Abstract
Both SDF-1 and CXCR4 disruption are lethal to mice at the embryonic stage and cause abnormalities in B lymphopoiesis, myelopoiesis, cardiogenesis, vasculogenesis, and cerebellar development. To investigate the role of SDF-1 and CXCR4 in hematopoiesis during the adult stage, mice reconstituted with bone marrow–derived hematopoietic progenitor cells transduced with either the SDF-1 or a genetically modified SDF-1–intrakine gene using a retroviral expression vector were analyzed. Flow cytometric (FCM) analysis showed a dramatic reduction of CXCR4 expression on the cells of intrakine-transduced mice, whereas CCR7 and CCR1 expression was unchanged or marginally decreased on splenocytes. Migration of splenocytes and bone marrow cells to SDF-1 was markedly suppressed in intrakine-transduced mice. FCM analysis of bone marrow cells of intrakine-transduced mice exhibited decreased numbers of pro-B (B220+ CD43+), pre-B (B220+CD43−), and immature B (B220+IgM+) cells and a decreased number of granulocytes/myeloid (Gr1+ CD11b+) cells. Impaired B lymphopoiesis and myelopoiesis in intrakine-transduced mice were confirmed by an in vitro colony-forming assay of bone marrow cells. In contrast, B lymphopoiesis and myelopoiesis were enhanced in SDF-1–transduced mice. Interestingly, T-cell maturation in the thymus was impaired both in intrakine- and SDF-1–transduced mice, suggesting that SDF-1 and CXCR4 play an important role in T lymphopoiesis as well as in B lymphopoiesis and myelopoiesis in adults. These results demonstrate an essential role of CXCR4 and its ligand SDF-1 in adult hematopoiesis, and they indicate the intrakine method as a powerful tool for functional analysis of chemokines/chemokine receptors in vivo and as a potential therapeutic approach for acquired immunodeficiency syndrome.
Introduction
Hematopoiesis consists of developmental cascades in which the hematopoietic stem cells (HSCs) generate lineage-committed cells and repeat the process of self-renewal.1,2 HSCs are defined as cells that have dual capability for self-renewal and multilineage differentiation. During embryonic development, hematopoietic progenitor cells (HPCs) sequentially appear in the yolk sac, para-aortic splanchnopleura mesoderm, aorta–gonad–mesonephros, fetal liver, and, finally, bone marrow.3 4 The molecular mechanism for the migration of HSCs and HPCs during development remains unknown.
Chemokines are a family of chemoattractive polypeptides that are classified into 4 groups, depending on the position of conserved cysteine residues. Chemokines mediate their effect by binding to 7 transmembrane G protein–coupled receptors, and they attract leukocyte subsets to inflammation sites.5,6 Recently, several chemokines were shown to be expressed constitutively in lymphoid tissues, indicating that these chemokines have homeostatic functions in regulating lymphocyte trafficking between and within lymphoid organs.7-9
Stromal cell–derived factor-1 (SDF-1), a CXC chemokine, was originally cloned from bone marrow stromal cells by using the signal sequence trap method10 and was later found to be pre-B-cell stimulating factor (PBSF).11 SDF-1 is chemoattractive for hematopoietic progenitor cells, B lymphocytes, T lymphocytes, and monocytes.12,13 CXCR4, a receptor for SDF-1, has been identified as an orphan receptor by several groups, including us,14-17 and is expressed constitutively in various tissues. Mice with disrupted SDF-1 or CXCR4 genes die in utero and show severe abnormalities in B lymphopoiesis, myelopoiesis in bone marrow, cardiogenesis, vascular development, and cerebellar development.18-20 CXCR4 also acts as a coreceptor for the T-tropic human immunodeficiency virus (HIV)-1, and SDF-1 has been also shown to competitively block the viral entry of T-tropic isolates of HIV-1.21 22
Genetically modified intracellular chemokines, or intrakines, which have been linked with an endoplasmic reticulum retention signal sequence (KDEL) on their carboxy termini, have been shown to remain inside cells, to bind intracellularly the newly synthesized cognate chemokine receptors, and to prevent the transport of the receptors to cell surfaces.23 24 Lymphocytes transduced with a gene expressing SDF-1–intrakine were found to be resistant to ligand stimulation and HIV-1 infection in vitro, indicating that intrakine can be used as a therapeutic approach for acquired immunodeficiency syndrome (AIDS). It remains to be established, however, whether intrakine blocks CXCR4 expression in vivo and how safe the down-regulation of CXCR4 by intrakine is in adults.
In this study, using mice reconstituted with bone marrow–derived hematopoietic progenitor cells (BM-HPCs) transduced with either SDF-1 or intrakine genes by a bicistronic retroviral expression vector, we demonstrated that the SDF-1–intrakine could successfully suppressed the expression and function of CXCR4 in vivo and that intrakine-transduced adult mice exhibited impaired lymphopoiesis and myelopoiesis.
Materials and methods
Cytokine and cell lines
Recombinant murine SCF and human Flt3L were kindly provided by Kirin Brewery (Tokyo, Japan). Preparations of recombinant murine IL-6, murine SLC, and human SDF-1 were described previously.25,26 Using a Toxicolor assay kit (Seikagaku-kogyo, Tokyo, Japan), endotoxin was found not to be detected in these cytokine preparations. Recombinant murine MIP-1α was purchased from R&D System (Rocky Hill, NJ). A retrovirus packaging cell line, BOSC23, for ecotropic retroviruses (ATCC CRL 11554) was maintained in Dulbecco minimum essential medium (DMEM) containing 10% fetal calf serum (FCS) and guanine phosphoribosyltransferase (GPT) selection reagents.27 Cells were transferred to DMEM 10% FCS without GPT selection reagents 2 days before transfection.
Construction of expression vectors
An internal ribosomal entry site (IRES) gene of the encephalomyocarditis virus from TFG-mIL-12 was amplified by polymerase chain reaction (PCR) reaction with the primers 5′-GCGGATCCCGAATTCCGCCCCTCT-3′ and 5′-GCGGATCCCCAAGGTTGTGGCCA-3′). The amplified DNA fragment was digested with EcoRI /NotI and inserted into the EcoRI /NotI site of the retrovirus expression vector pMY. This modified vector was designated pISMY. Enhanced green fluorescent protein (EGFP) gene from the pEGFP-N2 plasmid (Clontech, Palo Alto, CA) was amplified by PCR reaction with the primers 5′-GAATGCGGCCGCATGGTGAGCAAGGGCGAG-3′) and (5′-GTCGCGGCCGCTTTACTTGTACAGCTCGTA). The amplified DNA fragment was digested with NotI and inserted into the NotI site of the pISMY. This modified vector was designated pEGFPMY.
A murine SDF-1 gene was amplified by PCR reaction with the primers 5′-GCGAATTCCACCATG TGGACGCCAAGGTCGTC-3′ and 5′-GCGAATCCTTACTTGTTTAAAGCTTTCTG-3′). The murine SDF-1 gene was linked with an ER retention signal (SEKDEL) by a PCR reaction with the primers 5′-GCGAATTCCACCATGGACGCCAAGGTCGTC-3′ and 5′-GCGAATTCTTACAGCTCGTCCTTCTCGCTCTTGTTTAAAGCT- TTCTG-3′). These DNA fragments were digested with EcoRI and inserted into theEcoRI site of pEGFPMY. These bicistronic vectors were designated pSDFMY and pSDFKMY, respectively. All constructs were sequenced using the Big Dye Terminator Cycle Sequencing Kit (Applied Biosystems, Branchburg, NJ) and an Applied Biosystems model 377 DNA sequencer.
Preparation of antibody
The cDNA encoding the full length of mSDF-1 and NH2-terminal portion of mCXCR4 from mCXCR4-pBlue Script KS (+) was amplified by PCR reaction. PCR primers for SDF-1 were 5′-GCGGATCCATGGAGGCCAAGGTCGTC-3′ and 5′-GCCTCGAGTTACTTGTTTAAAGCTTTCTC-3′. Primers for CXCR4 were 5′-GCGGATCCATGGAACCGATCAGTGTG-3′ and 5′-GCCTCGAGTTAGGTGGGCAGGAAGATCCT-3′. Amplified DNA fragments were digested with BamHI/XhoI and subcloned into the GST-fusion protein expression vector, pGEX-4T-3 (Amersham Pharmacia Biotech, Bucks, UK). These expression vectors were introduced intoEscherichia coli BL21 (Trx) cells, and preparations of fusion protein were made according to a previously published method.28 Immunization of fusion protein and preparation of polyclonal antibodies to GST–mSDF-1 and GST-mCXCR4 fusion protein were performed according to a previously published method.29 Rabbit polyclonal antibodies against a synthetic peptide corresponding to the N-terminal region of mCCR7 were prepared according to standard methods.30 Preparation of antibodies against mCCR1 was described previously.31
The immunologic binding specificity of these prepared antibodies against a chemokine and chemokine receptors was confirmed using murine recombinant chemokines (mMIP-II and mSLC) and chemokine receptor transfectants (mCCR1, mCCR2, mCCR7, and mCXCR4), respectively.
Retrovirus-mediated bone marrow transduction and transplantation
Bone marrow (BM) mononuclear cells were obtained from the tibias and femurs of C57BL/6 mice and prepared by gradient centrifugation on LYMPHOPREP (Daiichi Pure Chemicals, Tokyo, Japan). For the purification of c-kit+ cells, BM cells were treated with a combination of anti–c-kit antibody and Microbeads in the MACS system (Miltenyi Biotech, Bergisch Gladbach, Germany). All isolated cells were confirmed to be highly purified (greater than 98%) by an immunofluorescence flow cytometry analysis using EPICS ELITE ESP cell sorter (Beckman Coulter, Fullerton, CA).
The c-kit+ cells were precultured in serum-free medium, SF03 (Sankou-junyaku, Tokyo, Japan) with 10 ng/mL murine SCF, 10 ng/mL murine IL-6, and 10 ng/mL human Flt3L for 48 hours. The production of supernatants containing retroviruses was performed according to the method described previously.27 For infection of c-kit+ cells, precultured 1 × 106 cells were transduced by centrifugation with 1 mL retrovirus supernatants containing 10 ng/mL mSCF, 10 ng/mL mIL-6, 10 ng/mL hFlt3L, and 8 μg/mL polybrene (Sigma, St. Louis, MO) at 2500g for 2 hours at 28°C to 30°C on day 1 and then cultured with DMEM containing 10% FCS, 10 ng/mL mSCF, 10 ng/mL mIL-6, and 10 ng/mL hFlt3L for 22 hours.
On day 2, cells were also transduced by the centrifugation method with retrovirus supernatants. On day 3, GFP+ cells were purified with a cell sorter. Purified 1 × 105 GFP+c-kit+ cells were transplanted by tail-vein injection into lethally irradiated (11 Gy total body irradiation) C57BL/6 recipient mice. Mice were maintained in a specific pathogen-free environment and with acidic water.
Reverse transcription–polymerase chain reaction
Total RNAs were extracted from cells using RNA-zolB (Biotex Laboratories, Houston, TX), according to the manufacturer's instructions. First-strand cDNA was synthesized at 37°C for 1 hour from 2 μg total RNA in 20 μL reaction mixture using random primers (Promega, Madison, WI). Thereafter, cDNA was amplified for 30 cycles at 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 60 seconds, with a pair of primers corresponding to each gene. Primers for GFP and SDF-1 were described above. Primers for G3PDH were described previously.31 Primers for CXCR4 were 5′-GCGAATTCATGGAACCGATCAGTGTG-3′ and 5′-GCGAATTCTTAGCTGGAGTGAAAACT-3′. Primers for intrakine were 5′-GCGAATCCACCATGTGGAC GCCAAGGTCGTC-3 and 5′-GCGAATTCTTACAGCTCGTCCTTCTCGCT-3′. PCR products were fractionated on 1.2% agarose gel and visualized by ethidium bromide staining.
Chemotaxis assay
Chemotaxis assays were performed using a 96-well chemotaxis chamber (Neuroprobe, Pleasanton, CA) with polycarbonate filter (5-μm pore size). Cells were suspended at a density of 1 × 106/mL in RPMI 1640 medium containing 20 mmol/L HEPES, pH 7.2, and 0.5% bovine serum albumin (BSA; Sigma). Twenty-five–microliter cell suspensions were added to upper chambers, and diluted chemokines (final volume, 29 μL) were added to lower chambers. Chemotaxis chambers were incubated for 2 hours at 37°C in 5% CO2. Cells that migrated to lower chamber were transferred to polystyrene tubes. Numbers of migrated cells were determined by EPICS ELITE ESP cell sorter (Beckman Coulter).
Flow cytometric analysis
Single-cell suspensions were prepared from tissues and peripheral blood after red blood cell depletion. Cells were incubated with monoclonal antibodies against cell surface markers for 30 minutes at 4°C. Phycoerythrin (PE), cy-chrome (CYC), or biotin-conjugated antibodies specific for murine CD11b, Gr-1, CD4, CD8, B220, CD43, and IgM (PharMingen, San Diego, CA) were used for flow cytometric analysis. Biotinylated antibodies were developed with CYC and allophycocyanin-conjugated streptavidin. Cells were analyzed with EPICS XL/XL-MCL System II and EPICS ELITE ESP cell sorter (Beckman Coulter). Dead cells were excluded by propidium iodide staining.
In vitro colony assay
Bone marrow cells were incubated in IMDM with 1.2% methylcellulose, 20% FCS, 1% BSA (Sigma), 50 μmol/L 2-mercaptoethanol, and 10 ng/mL of SCF or IL-3 or human EPO or mouse IL-7. Colony forming units (CFU) were monitored at 4 to 6 days (BFU-E) or 7 to 10 days (CFU-mix, CFU-GM, and CFU-IL-7) after inoculation.
ELISA for circulating immunoglobulin
Serum immunoglobulin levels were measured using antibody pairs specific for different mouse immunoglobulin isotypes (Southern Biotechnology Associates, Birmingham, AL) on microtiter plates. P-nitrophenylphosphatase–conjugated secondary antibodies were added, and the absorbance at 405 nm was measured in a microplate reader (Molecular Devices, Sunnyvale, CA). Concentrations were calculated by using purified immunoglobulin standards (Bio Pure AG, Bubendorf, Switzerland).
Results
Generation of GFP-, SDF-1–, and intrakine-transduced mice
We used a bicistronic retroviral expression vector system to generate mice reconstituted with BM-HPC transduced with either SDF-1 or SDF-1–intrakine genes. The long terminal repeats (LTRs) derived from PCC-4, cell–passaged myeloproliferative sarcoma virus (PCMV) and myeloproliferative sarcoma virus (MPSV) with wide host range were used to express SDF-1 or intrakine in vivo. The SDF-1 or intrakine genes to be expressed were placed upstream of the IRES and the GFP gene (Figure1A). The c-kit+ (BM-HPC) cells, which were precultured for 2 days in serum-free medium containing recombinant mouse SCF, IL-6, and human Flt3 ligand, were transduced by using the retroviruses expressing GFP, SDF-1–GFP, or intrakine-GFP by a centrifugal transduction method. After transduction, GFP+ cells were purified by cell sorting (Figure 1B), and 1 × 105 cells were injected into lethally irradiated recipient mice.
Generation of reconstituted mice using retrovirus vector.
(A) Diagrams of bicistronic retroviral expression vector constructs. (B) Flow cytometric analysis of c-kit+ cells 48 hours after retroviral transduction with retrovirus encoding GFP, SDF-1, or intrakine vector. GFP+ cells were collected by a cell sorter and subjected to transplantation. (C) Kinetic study on GFP-expressing cells in peripheral blood (■), bone marrow (⋄), spleen (○), and thymus (▵) of each reconstituted mouse transplanted with 1 × 105 GFP+ cells per mouse. Each point represents mean percentage ± SE from 4 separate experiments. (D). The mRNA expression level of transferred genes in the reconstituted mice. Total RNA was extracted from splenocytes. Total RNA was reverse transcribed with random primer and amplified using the specific primers for GFP, CXCR4, SDF-1, intrakine, and G3PDH. These results represent 3 independent experiments. ψ Indicates the packaging symbol.
Generation of reconstituted mice using retrovirus vector.
(A) Diagrams of bicistronic retroviral expression vector constructs. (B) Flow cytometric analysis of c-kit+ cells 48 hours after retroviral transduction with retrovirus encoding GFP, SDF-1, or intrakine vector. GFP+ cells were collected by a cell sorter and subjected to transplantation. (C) Kinetic study on GFP-expressing cells in peripheral blood (■), bone marrow (⋄), spleen (○), and thymus (▵) of each reconstituted mouse transplanted with 1 × 105 GFP+ cells per mouse. Each point represents mean percentage ± SE from 4 separate experiments. (D). The mRNA expression level of transferred genes in the reconstituted mice. Total RNA was extracted from splenocytes. Total RNA was reverse transcribed with random primer and amplified using the specific primers for GFP, CXCR4, SDF-1, intrakine, and G3PDH. These results represent 3 independent experiments. ψ Indicates the packaging symbol.
After transplantation, GFP expression in reconstituted mice was analyzed (Figure 1C). Fifty-six days after transplantation, reconstitution efficiency with transduced cells was up to 90.3% ± 7.8% and 95.5% ± 8.4% in the peripheral blood of the GFP- or SDF-1–transduced mice, respectively (Figure 1C). High-level reconstitution with GFP+ cells was also observed in the spleen, bone marrow, and thymus of these mice. On the other hand, reconstitution efficiency was 12% to 15% lower in intrakine-transduced mice than in others (Figure 1C). It has been reported that 56 to 84 days are required to establish full reconstitution in recipient mice; therefore,32 we used mice at 70 days after transplantation for further experiments. The absolute cell number in the peripheral blood, spleen, bone marrow, and thymus from GFP-transduced mice was 4.78 ± 0.47 × 106/mL, 1.56 ± 0.27 × 108 mL, 1.71 ± 0.29 × 107 mL, and 4.61 ± 0.46 × 107, respectively, 70 days after transplantation. In addition, the absolute cell number of those from SDF-1 and intrakine-transduced mice was 5.61 ± 0.49 × 106/mL, 2.06 ± 0.15 × 108 mL, 1.98 × 0.24 × 107, and 3.90 ± 0.72 × 107 mL versus 3.18 ± 0.77 × 106/mL, 1.18 ± 0.15 × 108, 1.15 ± 0.24 × 107, and 1.73 ± 0.42 × 107, respectively.
Next, we confirmed the mRNA expression level of the transferred genes in the reconstituted mice. Total RNA was extracted from splenocytes of reconstituted mice, and reverse transcription (RT)–PCR was performed using the primers for specific genes. High expression of SDF-1 mRNA was detected in the splenocytes from SDF-1–transduced mice compared with those from GFP- and intrakine-transduced mice (Figure 1D). On the other hand, specific and high expression of intrakine mRNA was detected in the splenocytes from intrakine-transduced mice but not in the GFP- or SDF-1–transduced mice (Figure 1D).
No differences were observed in the CXCR4 mRNA expression of these mice. Similar results were obtained from the peripheral blood, bone marrow, and thymus. These results suggested that genes transferred by retroviral vector were highly expressed in the reconstituted mice.
In vivo effects of SDF-1–intrakine on the expression and function of CXCR4 in adult reconstituted mice
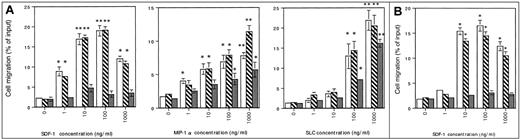
FCM analysis revealed that cell surface CXCR4 expression was dramatically decreased in the splenocytes of intrakine-transduced mice than in those of GFP- or SDF-1–transduced mice (Figure2Ai,iii,v). The suppression of CXCR4 expression was also observed in B220+ cells of peripheral blood (Figure 2A, panels ii, iv, vi), bone marrow, and thymus (data not shown). Similar expression levels of CXCR4 were observed in GFP- and SDF-1–transduced mice (Figure 2A, panels i-iv). In contrast, there were comparable expression levels of CCR7, a receptor for SLC/6Ckine and ELC/MIP-3β/CKβ-11, on the splenocytes of GFP-, SDF-1–, and intrakine-transduced mice (Figure 2B). The expression level of CCR1, a receptor for MIP-1α and RANTES, on the splenocytes of intrakine-transduced mice was lower than that of GFP- and SDF-1–transduced mice, though the decrease in CCR1 expression in intrakine-transduced mice was less prominent than that in CXCR4 expression (Figure 2C). Chemotaxis assay showed that in intrakine-transduced mice, splenocyte response to SDF-1 was dramatically decreased, whereas the responses to SLC and MIP-1α were slightly reduced (Figure 3A). Chemotaxis assay using bone marrow cells from intrakine-transduced mice also showed a dramatic decrease in response to SDF-1 (Figure 3B). Similar results were observed in the cells from peripheral blood, lymph node, and thymus (data not shown). Splenocytes derived from GFP- or SDF-1–transduced mice showed prominent responses to SDF-1, with profiles characterized by a typical sharp bell curve (Figure 3A). These results suggested that the dramatic inhibition of response to SDF-1 in intrakine-transduced mice resulted from the specific down-regulation of CXCR4 expression on the cell surfaces induced by the intrakine.
Effect of overexpressed SDF-1 or intrakine on the expression and function of CXCR4 in reconstituted mice.
(A) Cell surface expression of CXCR4 on the splenocytes (i, iii, v) and B220+ cells (ii, iv, vi) of peripheral blood from reconstituted mice. Cells from each reconstituted mouse at 70 days after transplantation were stained with control antibody (dotted lines), anti-mCXCR4 (bold lines), or without antibody (solid lines) and were subjected to flow cytometric analysis. Panels i and ii show cells from GFP-transduced mice. Panels iii and iv show cells from SDF-1–transduced mice. Panels v and vi show cells from intrakine-transduced mice. (B, C) Cell surface expression of CCR7 and CCR1 on the splenocytes from reconstituted mice. Cells were stained with control antibody (dotted lines), anti-chemokine receptor (bold lines), or without antibody (solid lines). These results represent 4 independent experiments.
Effect of overexpressed SDF-1 or intrakine on the expression and function of CXCR4 in reconstituted mice.
(A) Cell surface expression of CXCR4 on the splenocytes (i, iii, v) and B220+ cells (ii, iv, vi) of peripheral blood from reconstituted mice. Cells from each reconstituted mouse at 70 days after transplantation were stained with control antibody (dotted lines), anti-mCXCR4 (bold lines), or without antibody (solid lines) and were subjected to flow cytometric analysis. Panels i and ii show cells from GFP-transduced mice. Panels iii and iv show cells from SDF-1–transduced mice. Panels v and vi show cells from intrakine-transduced mice. (B, C) Cell surface expression of CCR7 and CCR1 on the splenocytes from reconstituted mice. Cells were stained with control antibody (dotted lines), anti-chemokine receptor (bold lines), or without antibody (solid lines). These results represent 4 independent experiments.
Chemotactic analysis.
(A) Splenocytes from GFP-transduced (open column), SDF-1–transduced (hatched column), or intrakine-transduced (gray column) mice were stimulated with the indicated concentration of SDF-1, SLC, or MIP-1α in a 96-well chemotaxis chamber. The assay was performed in triplicate, and the number of migrated cells was counted by a flow cytometer. Each point represents mean ± SE from 4 separate experiments. Statistical analysis was performed by Student t test (*P < .05; **P < .01). (B) Chemotactic analysis on bone marrow cells. Bone marrow cells from GFP-transduced (open column), SDF-1–transduced (hatched column), or intrakine-transduced (gray column) mice were stimulated with the indicated concentration of SDF-1 in a 96-well chemotaxis chamber. The assay was performed in triplicate, and the number of migrated cells was counted by a flow cytometer. Each point represents the mean ± SE from 4 separate experiments. *P < .01.
Chemotactic analysis.
(A) Splenocytes from GFP-transduced (open column), SDF-1–transduced (hatched column), or intrakine-transduced (gray column) mice were stimulated with the indicated concentration of SDF-1, SLC, or MIP-1α in a 96-well chemotaxis chamber. The assay was performed in triplicate, and the number of migrated cells was counted by a flow cytometer. Each point represents mean ± SE from 4 separate experiments. Statistical analysis was performed by Student t test (*P < .05; **P < .01). (B) Chemotactic analysis on bone marrow cells. Bone marrow cells from GFP-transduced (open column), SDF-1–transduced (hatched column), or intrakine-transduced (gray column) mice were stimulated with the indicated concentration of SDF-1 in a 96-well chemotaxis chamber. The assay was performed in triplicate, and the number of migrated cells was counted by a flow cytometer. Each point represents the mean ± SE from 4 separate experiments. *P < .01.
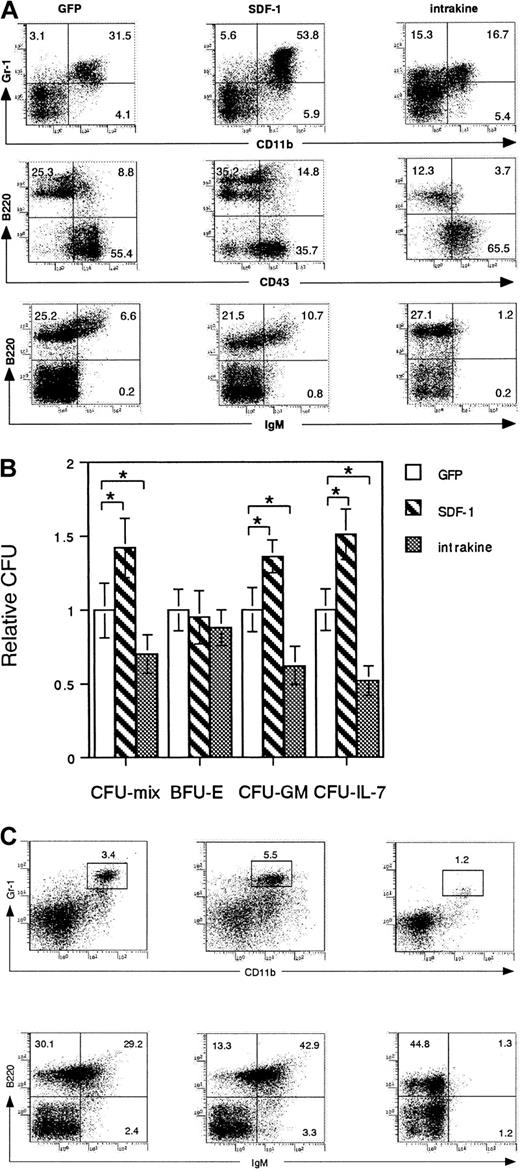
B lymphopoiesis and myelopoiesis in reconstituted mice
To investigate the role of SDF-1 in adult hematopoiesis, bone marrow cells from reconstituted mice were subjected to flow cytometric analysis. The numbers of pro-B (B220+ CD43+), pre-B (B220+ CD43−), and immature-B (B220+ IgM+) cells were significantly decreased in intrakine-transduced mice than in those in GFP-transduced mice (3.7%± 0.7% versus 8.8% ± 1.1%, 12.3% ± 1.7% versus 25.3% ± 2.2%, 1.2% ± 0.4% versus 6.6% ± 0.9%, respectively) (Figure 4A). Similar reductions in the B-cell number in the spleen and peripheral blood were observed in intrakine-transduced mice (Figure 4C). The numbers of granulocytes/myeloid cells (Gr-1+ CD11b+) in the bone marrow and peripheral blood were severely reduced in intrakine-transduced mice (16.7% ± 1.4% versus 31.5% ± 2.4%, respectively) (Figure 4A,C). The percentages of CD4+ and CD8+ T cells in peripheral blood of intrakine-transduced mice were 15.4% ± 1.3% and 10.7% ± 1.2%, respectively (data not shown).
B lymphopoiesis and myelopoiesis in reconstituted mice.
(A) Flow cytometric analysis of B-lymphoid and -myeloid cells in the bone marrow of reconstituted mice more than 70 days after transplantation. Dead cells were excluded by propidium iodide staining. Data are representative of 7 independent experiments. (B) Bone marrow cells from reconstituted mice were subjected to in vitro colony assay. Relative CFU colony numbers were calculated as the ratio of CFU colony numbers of bone marrow cells from GFP-transduced mice. Mean ± SE for 4 independent experiments are shown. Statistical analysis was performed by Student t test. *P < .05. (C) Flow cytometric analysis of B-lymphoid and -myeloid cells in peripheral blood. Dead cells were excluded by propidium iodide staining.
B lymphopoiesis and myelopoiesis in reconstituted mice.
(A) Flow cytometric analysis of B-lymphoid and -myeloid cells in the bone marrow of reconstituted mice more than 70 days after transplantation. Dead cells were excluded by propidium iodide staining. Data are representative of 7 independent experiments. (B) Bone marrow cells from reconstituted mice were subjected to in vitro colony assay. Relative CFU colony numbers were calculated as the ratio of CFU colony numbers of bone marrow cells from GFP-transduced mice. Mean ± SE for 4 independent experiments are shown. Statistical analysis was performed by Student t test. *P < .05. (C) Flow cytometric analysis of B-lymphoid and -myeloid cells in peripheral blood. Dead cells were excluded by propidium iodide staining.
On the other hand, the numbers of pro-B (B220+CD43+), pre-B (B220+ CD43−), and immature-B (B220+ IgM+) cells were significantly increased in the bone marrow (14.8% × 0.9% versus 8.8% ± 1.1%, 35.2% ± 2.7% versus 25.3% ± 2.2%, 10.7% ± 1.1% versus 6.6% ± 0.9%, respectively) and peripheral blood of SDF-1–transduced mice compared to GFP-transduced mice (42.9% ± 3.4% versus 29.2% ± 2.2%, respectively) (Figure4A,C). Numbers of granulocytes/myeloid cells (Gr-1+CD11b+) were also significantly increased in bone marrow and peripheral blood of these mice (53.8% ± 4.4% and 31.5% ± 2.4%, 5.5% ± 0.4% versus 3.4% ± 0.4%, respectively) (Figure 4A,C). Percentages of CD4+ and CD8+ T cells in peripheral blood of SDF-1–transduced mice were 16.4% ± 1.4% and 11.2% ± 0.6%, respectively (data not shown).
In vitro colony assay revealed that the relative CFU of CFU-mix, CFU-GM, and CFU–IL-7 were reduced in intrakine-transduced mice compared to GFP-transduced mice (0.70 ± 0.13 versus 1.0 ± 0.18, 0.62 ± 0.12 versus 1.0 ± 0.15, and 0.52 ± 0.10 versus 1.0 ± 0.14, respectively) (Figure 4B). In contrast, the relative CFU of CFU-mix, CFU-GM, and CFU-IL-7 were increased in SDF-1–transduced mice compared to those in GFP mice (1.43 ± 0.20 versus 1.0 ± 0.18, 1.38 ± 0.11 versus 1.0 ± 0.15, and 1.52 ± 0.17 versus 1.0 ± 0.1, respectively) (Figure 4B). Percentages of c-kit+ cells in the bone marrow was increased in the SDF-1–transduced mice (9.4% ± 0.3%), whereas it was decreased in intrakine-transduced mice (4.1% ± 0.1%) compared to GFP-transduced mice (6.4% ± 0.3%). These results suggested that the inhibition of CXCR4 expression on the cell surfaces by intrakine results in impaired B lymphopoiesis and myelopoiesis in adult mice and that the overexpression of SDF-1 in SDF-1–transduced mice induces enhanced B lymphopoiesis and myelopoiesis in bone marrow. As shown in Table 1, serum immunoglobulin concentrations in reconstituted mice were measured by isotype-specific enzyme-linked immunosorbent assay (ELISA). The mean concentrations of IgG, IgM, and IgA in sera were decreased in intrakine-transduced mice compared to those of GFP-transduced mice, whereas the mean concentrations of IgG, IgM, and IgA in sera were increased in SDF-1–transduced mice (Table 1).
Serum Ig concentrations
| Type of mouse . | Number of mice . | IgG (μg/ml) . | IgM (μg/ml) . | IgA (μg/ml) . |
|---|---|---|---|---|
| GFP | 5 | 1789 ± 312 | 709 ± 103 | 207 ± 26 |
| SDF-1 | 5 | 2745 ± 418* | 1197 ± 214* | 288 ± 45* |
| Intrakine | 5 | 980 ± 310* | 356 ± 123* | 144 ± 36* |
| Type of mouse . | Number of mice . | IgG (μg/ml) . | IgM (μg/ml) . | IgA (μg/ml) . |
|---|---|---|---|---|
| GFP | 5 | 1789 ± 312 | 709 ± 103 | 207 ± 26 |
| SDF-1 | 5 | 2745 ± 418* | 1197 ± 214* | 288 ± 45* |
| Intrakine | 5 | 980 ± 310* | 356 ± 123* | 144 ± 36* |
Serum Ig concentrations were reduced in intrakine-transduced mice but increased in SDF-1–transduced mice. Concentrations of different Ig isotypes were determined by ELISA. The mean ± SE for 3 independent experiments is shown. Statistical analysis was conducted (Student t test) to determine whether Ig concentrations from intrakine- or SDF-1–transduced mice were significantly different from EGFP-1–transduced mice.
P < .01.
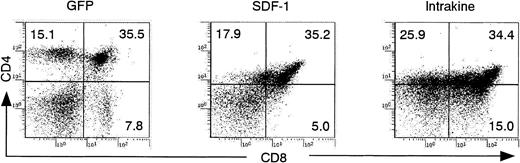
T lymphopoiesis in reconstituted mice
FCM analysis of the thymocytes revealed that the number of CD4+dull CD8+dull double-positive cells was significantly increased in SDF-1–transduced mice in comparison with GFP-transduced mice and that CD4+high and CD8+high single-positive cells were extremely few in these mice (Figure 5). In contrast, an aberrant increase in CD4−/dull CD8+/− cells was observed in the thymi of intrakine-transduced mice (Figure 5). FCM analysis showed no difference in thymocyte size. The total number of thymocytes in intrakine-transduced mice was decreased by approximately 30% to 40% compared with GFP-transduced mice. Histologic analysis of the thymus revealed hyperplasia but no increase in total cell number in the cortices of SDF-1–transduced mice compared with GFP-transduced mice. Similar patterns were observed in the thymic medullas of SDF-1–transduced mice. On the other hand, histologic studies revealed thin cortical areas and hypoplasia in the medullas of intrakine-transduced mice.
T lymphopoiesis in reconstituted mice.
Flow cytometric analysis on the thymocytes from reconstituted mice 70 days after transplantation. Dead cells were excluded by propidium iodide staining. These results represent 5 independent experiments.
T lymphopoiesis in reconstituted mice.
Flow cytometric analysis on the thymocytes from reconstituted mice 70 days after transplantation. Dead cells were excluded by propidium iodide staining. These results represent 5 independent experiments.
Discussion
We have demonstrated in this study that the expression and function of CXCR4 was blocked in mice reconstituted with BM-HPCs expressing SDF-1 intrakine and that hematopoiesis, including T lymphopoiesis, was markedly impaired in intrakine-transduced mice. This is the first in vivo study that shows the successful use of an intrakine to regulate the function of specific chemokines and chemokine receptors. Ma et al33 reported that CXCR4 is required for the retention of the B lineage and granulocytic precursors in the bone marrow microenvironment. They detected a decrease in the number of B-cell lineages (B220+) in bone marrow and an increase in the number of immature B cells (B220+ CD43+IgM−, B220+ CD43−IgM−) in the peripheral blood of reconstituted mice with CXCR4-deficient fetal liver cells 4 weeks after transplantation. These cells (B220+ IgM−) in peripheral blood, which normally reside in bone marrow, differentiate into IgM+cells under Whitlock-Witte culture conditions. Ma et al33also detected a decrease in the number of immature granulocytic precursors (Gr-1+) in bone marrow and an increase of morphologically immature granulocytic precursors in peripheral blood. From these results, they concluded that CXCR4 is required for the retention of the B lineage and of granulocytic precursors in the bone marrow microenvironment but not for the differentiation and proliferation of B cells. Kawabata et al34 also reported that CXCR4 in hematopoietic cells is essential for long-term lymphoid and myeloid reconstitution. They detected a severe decrease in the numbers of B220+ B cells, Mac-1+granulocytes–monocytes, Gr-1+ granulocytes in bone marrow, and CD4+ thymocytes. They also observed that the number of c-kit+ Sca-1−/lin− cells, but not c-kit+ Sca-1+/lin− cells, was significantly decreased in reconstituted mice. Because c-kit+ Sca-1−/lin− cells are generally considered to be more committed hematopoietic progenitors than c-kit+ Sca-1+/lin− cells in mice,35 they speculated that CXCR4 might play an essential role in the generation, expansion, or both of early hematopoietic progenitors within bone marrow.34 In our study, all B-cell stages (B220+ CD43+, B220+CD43−, and B220+ IgM+) and myeloid cells (CD11b+ Gr-1+) were decreased in the bone marrow, peripheral blood, and spleen of intrakine-transduced mice 70 days after transplantation. In parallel to the decrease of B cells, concentrations of serum immunoglobulins were decreased in these mice. In contrast, B lymphopoiesis and myelopoiesis were increased in SDF-1–transduced mice. We also found that T lymphopoiesis was impaired in the intrakine-transduced mice. It has been reported that 4 weeks after transplantation is not long enough to establish reconstitution, especially in lymphopoiesis.32 Therefore, the differences between our results and those reported by other groups may result from the different time points of analysis after reconstitution. Based on these results, we hold that SDF-1/CXCR4 is essentially involved in the differentiation and proliferation of B cells, T cells, and myeloid cells. The responses to MIP-1α and SLC in the cells of intrakine-transduced mice were slightly reduced than those in GFP- and SDF-1–transduced mice. But the effect of intrakine in the response to SDF-1 of the cells from intrakine-transduced mice was much greater than that observed in MIP-1α and SLC. Most strikingly, the response to SDF-1 of bone marrow cells from intrakine-transduced mice was almost completely blocked. There are comparable expression levels of CCR7 on the splenocytes from these mice, suggesting that the inhibition of CXCR4 expression in intrakine-transduced mice is specific. The expression level of CCR1 on splenocytes in intrakine-transduced mice was significantly, but not dramatically, decreased compared with that in GFP- and SDF-1–transduced mice. This could be the result of a dramatic change in splenic population caused by impaired myelopoiesis and B lymphopoiesis in intrakine-transduced mice. Alternatively, immature B cells and myeloid cells may express lower levels of CCR1 on the cell surface.
Because an ELISA system for measuring serum levels of murine SDF-1 is unavailable, we could not confirm higher concentrations of SDF-1 in SDF-1–transduced mice than in GFP-transduced control mice. Although a higher expression of messenger RNA for SDF-1 was observed in SDF-1–transduced mice than in GFP-transduced mice by RT-PCR analysis, similar expression levels of CXCR4 on the cells by FACS analysis and migration activity in GFP- and SDF-1–transduced mice were observed. This is rather unexpected because the overexpression of SDF-1 could be expected to result in the down-regulation of CXCR4 expression on leukocytes in SDF-1–transduced mice. The reason for this phenotype is unclear, but it could be because of the accelerated turnover of CXCR4 in SDF-1–transduced mice.
Interestingly, we found abnormalities in T-cell maturation in the thymus, both in SDF-1– and intrakine-transduced mice. There was an increase of CD4+dull CD8+dull cells in the thymi of SDF-1–transduced mice in contrast to an increase of CD4−/dull CD8+/− cells in the thymi of intrakine-transduced mice.
Preliminary results from immunohistochemical staining showed that SDF-1 is highly expressed in the thymic medulla, whereas CXCR4 is expressed in the thymic cortex. This suggests that preferential localization of SDF-1 in the thymic cortex plays a role in positive selection and that down-regulation of CXCR4 during positive selection enables selected thymocytes to move toward the thymic medulla. Kim et al36reported that high levels of mRNA of SDF-1 and CXCR4 were detected in the thymus and that SDF-1 had a chemotactic activity for immature thymocytes (subsets of triple-negative thymocytes and double-positive thymocytes) over mature single-positive thymocytes. They speculated that SDF-1 might be a chemoattractant regulating the migration of immature double-negative and double-positive thymocytes at premedullar stage (cortex) migration. Based on these results and our own, we speculate that the overexpression of SDF-1 may cause the retention of immature thymocytes in the cortex and inhibit intrathymic trafficking required for positive selection. On the other hand, the migration of thymocytes in intrakine-transduced mice is also interfered with by down-regulated CXCR4 expression on those by intrakine.
FCM analysis on thymocytes in intrakine-transduced mice revealed the accumulation of the CD4−/dull CD8+/−population. This may represent an arrest of thymocyte maturation at the step from double-negative to double-positive cells in intrakine-transduced mice. It is also likely that SDF-1/CXCR4 may regulate the migration of T-cell progenitor cells from bone marrow to thymus because the total number of thymocytes in intrakine-transduced mice decreased approximately 30% to 40% compared with that in GFP-transduced mice. Ma et al33 did not detect any abnormalities of T lymphopoiesis, and Kawabata et al34described only a decrease in the number of CD4+ T cells in the thymus of reconstituted mice, using fetal liver cells from CXCR4 knockout embryos. It is possible that some differences between our results and those of other groups are caused by differences in donor cells and the time point of analysis. We used bone marrow–derived c-kit+ cells instead of whole fetal liver cells as donor cells and analyzed mice 70 days after reconstitution. Furthermore, the fetal liver cells from CXCR4 knockout mice used in other studies might have had different genetic backgrounds. Further studies are needed to elucidate the role of SDF-1/CXCR4 in thymic selection.
The discovery that several chemokine receptors act as coreceptors for HIV-1 provides multiple opportunities for new therapeutic interventions into the process of viral pathogenesis in AIDS.37,38T-tropic HIV uses the chemokine receptor CXCR4 as a coreceptor and emerges during the late stages of AIDS progression associated with a decline in CD4+ T lymphocytes.39 40 This suggests that CXCR4 may be related to AIDS pathogenesis and that it is a suitable target for AIDS therapeutic application. Our results may provide a new therapeutic approach to AIDS through the use of the intrakine gene. It should be noted, however, that the transduction of an intrakine into human hematopoietic stem cells from patients and the transplantation of those into AIDS patients might result in impaired hematopoiesis and lymphopoiesis.
In summary, this study represents the first successful use of intrakine in vivo to down-regulate the expression and function of specific cognate receptors. By using this novel approach, we have demonstrated that SDF-1/CXCR4 has an important role in B lymphopoiesis, myelopoiesis, and T lymphopoiesis in adult hematopoiesis.
Acknowledgment
We thank Dr Masataka Osawa (Kirin Brewery, Tokyo, Japan) for valuable technical advice on bone marrow transplantation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kouji Matsushima, Department of Molecular Preventive Medicine, School of Medicine, University of Tokyo, 7-3-1 Hongo, Bonkyo-ku, Tokyo 113-0033, Japan; e-mail:koujim@m.u-tokyo.ac.jp.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal