences in the abundance of individual transcripts between the 2 populations, thus facilitating the identification of a minority of differentially expressed genes distributed among numerous high-copy mRNA species. As shown in Figure1, the suppression PCR method selectively suppresses amplification of abundant transcripts present in both populations and leads to a subtracted population containing a high frequency of up-regulated transcripts.12
Portfolio of genes identified in the subtracted cDNA library
Using genes known to be differentially expressed in Gaucher tissue, we show that the cDNA subtraction method identifies genes which are specifically upregulated; these include at least one cytokine influencing macrophage proliferation and expression35 as well as genes encoding lysosomal proteins. Several of the human genes identified in the Gaucher library correspond to those recently reported to be upregulated in human monocyte-derived macrophages obtained after exposure to growth factors in vitro. These include lysosomal acid lipase, cathepsin B, β2-microglobulin, NMB, Acp 5, and chitotriosidase.42
As expected, the genes overexpressed in Gaucher spleen included genes encoding the lysosomal proteins α-fucosidase and lysosomal acid lipase as well as the isozyme of tartrate-resistant Acp 5 and chitotriosidase. The products of these latter 2 genes are known to be increased in the serum of patients with Gaucher disease.11However, the finding of increased expression at the level of the RNA protein and enzyme of a single class of cysteine proteinases (cathepsins B, K, and S), with evidence of their increased secretion and release, was unexpected. This is the first time these proteins have been identified in Gaucher disease, and specifically, they are proteins that characterize the condition and that we have directly demonstrated in the storage cells. Cathepsins participate in enzyme and hormone activation and protein processing in signaling pathways including those of cell death, tumor metastasis, antigen processing, and, especially in the newly described osteoclast-specific protein cathepsin K, bone resorption.19 43-49
Disease-related specificity of the expressed genes identified
Evidence that the changes observed were not related to overt differences in tissue composition in the spleens examined and that they reflect Gaucher disease activity in other organs was provided by electrophoretic SDS-PAGE (not shown). This revealed closely similar polypeptide compositions and abundance between the 7 spleen samples used in this study. In addition, elevated cathepsin activities were found in sera obtained from patients with Gaucher disease, which was irrespective of splenectomy, including a pair of monozygotic twins homozygous for the N370S glucocerebrosidase allele; these were discordant for disease manifestations, and only one twin with overt disease had been treated by splenectomy.
Elevated plasma and tissue cathepsin activities are directly related to the presence of Gaucher cells, as indicated by the following: (1) Gaucher cells in situ show strong immunostaining for cathepsin antigens. (2) Plasma activities of cathepsins B and K are not significantly elevated in patients with nonglycolipid lysosomal diseases or in patients with glycosphingolipidoses other than Gaucher disease. (Although cathepsin S activities may be elevated in these disorders, the relative increases are less striking.) (3) Specific therapy of Gaucher disease with macrophage-targeted human glucocerebrosidase correlated with a significant decrease in serum cathepsin activities. (4) Patients with symptomatic Gaucher disease showed a progressive decrease in their serum cathepsin activities toward normal levels. However, presymptomatic Gaucher disease patients not yet offered enzyme therapy had less elevated cathepsin activities which remained stable or increased gradually during prolonged monitoring, indicating a relationship between cathepsin activity and disease activity.
Possible significance of cathepsins in Gaucher disease
Cathepsin B is an abundant lysosomal protein that displays diverse peptidase activities.50,51 Its physiologic role appears to be in the intralysosomal degradation of proteins; indeed cathepsin B is responsible for the activation of some lysosomal precursor enzymes52 as well as peptide prohormone processing.53,54 Cathepsin B is implicated in certain tissue destructive states including arthritis, bone resorption, and metastasis.55-61
Cathepsin K has been recently identified as the principal expressed protein of the osteoclast,48 and its pattern of expression is restricted to osteoclasts, the ovary, and colonic tissue.27 Cathepsin K is highly active in the cleavage of the bone matrix proteins collagen 1 and osteonectin and its role in bone resorption, modeling, and turnover is clearly demonstrated by the occurrence of an osteopetrotic syndrome in mice homozygous for a disrupted allele of cathepsin K.62 The role of cathepsin K in humans is vividly illustrated by the recent description of diverse mutations in the cathepsin K gene of patients with pycnodysostosis, a rare recessive trait characterized by osteosclerosis, short stature, skull deformities, and increased regions of demineralized bone, which indicate that the defective osteoclasts have impaired ability to degrade organic bone matrix.63 The development of specific inhibitors of cathepsin K activity is an active area of current pharmaceutical research.64 Given the intractable nature of the skeletal manifestations of Gaucher disease and the severity of the osteolytic lesions once established, enhanced cathepsin K expression associated with the condition immediately suggests the potential for selective cathepsin K inhibitors in this disorder.
Human cathepsin S is another cysteine proteinase whose expression was found to be greatly enhanced in the tissues, plasma, and serum of patients with Gaucher disease. Cathepsin S, like cathepsin K, shows a restricted pattern of tissue expression with the highest levels in the spleen, heart, and lung.65 In the latter tissue, detectable cathepsin S staining has only been identified in pulmonary alveolar macrophages, suggesting that the protein may have a specific role in the innate immune system including a role in antigen processing.47,66,67 Cathepsin S has diverse endopeptidase, di-peptidyl-peptidase, and amino-peptidase activities and a broad substrate activity range.68-70 Unlike other cathepsins, cathepsin S is stable and active at a neutral pH. Recent studies indicate that cathepsin S is highly expressed in lymphocytes, monocytes, and other megahistocompatability complex (MHC) class II–expressing cells, where it is readily induced by interferon-γ.71 The greatly increased activity of cathepsin S in Gaucher spleen and in the plasma of patients with this disorder is of interest because the protein may be involved in the abnormal immune regulation that characterizes chronic type I Gaucher disease.10 11
Other macrophage-specific genes in Gaucher disease
The human C-C chemokine PARC has close homology to MIP-1–α and is abundant in pulmonary alveolar macrophages and follicular dendritic cells. This protein may contribute to the recruitment of lymphoid precursors and to B-cell activation, which characterize Gaucher infiltrates.8-11 Similarly, the cDNA fragment related to a human gene located on chromosome 13 and isolated from human macrophages indicates the extent to which macrophage activation is generalized in Gaucher tissue. The gene encoding the NMB protein may also merit further study because it is a tumor suppressor of unknown mechanism.
Pathways for gene activation in Gaucher disease
We and others have recently shown enhanced concentrations of proinflammatory cytokines including interleukin-6 (IL-6) in the serum of patients with Gaucher disease.8-11 We suggested that IL-6 hypersecretion may be a critical triggering factor for plasmacytoma and multiple myeloma in patients with Gaucher disease.10 IL-6 may also be a mediator of osteoclastic activation at the surface of the bone adjacent to infiltrating Gaucher cells. It is therefore significant that the human ACP5 gene encoding tartrate-resistant Acp-5 and the cathepsin K gene both harbour an IL-6 response element in their upstream 5′-untranslated region and cathepsin B transcription is stimulated by IL-6, which may represent a common pathogenetic pathway for phagocytic activation in Gaucher disease.72 73
In summary, analysis of an enriched population of cDNA sequences that are enhanced in Gaucher disease has identified genes encoding lysosomal proteins which show selective expression in the plasma, serum, and tissues of Gaucher disease patients and, specifically, in their pathologic macrophages. The cluster of cDNAs representing cysteine proteases (cathepsins B, K, and S) have biologic functions that immediately suggest a pathogenic role. In particular cathepsin K, previously thought to be restricted in expression to the osteoclast,27,28,43,48 49 is highly active in Gaucher spleen and is expressed in the Gaucher cell.
The systematic identification of gene expression occurring as a result of glycolipid storage may ultimately improve methods for selecting patients at risk from complications of Gaucher disease and facilitate the development of protein markers with which to monitor its severity and long-term effects.
Acknowledgments
We thank Joan Grantham for preparing the manuscript, for secretarial assistance, and for supporting our Gaucher services. Dr John Grant kindly carried out the immunohistochemistry on fixed spleen sections. We are also indebted to the UK Gaucher Association for continued encouragement and support.
Supported by the U.K. Gaucher's Association; the Medical Research Council; and the Arthritis and Rheumatism Council of London, UK.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
T. M. Cox, Department of Medicine, Box 157, Addenbrooke's Hospital, Hills Road, Cambridge CB2 2QQ, England; e-mail: jbg20@medschl.cam.ac.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal