Abstract
We studied the role of adenosine (Ado), which is generated from adenine nucleotides via the activity of ecto-5′-nucleotidase (ecto-5′-NT), in the inhibition of platelet aggregation by endothelial cells (ECs). The enzymatic activity of nucleotidases on human umbilical vein endothelial cells (HUVECs) was examined with regard to (1) the inhibition of adenosine diphosphate (ADP)–induced platelet aggregation and (2) the liberation of inorganic phosphate from adenine nucleotides. Adenosine 5′-monophosphate (AMP) preincubated with HUVECs significantly inhibited ADP-induced platelet aggregation. This was completely blocked by the treatment of HUVECs with a specific inhibitor of ecto-5′-NT, 5′-[αβ-methylene] diphosphate (APCP), or by the addition of an A2a receptor antagonist. Neither nitric oxide nor prostacyclin was involved in this inhibitory activity, suggesting that Ado generated in the incubation medium by the activity of 5′-NT on HUVECs inhibited platelet aggregation. When ADP was incubated on HUVECs, it lost most of its agonistic activity for platelets. Pretreatment of HUVECs with APCP at a concentration that abolished ecto-5′-NT activity partially restored ADP-induced platelet aggregation. Ecto-5′-NT contributes to EC function by inhibiting platelet aggregation in cooperation with ATP diphosphohydrolase, which degrades ADP to AMP.
Introduction
It has been well established that quiescent endothelial cells (ECs) exert anticoagulant effects both through their expression of thrombomodulin1 and heparan sulfate proteoglycans2 and by the release of tissue factor pathway inhibitor.3 ECs also inhibit platelet aggregation through the production of nitric oxide (NO)4 and prostacyclin (PGI2)5 and through degradation of adenosine diphosphate (ADP) by adenosine triphosphate (ATP) diphosphohydrolase (ATPDase), which is expressed on the luminal surface of ECs.6-8 However, ATPDase can inhibit platelet aggregation and recruitment in the absence of NO and PGI2.6-8 ADP released from activated platelets induces platelet recruitment followed by further platelet aggregation via binding to platelet P2X, P2T, and P2Y receptors.9,10 ATPDase (molecular mass, 70-100 kd) is a glycoprotein belonging to the E-type ATPase family.11 This enzyme hydrolyzes ATP and ADP to ADP and adenosine 5′-monophosphate (AMP), respectively. Enzyme activity is dependent on calcium (Ca2+) and magnesium (Mg2+), and is inhibited by chelating agents, azides, and ATP analogues, although it is not affected by inhibitors of P-, F- and V-type ATPases.6,8,11,12 Degradation of extracellular ADP by ATPDase has been recognized as important for the inhibition of platelet aggregation by ECs, because of the critical role ADP plays as an agonist for platelet aggregation.6-8 13
Ecto-5′-nucleotidase (ecto-5′-NT) (CD73) also participates in adenine nucleotide metabolism on the surface of ECs.14,15AMP, generated from ADP by the action of ATPDase, is subsequently hydrolyzed to adenosine (Ado) by ecto-5′-NT. Widely distributed in bacteria, plant cells, and vertebrate tissues, 5′-NT is classified into 4 groups according to cellular location and biochemical properties: a membrane-anchored ecto-5′-NT, a soluble form derived from ecto-5′-NT, and 2 cytoplasmic forms. Ecto-5′-NT, anchored to the plasma membranes via glycosyl-phosphatidylinositol (GPI) moiety,16 is distributed in a variety of cells including hepatocytes, fibroblasts, endothelial cells, lymphocytes, and glial cells.17 The catalytic activity of 5′-NT controls intracellular and extracellular levels of AMP and Ado, thereby allowing Ado to be metabolized for the synthesis of adenine nucleotides in the purine salvage pathway.18 In addition to its enzymatic activity, ecto-5′-NT is involved in cell-cell and cell-matrix interactions and transmembrane signaling.19 20
The disulfide-linked homodimer form of ecto-5′-NT is essential for its enzymatic activity. Although ecto-5′-NT hydrolyzes a variety of nucleoside 5′-monophosphates, it has greatest affinity for AMP, with Km values in the micromolar range. Enzymatic activity is not dependent on added divalent cations but is inhibited by metal ion chelating agents because of the presence of several potential zinc (Zn2+) binding sites that are important for its activity.21 Adenosine 5′-[α,β-methylene] diphosphate (APCP) is a potent inhibitor of ecto-5′-NT with Ki values in the nanomolar range.14 22 ATP and ADP also inhibit ecto-5′-NT with Ki values in the micromolar range.
Ado inhibits a variety of cellular functions including platelet aggregation,23 expression of tissue factor or adhesion molecules and cytokine release by activated ECs,24,25neutrophil adherence and injury to ECs,26 and release of superoxide from neutrophils.27 However, Ado also enhances NO production by ECs.28 These effects are mediated through the binding of Ado to the A1, A2, and A3 receptors that are expressed on cells in a variety of tissues. The inhibition of platelet aggregation by Ado is thought to be mediated by the stimulation of adenylate cyclase through A2a receptors expressed on platelets.23Although the inhibitory aggregation effect of Ado on platelet is evident in vitro,23 its effect in vivo has remained controversial because of its rapid transport into cells29,30 and its rapid degradation by Ado deaminase (ADA).31 However, studies showing that both platelet aggregation ex vivo and thrombosis formation in vivo were inhibited by the administration of Ado analogues to humans and dogs32and that an Ado receptor antagonist caused thrombosis in dogs33 suggest a critical involvement of Ado in the inhibition of platelet aggregation in vivo.
In the present study, we examined ecto-5′-NT activity on the surface of human umbilical vein endothelial cells (HUVECs) and confirmed the cooperative function of ecto-5′-NT with ATPDase in the regulation of platelet aggregation.
Materials and methods
Materials
We purchased the following materials: MCDB131 medium, gelatin, bovine serum albumin (BSA), aspirin, levamisole, and APCP (Sigma Chemical Co., St Louis, MO); AMP, Ado, malachite green, and NG-nitro-L-arginine-methyl ester (L-NAME) (Wako Pure Chemical, Osaka, Japan); 8-(3-chlorostyryl) caffeine (CSC) and dipyridamole (DIP) (Research Biochemicals International, Natick, MA); nitrate/nitrite colorimetric assay kit (Cayman Chemical, Ann Arbor, MI); fetal calf serum (FCS) (Nichirei, Tokyo, Japan); acidic fibroblast growth factor (aFGF) (Austral Biologicals, San Ramon, CA); heparin (Daiichi Pure Chemicals, Tokyo, Japan); ADP (Oriental Yeast, Tokyo, Japan); Urinary Prostacyclin Enzyme Immunoassay Kit (Assay Designs Inc., Ann Arbor, MI); and recombinant human tumor necrosis factor–α (TNF-α) (Peprotech EC, London, England).
Cell culture of HUVECs
HUVECs were prepared from umbilical cords according to the methods described previously.34 The cells were cultured in MCDB131 medium supplemented with 15% FCS, 10 ng/mL aFGF, and 5 μg/mL heparin on gelatin-coated flasks (Nalge Nunc International, Naperville, IL) at 37°C under 5% carbon dioxide (CO2). Second- to fourth-passage cells were used in the present study.
Inhibition of platelet aggregation by ADP preincubated on HUVECs
Nucleotidase activity.
HUVECs were grown to confluence in a gelatin-coated 96-well plate. After removal of the culture medium, the HUVEC-containing wells and the cell-free (blank) wells were washed 3 times with 50 mmol/L phosphate-free buffer containing Tris HCl (tris[hydroxymethyl] aminomethane hydrochloride) buffer (pH 8.0) and 150 mmol/L sodium chloride (NaCl), 5 mmol/L calcium dichloride (CaCl2), 5 mmol/L magnesium dichloride (MgCl2), and 0.1 mg/mL BSA (buffer A). The cells were then incubated with 200 μL buffer A containing 1-75 μmol/L ADP at 37°C for 15 minutes. The amounts of inorganic phosphate (Pi) liberated into the supernatants were measured by the malachite green colorimetric assay as described by Baykov et al.35 The agonistic activity of the incubation buffer on platelets following incubation with HUVECs or blank wells was also examined as described below. In some experiments, HUVECs were treated with 0.1-10 mmol/L levamisole, an inhibitor of alkaline phosphatase,36 37 or 1-100 μmol/L APCP before incubation with ADP.
Inhibition of platelet aggregation.
Blood from healthy volunteers was drawn into a 0.1 volume of sodium citrate (3.8%). Platelet-rich plasma (PRP) was obtained by differential centrifugation of the blood. PRP containing 6 × 107 platelets per 200 μL was preincubated at 37°C in an aggregometer cuvette. Platelet activation was then started by the addition of 22 μL of an ADP-containing (1-75 μmol/L) buffer preincubated on the HUVECs. Platelet aggregation was monitored by the increase of light transmission using an aggregometer (Hema Tracer I, Niko Bio Science, Tokyo, Japan). The percentage of aggregation was defined as the maximal change of light transmission within 10 minutes.
Inhibition of ADP-induced platelet aggregation by HUVECs exposed to AMP
Nucleotidase activity.
HUVECs were grown to confluence in a gelatin-coated 96-well plate. After removal of the medium, the HUVEC-containing wells or the blank wells were washed twice with the wash buffer (buffer B) containing 30 mmol/L Tris HCl (pH 7.4) with 0.25 mmol/L ethylenediamine tetraacetic acid (EDTA), 0.125 mmol/L ethyleneglycotetraacetic acid (EGTA), 130 mmol/L sodium chloride (NaCl), and 5.5 mmol/L glucose. The wells were then washed with the incubation buffer (buffer C) containing 50 mmol/L Tris HCl (pH 7.4) with 130 mmol/L NaCl, 5 mmol/L MgCl2, and 5.5 mmol/L glucose. Buffer C, containing 1-100 μmol/L AMP, was then added to the wells and incubated at 37°C for 15 minutes. The amounts of Pi liberated into the supernatants were measured as described above. The inhibitory effect of the preincubated buffer on HUVECs or blank wells on ADP-induced platelet aggregation was also examined as described below. In some experiments, HUVECs were treated with one of the following inhibitors prior to incubation with AMP: 0.1-10 mmol/L L-NAME, an inhibitor of NO synthase; 0.01-1 mmol/L aspirin, an inhibitor of cyclo-oxygenase5,38; 1-100 μmol/L DIP, an inhibitor of adenosine transporter39; 0.1-10 mmol/L levamisole; or 100 μmol/L APCP.
Inhibition of ADP-induced platelet aggregation by HUVECs exposed to AMP.
We added 22 μL of AMP-containing buffer (1-100 μmol/L) preincubated on HUVECs or blank wells to 200 μL PRP immediately before the activation of PRP by ADP (final concentrations, 2-3 μmol/L); then platelet aggregation was monitored as described above. In some experiments, 0.5-10 μmol/L CSC, a selective antagonist for the A2a receptor,40 was added to PRP with the incubation buffer from HUVECs or blank wells immediately before the activation of platelets by ADP.
Production of NO and PGI2 by HUVECs
The concentration of NO in the incubation buffer was measured as total nitrate/nitrite concentration using a nitrate/nitrite colorimetric assay kit. In some experiments, HUVECs were pretreated with 0.01-10 mmol/L L-NAME. The production of PGI2 during the incubation period was evaluated by measuring its stable breakdown products, 6-keto-prostaglandin F1α and 2,3-dinor-6-keto-prostaglandin F1α in the incubation buffer using a urinary prostacyclin enzyme immunoassay kit. In some experiments, HUVECs were pretreated with 0.001-1 mmol/L aspirin.
Statistical analyses
The Student unpaired t test was used for all statistical analyses.
Results
Inhibition of platelet aggregation by ADP preincubated on HUVECs
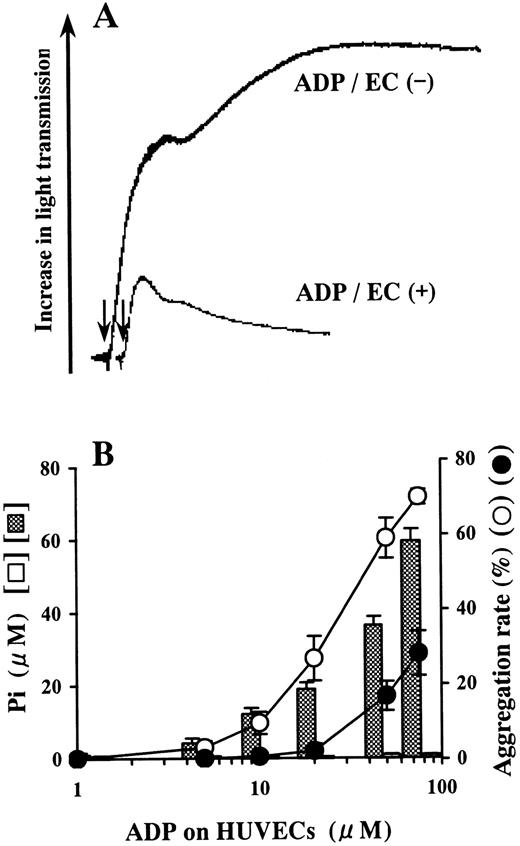
ADP incubated on the blank wells did not release Pi and induced aggregation of platelets (Figure1). However, Pi was liberated from ADP on HUVECs in a dose-dependent manner relative to the concentration of ADP added (Figure 1B). Platelet aggregation induced by the ADP-containing buffer preincubated on HUVECs was inhibited compared with aggregation induced by the buffer preincubated on blank wells (Figure 1).
Inhibition of platelet aggregation by ADP preincubated on HUVECs.
HUVEC-containing wells and blank wells were incubated with a phosphate-free buffer containing 1-75 μmol/L ADP at 37°C for 15 minutes. (A) When 50 μmol/L ADP was incubated on HUVECs, it lost most of its agonistic activity for platelets. The arrows indicate addition of the incubation buffer from HUVECs or blank wells. The results are representative of 3 experiments. (B) Pi was liberated from ADP preincubated on HUVECs in a dose-dependent manner relative to the concentration of ADP added (▩). Almost no Pi was liberated from ADP preincubated on blank wells (open columns). The ADP-containing buffer preincubated on HUVECs (●) lost its agonistic activity for platelets in comparison with the ADP-containing buffer preincubated on blank wells (○). The ADP-treated incubation buffer was added to PRP in a 10-fold dilution. The results are the mean ± SD of 3 separate experiments.
Inhibition of platelet aggregation by ADP preincubated on HUVECs.
HUVEC-containing wells and blank wells were incubated with a phosphate-free buffer containing 1-75 μmol/L ADP at 37°C for 15 minutes. (A) When 50 μmol/L ADP was incubated on HUVECs, it lost most of its agonistic activity for platelets. The arrows indicate addition of the incubation buffer from HUVECs or blank wells. The results are representative of 3 experiments. (B) Pi was liberated from ADP preincubated on HUVECs in a dose-dependent manner relative to the concentration of ADP added (▩). Almost no Pi was liberated from ADP preincubated on blank wells (open columns). The ADP-containing buffer preincubated on HUVECs (●) lost its agonistic activity for platelets in comparison with the ADP-containing buffer preincubated on blank wells (○). The ADP-treated incubation buffer was added to PRP in a 10-fold dilution. The results are the mean ± SD of 3 separate experiments.
Inhibition of ADP-induced platelet aggregation by AMP preincubated on HUVECs
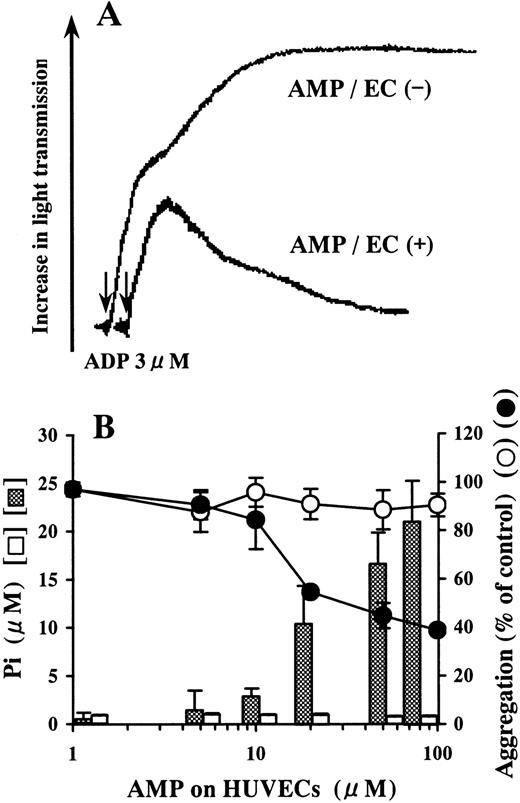
AMP was not hydrolyzed on blank wells (Figure 2B). Pi was liberated by the incubation of AMP on HUVECs in a dose-dependent manner relative to the concentration of AMP added (Figure2B). The AMP-containing buffer preincubated on HUVECs inhibited ADP-induced platelet aggregation compared with that preincubated on blank wells (Figure 2).
Inhibition of ADP-induced platelet aggregation by AMP degraded on HUVECs.
HUVEC-containing wells and blank wells were incubated with a phosphate-free buffer containing 1-100 μmol/L AMP at 37°C for 15 minutes. (A) We added 50 μmol/L AMP buffer preincubated on HUVECs to PRP in 10-fold dilution just before activation by ADP (final concentration, 3 μmol/L). Platelet aggregation was inhibited compared with the AMP buffer preincubated on blank wells. The arrows indicate the addition of ADP. The result is representative of 3 experiments.(B) Although AMP was not hydrolyzed on blank wells (■), Pi was liberated from AMP on HUVECs in a dose-dependent manner relative to the concentration of AMP added (▩). AMP buffer preincubated on HUVECs inhibited ADP-induced platelet aggregation (●), whereas AMP buffer preincubated on blank wells did not affect aggregation significantly (○). The results are the mean ± SD of 3 separate experiments.
Inhibition of ADP-induced platelet aggregation by AMP degraded on HUVECs.
HUVEC-containing wells and blank wells were incubated with a phosphate-free buffer containing 1-100 μmol/L AMP at 37°C for 15 minutes. (A) We added 50 μmol/L AMP buffer preincubated on HUVECs to PRP in 10-fold dilution just before activation by ADP (final concentration, 3 μmol/L). Platelet aggregation was inhibited compared with the AMP buffer preincubated on blank wells. The arrows indicate the addition of ADP. The result is representative of 3 experiments.(B) Although AMP was not hydrolyzed on blank wells (■), Pi was liberated from AMP on HUVECs in a dose-dependent manner relative to the concentration of AMP added (▩). AMP buffer preincubated on HUVECs inhibited ADP-induced platelet aggregation (●), whereas AMP buffer preincubated on blank wells did not affect aggregation significantly (○). The results are the mean ± SD of 3 separate experiments.
Involvement of NO and PGI2 in the inhibition of ADP-induced platelet aggregation by AMP preincubated on HUVECs
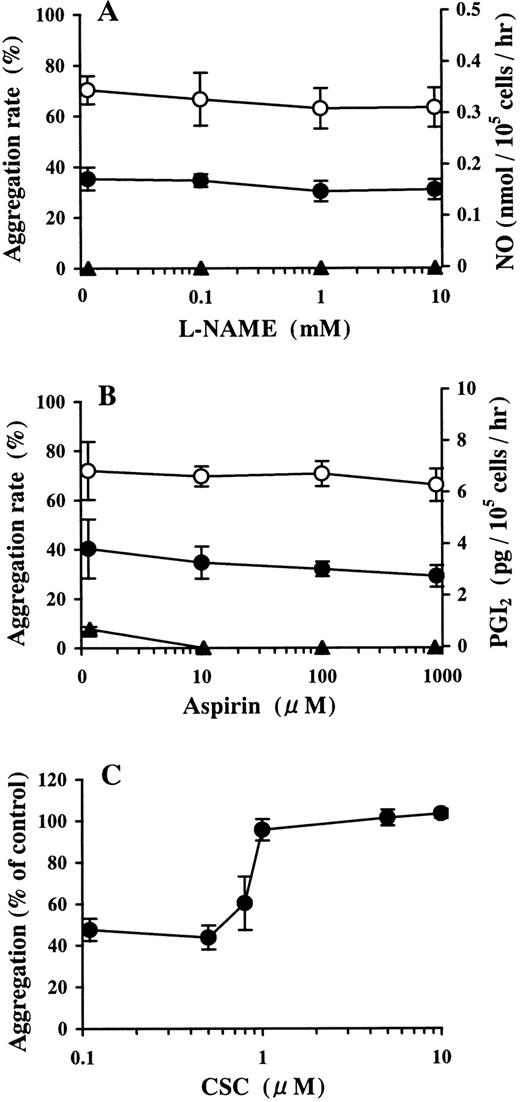
The 30 μmol/L AMP buffer preincubated on HUVECs did not contain detectable amounts of nitric oxide. Pretreatment with more than 10 μmol/L L-NAME, which completely inhibited NO production in the growth medium of HUVECs, did not affect the inhibition of ADP-induced platelet aggregation by the AMP buffer preincubated on HUVECs (Figure3A). This buffer contained 0.76 ± 0.09 pg/105 cells per hour (the mean ± SD) of PGI2, which is at the lowest limit of detection of the assay used. Pretreatment with more than 10 μmol/L aspirin completely blocked PGI2 production in the growth medium of HUVECs. Under these conditions, inhibition of ADP-induced platelet aggregation by AMP buffer preincubated on HUVECs was not affected (Figure 3B).
Involvement of the NO, PGI2, and Ado receptor in the inhibition of ADP-induced platelet aggregation by AMP preincubated on HUVECs.
HUVEC-containing wells and blank wells were treated with (A) 0.1-10 mmol/L L-NAME or (B) 0.01-1 mmol/L aspirin. After washing, the wells were incubated with 30 μmol/L AMP at 37°C for 15 minutes. The inhibitory activity of the AMP buffer preincubated on HUVECs against ADP-induced platelet aggregation was examined. ● and ○ denote the AMP buffer preincubated on HUVECs and blank wells, respectively. The concentrations of NO (panel A, ▴) and PGI2 (panel B, ▴) in the buffer incubated on HUVECs are also shown. The results are the mean ± SD of 3 separate experiments. (C) HUVEC-containing wells and blank wells were treated with 30 μmol/L AMP at 37°C for 15 minutes. CSC, a selective A2a receptor antagonist, was added with the AMP-containing buffer preincubated on HUVECs to PRP in 10-fold dilution immediately before activation by ADP (final concentration, 2 μmol/L). The results are the mean ± SD of 3 separate experiments.
Involvement of the NO, PGI2, and Ado receptor in the inhibition of ADP-induced platelet aggregation by AMP preincubated on HUVECs.
HUVEC-containing wells and blank wells were treated with (A) 0.1-10 mmol/L L-NAME or (B) 0.01-1 mmol/L aspirin. After washing, the wells were incubated with 30 μmol/L AMP at 37°C for 15 minutes. The inhibitory activity of the AMP buffer preincubated on HUVECs against ADP-induced platelet aggregation was examined. ● and ○ denote the AMP buffer preincubated on HUVECs and blank wells, respectively. The concentrations of NO (panel A, ▴) and PGI2 (panel B, ▴) in the buffer incubated on HUVECs are also shown. The results are the mean ± SD of 3 separate experiments. (C) HUVEC-containing wells and blank wells were treated with 30 μmol/L AMP at 37°C for 15 minutes. CSC, a selective A2a receptor antagonist, was added with the AMP-containing buffer preincubated on HUVECs to PRP in 10-fold dilution immediately before activation by ADP (final concentration, 2 μmol/L). The results are the mean ± SD of 3 separate experiments.
Involvement of Ado receptor in the inhibition of ADP-induced platelet aggregation by AMP preincubated on HUVECs
In the presence of CSC, inhibition of ADP-induced platelet aggregation by purified Ado was abrogated in a dose-dependent manner (data not shown). The inhibition of ADP-induced platelet aggregation by 30 μmol/L AMP buffer preincubated on HUVECs was blocked by more than 1 μmol/L CSC (Figure 3C).
Involvement of ecto-5′-NT in the inhibition of ADP-induced platelet aggregation by AMP preincubated on HUVECs
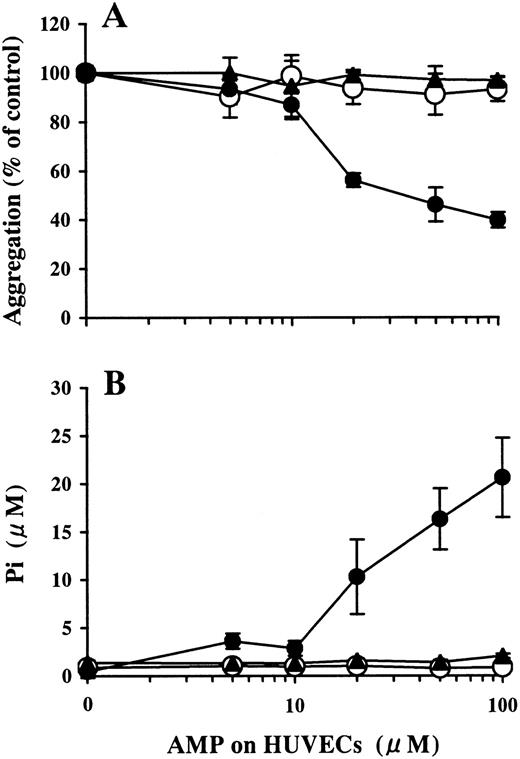
Levamisole (10 mmol/L) did not affect AMP degradation on HUVECs (data not shown). APCP inhibited AMPase activity on HUVECs in a dose-dependent manner, as measured by the mean ± SD: Pi release without APCP, 21.0 ± 1.9 μmol/L; Pi release with 1 μmol/L APCP, 11.6 ± 2.2 μmol/L; Pi release with 10 μmol/L APCP, 5.6 ± 1.3 μmol/L. Pi was not liberated with 100 μmol/L APCP (Figure 4B). Inhibition of platelet aggregation by the AMP buffer preincubated on HUVECs was also attenuated by APCP in a dose-dependent manner (data not shown) and was completely blocked by 100 μmol/L APCP (Figure 4A).
Involvement of ecto-5′-NT in the inhibition of ADP-induced platelet aggregation by AMP preincubated on HUVECs.
HUVEC-containing wells and blank wells were pretreated with 100 μmol/L APCP, an inhibitor of ecto-5′-NT, then incubated with a phosphate-free buffer containing 1-100 μmol/L AMP at 37°C for 15 minutes. (A) The inhibitory activity of the AMP buffer preincubated on HUVECs for ADP-induced platelet aggregation (●) was completely blocked by the treatment of HUVECs with APCP (▴) to the levels found for the blank wells (○). The results are the mean ± SD of 3 separate experiments. (B) Pi was liberated on HUVECs in a dose-dependent manner relative to the concentration of the AMP added (●). The treatment of the HUVECs with APCP completely inhibited Pi liberation from AMP (▴), comparable to the results from the blank wells (○). The results are the mean ± SD of 3 separate experiments.
Involvement of ecto-5′-NT in the inhibition of ADP-induced platelet aggregation by AMP preincubated on HUVECs.
HUVEC-containing wells and blank wells were pretreated with 100 μmol/L APCP, an inhibitor of ecto-5′-NT, then incubated with a phosphate-free buffer containing 1-100 μmol/L AMP at 37°C for 15 minutes. (A) The inhibitory activity of the AMP buffer preincubated on HUVECs for ADP-induced platelet aggregation (●) was completely blocked by the treatment of HUVECs with APCP (▴) to the levels found for the blank wells (○). The results are the mean ± SD of 3 separate experiments. (B) Pi was liberated on HUVECs in a dose-dependent manner relative to the concentration of the AMP added (●). The treatment of the HUVECs with APCP completely inhibited Pi liberation from AMP (▴), comparable to the results from the blank wells (○). The results are the mean ± SD of 3 separate experiments.
Involvement of ecto-5′-NT in the inhibition of platelet aggregation by ADP preincubated on HUVECs
Pretreatment of HUVECs with APCP partially inhibited Pi liberation from ADP preincubated on HUVECs (Figure5B). Platelet aggregation induced by the ADP buffer preincubated on HUVECs was restored by the pretreatment of HUVECs with APCP (Figure 5A).
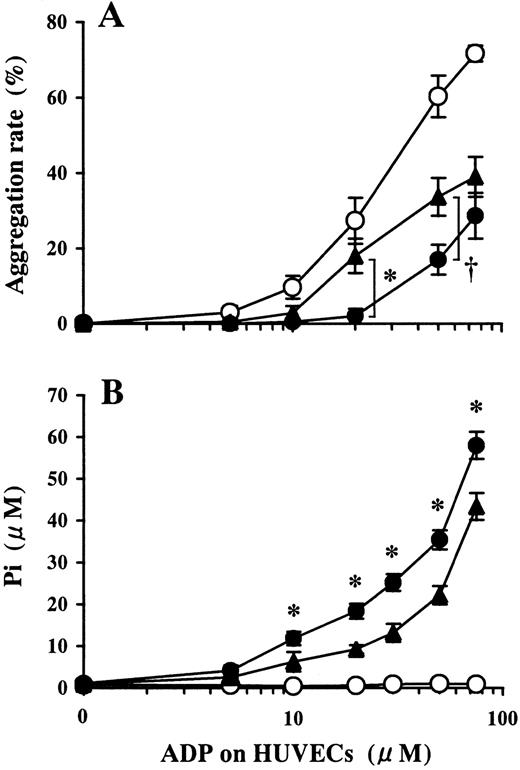
Involvement of ecto-5′-NT in the inhibition of platelet aggregation induced by ADP preincubated on HUVECs.
HUVEC-containing wells and blank wells were pretreated with 100 μmol/L APCP, an inhibitor of ecto-5′-NT, then incubated with a phosphate-free buffer containing 1-75 μmol/L ADP at 37°C for 15 minutes. (A) Platelet aggregation induced by ADP buffer preincubated on HUVECs pretreated with APCP (▴) induced platelet aggregation more than that on APCP-untreated HUVECs (●). ○ denotes the data of ADP buffer preincubated on blank wells. The results are the mean ± SD of 3 separate experiments. The asterisk indicates the meanP < .01, and the dagger indicates the meanP < .02. (B) Pi was liberated on HUVECs in a dose-dependent manner relative to the concentration of ADP added (●). Pretreatment of HUVECs with APCP partially inhibited Pi liberation from ADP (▴) (P < .01, at 10-100 μmol/L ADP). ○ indicates data from the blank wells. The results are the mean ± SD of 3 separate experiments.
Involvement of ecto-5′-NT in the inhibition of platelet aggregation induced by ADP preincubated on HUVECs.
HUVEC-containing wells and blank wells were pretreated with 100 μmol/L APCP, an inhibitor of ecto-5′-NT, then incubated with a phosphate-free buffer containing 1-75 μmol/L ADP at 37°C for 15 minutes. (A) Platelet aggregation induced by ADP buffer preincubated on HUVECs pretreated with APCP (▴) induced platelet aggregation more than that on APCP-untreated HUVECs (●). ○ denotes the data of ADP buffer preincubated on blank wells. The results are the mean ± SD of 3 separate experiments. The asterisk indicates the meanP < .01, and the dagger indicates the meanP < .02. (B) Pi was liberated on HUVECs in a dose-dependent manner relative to the concentration of ADP added (●). Pretreatment of HUVECs with APCP partially inhibited Pi liberation from ADP (▴) (P < .01, at 10-100 μmol/L ADP). ○ indicates data from the blank wells. The results are the mean ± SD of 3 separate experiments.
Discussion
The ability of HUVECs to inhibit platelet aggregation by ADP degradation was confirmed6 (Figure 1). The ADP- or AMP-treated buffers preincubated on HUVECs could contain not only adenine nucleotides but also NO and PGI2 produced in the incubation periods. Because both NO and PGI2 are major substances released from ECs that inhibit platelet aggregation, it was possible that NO and/or PGI2 were involved in the mechanisms that inhibited platelet aggregation under our experimental conditions. HUVECs released NO in the growth medium at 0.14 ± 0.02 (mean ± SD) nmol/105 cells per hour, at the lowest limit of detection of the assay. However, we removed the medium followed by 3 washes of the wells prior to incubation with ADP- or AMP-containing buffer for 15 minutes. These incubation buffers did not contain any detectable amounts of nitric oxide. Pretreatment of HUVECs with more than 10 μmol/L L-NAME, which was enough to block NO production in the growth medium, did not change the inhibitory effect on platelet aggregation of ADP buffer preincubated on HUVECs (data not shown). This suggested that NO produced in the buffer during the incubation phase did not affect platelet aggregation under our experimental conditions.
Basal levels of PGI2 in the growth medium of HUVECs were 1.34 ± 0.25 pg/105 cells per hour, and the incubation buffer contained 0.51 ± 0.07 pg/105 cells per hour, the limit of detection of the assay. Aspirin completely inhibited PGI2 production during the incubation of buffer at 0.01-1 mmol/L aspirin (data not shown). Because pretreatment of HUVECs with 1 mmol/L aspirin did not influence the inhibition of platelet aggregation by the ADP buffer preincubated on HUVECs (data not shown), it is likely that PGI2 in the incubation buffer did not affect the inhibitory effect on aggregation in our experiments.
On the surface of HUVECs, ATPDase, ecto-5′-NT, and ALP can hydrolyze adenine nucleotides.11,14,37,41 However, pretreatment of HUVECs with 0.1-10 mmol/L levamisole, a specific inhibitor of ALP,36,37 did not affect Pi liberation from ADP in our experiments. This may be due to our experimental condition being at a lower pH than the optimal pH for ALP. Therefore, the role of HUVECs in the inhibition of platelet aggregation shown in Figure 1could be explained as ADP degradation by nucleotidases on HUVECs. Undoubtedly, hydrolysis of ADP by ATPDase plays a pivotal role in the inhibition of platelet aggregation (Figure 1). However, we hypothesized that ecto-5′-NT was involved in this cooperative function with ATPDase because AMP generated by ATPDase is subsequently hydrolyzed to Ado by the action of ecto-5′-NT. In a preliminary experiment, we found that purified Ado, but not AMP, inhibited ADP-induced platelet aggregation in a dose-dependent manner in vitro and that this effect was completely blocked by CSC, a selective antagonist for the A2a receptor (data not shown). Previous studies showed that Ado inhibits platelet aggregation by binding to the A2a receptor on the platelets, thereby activating adenylate cyclase and elevating the cAMP level.23
We first examined enzymatic activity of ecto-5′-NT on HUVECs. Although AMP was not hydrolyzed on blank wells, Pi was liberated from AMP in a dose-dependent manner relative to the concentration of AMP added to HUVECs (Figure 2B). This AMP buffer preincubated on HUVECs inhibited ADP-induced platelet aggregation in proportion to the amounts of Pi liberated (Figure 2B). The incubation buffer could contain the remaining AMP, Ado generated, and several substances, including NO and PGI2, which were produced in the incubation phase. Our system did not contain strong agonists for either PGI2 or NO production. Any quantities that were produced were at baseline levels (Figure 3).
The ineffectiveness of L-NAME on the function of AMP buffer preincubated on HUVECs to inhibit ADP-induced aggregation (Figure3A) indicated that NO was not a major contributor under our experimental conditions. This might be due to the conditions of HUVECs in the confluent layer because previous studies revealed that NO synthase expression and NO release declined in the confluent phase.42 As for PGI2, AMP buffer preincubated on HUVECs contained little PGI2, and pretreatment of HUVECs with aspirin did not affect the inhibitory effects of the incubation buffer (Figure 3B). This suggests that PGI2 produced during the incubation phase did not influence ADP-induced platelet aggregation in our experiments. The IC50 value of PGI2 for ADP-induced platelet aggregation is about 100-fold higher than the concentration of the preincubated buffer.43 The production of PGI2by ECs may decrease in the subculture and confluent states in our experiments, as previously shown in several studies.44 45
Because we hypothesized that the hydrolysis of AMP by ecto-5′-NT was the main mechanism by which AMP buffer preincubated on HUVECs inhibited ADP-induced platelet aggregation (Figure 2), we expected that the Ado receptor antagonists would block this effect. As expected, CSC, a selective A2a receptor antagonist,40 abrogated this effect in a dose-dependent manner (Figure 3C). Therefore, it is likely that the inhibitory effect of AMP buffer preincubated on HUVECs is derived via an interaction of Ado with the Ado receptor on platelets.
The remaining question was whether some portions of Ado produced on the luminal surface may be taken up by HUVECs or deaminated by ADA before exerting any biological effects. Pretreatment of HUVECs with 1-100 μmol/L DIP, an inhibitor of Ado transport,39 did not affect AMP degradation or the inhibitory effect of the buffer on platelet aggregation (data not shown). This indicated that transport of Ado across the membranes had a limited effect on the extracellular levels of Ado, at least in our experimental conditions. Taken together with the report by Aalto et al,46 which showed that the effect of deamination of Ado by ADA on cultured HUVECs is limited, it is likely that in our experiments, the extracellular levels of Ado are regulated mainly by the generation of Ado from adenine nucleotides on HUVECs. Because treatment with levamisole, an inhibitor of ALP, did not interfere with the AMPase activity on HUVECs, we estimated that ecto-5′-NT was mainly involved in the hydrolysis of AMP. APCP, an ADP analogue modified on the phosphate chain by substituting a bridging oxygen with a methylene, is a specific inhibitor of 5′-NT (Ki = 6 nmol/L).14,22 It was demonstrated that APCP is neither affected by the hydrolysis of ADP by ATPDase nor is it hydrolyzed by ATPDase.47 APCP displayed almost no affinity for the P2X receptor,48 which was also supported by our findings that ADP-induced platelet aggregation was not influenced by the addition of APCP (data not shown).
As shown in Figure 5, pretreatment of HUVECs with APCP resulted in a partial decrease in Pi liberation from HUVECs that were treated with ADP. In addition, platelet aggregation was partially restored at comparatively low concentrations of ADP. This might be explained by the fact that while a low concentration of ADP could be completely hydrolyzed by ATPDase on HUVECs, excessive ADP, which was undigested by ATPDase, inhibited the activity of ecto-5′-NT. Therefore, it seems reasonable to suppose that ecto-5′-NT is involved in the HUVEC function of inhibiting platelet aggregation in cooperation with ATPDase, especially at comparatively low concentrations of ADP. Inhibition of remnant ADP, which is not hydrolyzed by ATPDase, by Ado generated by the activity of ecto-5′-NT should lead to an increase in the threshold for platelet aggregation.
In conclusion, we identified a contribution of ecto-5′-NT to the inhibitory effect of HUVECs on platelet agregation in cooperation with ATPDase at a comparatively low concentration of ADP. Ecto-5′-NT seems to be effective for prevention of thrombosis formation by increasing the threshold for platelet aggregation, although its role in vivo remains to be clarified. Results from these studies verify the concept that platelets in motion and in close proximity to endothelial cells do not respond to standard platelet agonists. This is probably due to the combined action of ATPDase and ecto-5′-NT described in our studies.
Acknowledgments
We thank Dr T. Kubo and all the doctors in the Division of Gynecology and Obstetrics, Institute of Clinical Medicine, University of Tsukuba, Tsukuba, Ibaraki, Japan, for their generous provision of human umbilical cord.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Haruhiko Ninomiya, College of Medical Technology, University of Tsukuba, Tennodai 1-1-1, Tsukuba, Ibaraki 305-8577, Japan; e-mail: hninomiya@itan.tsukuba.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal