Abstract
Activation of T cells can be initiated through cell surface molecules in addition to the T-cell receptor-CD3 (TCR-CD3) complex. In human T cells, ligation of the CD2 molecule by mitogenic pairs of anti-CD2 monoclonal antibodies activates T cells via biochemical signaling pathways similar but not identical to those elicited on TCR engagement. This study describes a key role for the p36/38 membrane adapter protein linker for T cell activation (LAT) in CD2-mediated T-cell activation. Following ligation of CD2 on the surface of the Jurkat T-cell line and human purified T cells, LAT was tyrosine phosphorylated and shown to associate in vivo with a number of other tyrosine phosphorylated proteins including PLCγ-1, Grb-2, and SLP-76. Using Jurkat cell lines deficient in ZAP70/Syk (P116) or LAT (ANJ3) expression, CD2-dependent PLCγ-1 and SLP-76 tyrosine phosphorylation required expression both of ZAP70 or Syk and of LAT. As predicted, the absence of either LAT or ZAP70/Syk kinases correlated with a defect in the induction of nuclear factor of activated T cells (NFAT) transcriptional activity, activation of the interleukin-2 promoter, and ERK phosphorylation following CD2 stimulation. These data suggest that LAT is an adapter protein important for the regulation of CD2-mediated T-cell activation.
Introduction
Engagement of the T-cell antigen receptor (TCR) by specific antigen–major histocompatibility complexes (MHC) or anti-TCR monoclonal antibody (mAb) triggers an intracellular cascade of biochemical events that culminate in T-cell activation.1-3Many of these events are now well characterized, although incompletely understood. One of the earliest biochemical responses elicited by the TCR is the activation of protein tyrosine kinases (PTK) of the Src family, Lck and/or Fyn, that mediate the phosphorylation of the immunoglobulin receptor family tyrosine-based activation motifs (ITAMs) on the intracellular tails of the CD3 and ζ molecules of the TCR complex.4 A consequence of ITAM phosphorylation is the recruitment, tyrosine phosphorylation, and activation of the Syk family members ZAP70 and Syk kinases. Following activation of Src and Syk family kinases, a multicomponent signaling complex is assembled that is composed both of active signaling molecules (tyrosine and serine/threonine kinases and phosphatases)5-8 as well as adapter molecules that exhibit no intrinsic enzymatic activity but rather serve to recruit additional proteins to the complex.9 A number of substrates are recruited to, or activated downstream of, this signaling complex, including enzymes such as phospholipase Cγ-1 (PLCγ-1) and Vav.
Linker for T-cell activation (LAT) is a recently identified 36- to 38-kd membrane adapter protein expressed in T cells, natural killer (NK) cells, mast cells, and megakaryocytes, but not in B cells or monocytes.10,11 Following ligation of the TCR-CD3 complex, ZAP70 or Syk kinase mediates the tyrosine phosphorylation of LAT that, in turn, is required for Ras activation as for the induction of nuclear factor of activated T cells (NFAT) and AP1 transcriptional activation.10,12,13 LAT tyrosylphosphorylation results in the recruitment and SH2-dependent binding of the adapter molecule Grb2 and thus binding of mSos (an upstream activator of Ras), Cbl, and a complex of proteins including SLP-76 and Vav (an upstream activator of Rac). Moreover, in vivo, tyrosine phosphorylation of LAT results in its association with PLCγ-1, and subsequent PLCγ-1 tyrosine phosphorylation and activation.10 Studies have also shown that LAT is palmitoylated and localized to the glycolipid-enriched microdomains (GEMs) of plasma membranes14 and that the localization of LAT in the GEMs is required for TCR-mediated T-cell activation.13 15 Thus, ZAP70/Syk kinase-dependent tyrosine phosphorylation of LAT appears to couple TCR engagement, via Grb2, mSos, PLCγ-1, and other signaling molecules, to downstream signaling events such as the increase in intracellular calcium, Ras activation, and the induction of AP1 and NFAT transcriptional activity.
Our present studies were undertaken to determine whether LAT plays a role in T-cell activation induced through ligation of the CD2 molecule. CD2 is a 50- to 55-kd glycoprotein expressed on a majority of thymocytes, T cells, and NK cells. Through physiologic interactions with its ligands CD58 (lymphocyte function-associated antigen-3 [LFA-3]), CD48, and CD59, the CD2 molecule plays a role in T-cell signaling and promotes lymphocyte adhesion. Specific pairs of CD2-specific mAbs were demonstrated to be able to induce interleukin (IL)-2 production and T-cell proliferation in the absence of engagement of the TCR-CD3 complex.16 17 In our studies, biochemical and functional data support a critical role for LAT in CD2-mediated T-cell activation. In particular, we show, on CD2 stimulation of human purified T cells and of Jurkat T cells, the time- and activation-dependent tyrosine phosphorylation of LAT and its association in vivo with several phosphotyrosine-containing proteins, including PLCγ-1, Grb-2, and SLP-76. The CD2-dependent tyrosine phosphorylation of PLCγ-1 and SLP-76 required LAT tyrosine phosphorylation that, in turn, required Syk/ZAP70 kinase family expression. Furthermore, we demonstrate that LAT is required for Erk1/2 activation, induction of NFAT transcriptional activity, and IL-2 promoter activation on CD2 stimulation.
Materials and methods
Cells and reagents
Jurkat cell lines were cultured in RPMI 1640 (MediaTech, Herndon, VA) supplemented with 10% heat-inactivated fetal calf serum (FCS) (GibcoBRL, Life Technologies, Gaithersburg, MD), 2 mmol/Ll-glutamine, 10 mmol/L Hepes, 100 U/mL penicillin and 100 μg/mL streptomycin (MediaTech), and 50 μmol/L 2-mercaptoethanol (Biorad, Hercules, CA), termed complete RPMI medium. The Jurkat T-cell leukemia cell line (clone J77) was the generous gift of Kendall Smith (Cornell University, New York, NY). The ZAP70/Syk-deficient clone, P116, and the ZAP70-reconstituted clone, P116-ZAP70, were the gift of Robert Abraham (Duke University, Durham, NC). The JCaM1.6 cells reconstituted with wild-type Lck (JCaM1.6-Lck), a generous gift of David B. Straus (University of Chicago, Chicago, IL), were grown in complete RPMI medium containing 1 mg/mL geneticin (GibcoBRL) and 250 μg/mL hygromycin B (Calbiochem, La Jolla, CA). In the JCaM1.6 transfectant, the Lck kinase was expressed under the control of a tetracycline-repressible TetR-VP16 fusion protein.18 The LAT-deficient cells, ANJ3, and the LAT-reconstituted clone, ANJ3-LATwt, were described previously.13
For all the cell lines, surface expression of both CD3 and CD2 was routinely evaluated by direct immunofluorescence; cells were incubated on ice for 20 minutes with phycoerythrin (PE)-conjugated anti-CD3 mAb, clone UCHT1 (Beckman Coulter Inc, Fullerton, CA), and fluorescein isothiocyanate (FITC)-conjugated anti-CD2 mAb, clone RPA-2.10 (BD PharMingen, San Diego, CA) or appropriate PE- and/or FITC-conjugated IgG isotype control (Beckman Coulter). Cells then were washed and analyzed using a Coulter Epics flow cytometer (Beckman Coulter). CD3 and CD2 expressions were equivalent between wild-type Jurkat, P116, P116-ZAP70, and JCaM1.6-Lck and comparable between ANJ3 and ANJ3-LATwt (not shown). CD3 and CD2 cell surface expression remained constant with time.
Resting human peripheral blood lymphocytes (PBLs) were isolated from adult healthy donors by density-gradient centrifugation on Ficoll-Paque PLUS (Amersham Pharmacia Biotech, Uppsala, Sweden) and plastic adherence, resuspended in complete RPMI medium, and used within 24 hours of isolation.
The anti-CD2 mAbs T112 and T113 were the kind gift of Ellis Reinherz (Dana-Farber Cancer Institute, Boston, MA); the murine antihuman CD3ε mAb OKT3 (American Type Culture Collection, Rockville, MD) was used as purified ascites fluid; the antiphosphotyrosine mAb 4G10 was a kind gift of Thomas Roberts (Dana-Farber Cancer Institute); the SLP-76 antiserum was a kind gift of Gary Koretzky (University of Pennsylvania, Philadelphia, PA). The rabbit polyclonal anti-LAT antiserum was previously described.10 The anti-PLCγ-1 mAb, the anti-Vav mAb, and the rabbit polyclonal anti-ERK1/2 and anti-Lck antibodies were purchased from Upstate Biotechnology, Inc (Lake Placid, NY); the rabbit polyclonal anti-Cbl antiserum, antiphosphotyrosine (PY99) mAb directly conjugated to agarose beads, and unconjugated protein A–agarose beads were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA); the anti-Grb-2 and anti-ZAP70 mAbs were purchased from Transduction Laboratories (San Diego, CA); and the mAbs specific for the dual-phosphorylated (Thr202 and Tyr204) activated p44 and p42 MAP kinase (Erk1 and Erk2) were obtained from New England Biolabs, Inc (Beverly, MA). Phorbol myristate acetate (PMA) and ionomycin were purchased from Calbiochem. The selective Src family kinase inhibitor PP1 was purchased from BIOMOL (Plymouth Meeting, PA).
Cell stimulation and immunoprecipitation
For stimulation, Jurkat cells (1 × 107) or PBLs (2-3 × 107) were washed in RPMI 1640 (without additives); resuspended in 0.5 mL of ice-cold RPMI 1640; incubated with RPMI 1640 medium alone (termed unstimulated), 1 μg of OKT3, or 1 μL of T112 plus 1 μL of T113 purified ascites on ice for 15 minutes; and then transferred to 37°C for the indicated time periods. For experiments involving the Scr kinase inhibitor PP1, cells were preincubated with either vehicle (DMSO) or PP1 20 μmol/L at 37°C for 30 minutes prior to stimulation. The 0 time point was defined as the time of addition of the stimulating antibody. The cells were then washed with cold RPMI 1640 and lysed by addition of 1 mL of ice-cold lysis buffer (1% Brij97, 150 mmol/L NaCl, 25 mmol/L Tris [pH 7.5], 1 mmol/L EDTA, 1 mmol/L Na3VO4, 10 μg/mL leupeptin, 10 μg/mL aprotinin, and 1 mmol/L phenylmethylsulfonyl fluoride) on ice for 20 minutes. Cell lysates were clarified by centrifugation at 14,000 × g for 10 minutes at 4°C. When indicated, 30 μL of postnuclear lysate (PNL) from each sample was set aside before immunoprecipitation.
For LAT immunoprecipitations, 2 μL of anti-LAT polyclonal Ab and 20 μL of protein A–agarose beads were added together to the clarified cell lysates and incubated at 4°C for 2 hours. The lysates were not precleared by preincubation with beads alone. Therefore clarified cell lysates from the same conditions of stimulation incubated with 20 μL protein A–agarose beads alone served as the control for carryover of stimulating mAbs, as indicated. For immunoprecipitation of phosphotyrosine-containing proteins, 20 μL of anti-pTyr (PY99) directly conjugated to agarose beads were used. The beads were then washed 3 times with washing buffer (0.1% Brij97, 150 mmol/L NaCl, 25 mmol/L Tris [pH 7.5], 1 mmol/L Na3VO4). Proteins were boiled in sodium dodecyl sulfate (SDS) sample buffer, separated by SDS–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene difluoride (PVDF; Millipore, Bedford, MA), and probed with primary antibodies followed by horseradish peroxidase-conjugated secondary antibodies. Polypeptides recognized in the Western blot were detected using the enhanced chemiluminescence (ECL) methods according to manufacturer's instructions (Amersham Pharmacia Biotech Inc, Piscataway, NJ). Quantification of band density was determined by densitometry using the Imagequant software (Molecular Dynamics, Sunnyvale, CA) when indicated.
Transient transfection and luciferase assay
Jurkat cells (2 × 107) were removed from culture, washed, resuspended in 0.5 mL RPMI 1640 to which 10% FCS had been added (termed 10% FCS-RPMI) and incubated with 10 μg of a reporter plasmid p3xNFAT-luc19 carrying the luciferasegene driven by 3 tandem repeats of the distal NFAT sequences derived from the IL-2 promoter and 1 μg of pRL-TK vector (Promega, Madison, WI), which provides constitutive expression of Renillaluciferase, for 15 minutes at room temperature. Cells were then electroporated at 250 V, 800 microfarads, low Ω (Life Technologies, Inc); incubated for 15 minutes at room temperature; and then transferred to complete RPMI medium and incubated at 37°C. Twenty-four hours after the transfection, 1 × 106cells/test were left unstimulated or stimulated with plate-bound OKT3, 1 μL of T112 plus 1 μL of T113, each either alone or in combination with PMA (10 ng/mL) or ionomycin (1.5 μmol/L), or PMA plus ionomycin for 6 hours. Cells were then washed with PBS, and samples were prepared using the Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer's instructions. Results are reported as the activity of firefly luciferase normalized to that of Renilla luciferase (to correct for the efficiency of transfection) in the lysates, and expressed as the percentage of maximal response obtained with PMA plus ionomycin.
Reverse transcriptase–polymerase chain reaction
To examine the induction of IL-2 transcription, Jurkat cells (1 × 106/test) were either left unstimulated or stimulated with plate-bound OKT3, 1 μL of T112 plus 1 μL of T113, each either alone or in combination with PMA (10 ng/mL), or PMA plus ionomycin for 4 hours. Total RNA was then extracted from cells with TRIAZOL (GibcoBRL, Life Technologies) according to the manufacturer's instructions. Reverse transcriptase–polymerase chain reaction (RT-PCR) was performed using the human IL-2–specific primers 5′-CATTGCACTAAGTCTTGCACTTGTCA-3′ and 5′-CGTTGATATTGCTGATTAAGTCCCTG-3′ using the QUIAGEN OneStep RT-PCR kit (QUIAGEN Inc, Valencia, CA) according to the manufacturer's instructions.
Results
CD2 stimulation induced LAT tyrosine phosphorylation and association with PLCγ-1 and other tyrosine phosphorylated proteins
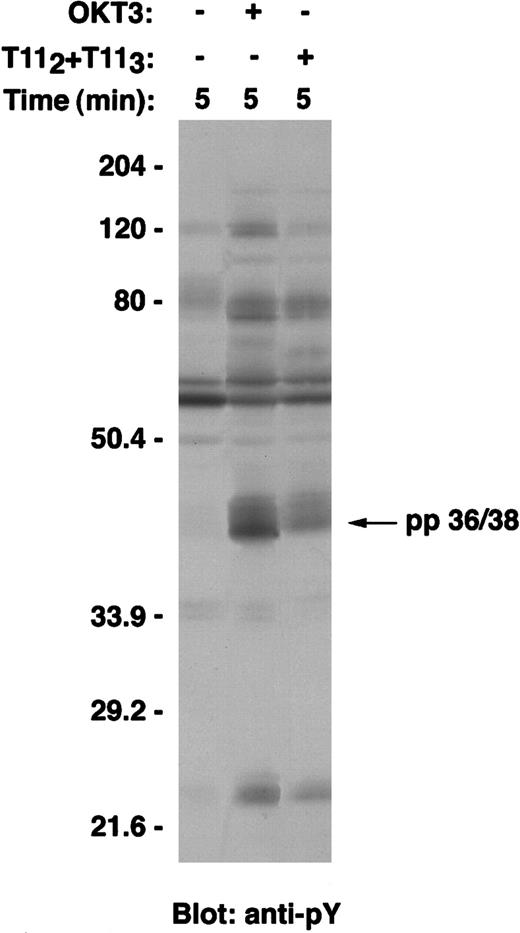
Previously, LAT was shown to be a critical adapter molecule for T-cell development20 and T-cell activation via the TCR-CD3 complex.10,12,13 Its adapter function depends on its ability to become tyrosine phosphorylated and to associate, either directly or indirectly, with a number of signaling molecules. To determine whether LAT is tyrosine phosphorylated on CD2 engagement, wild-type Jurkat T cells were left untreated; stimulated with a mitogenic pair of anti-CD2 mAbs T112 and T113; or, for comparison, stimulated with anti-CD3 mAb (Figure1). Clarified postnuclear lysates were separated by SDS-PAGE, transferred to PVDF membranes, and subjected to Western blot analysis using an antiphosphotyrosine-specific mAb. The pattern of tyrosine phosphorylated proteins on CD2 stimulation is similar, but not identical, to that following TCR-CD3 stimulation, as has been previously observed21-25; specifically, a prominent 36/38-kd protein is observed (Figure 1). To determine whether LAT was the pp36/38 phosphoprotein, lysates of stimulated cells were immunoprecipitated with rabbit anti-LAT antibody (Figure2). Like TCR-CD3, CD2 stimulation induced the rapid and time-dependent tyrosine phosphorylation of LAT, reaching maximal phosphorylation at about 5 minutes (Figure 2A, upper panel). Similar amounts of LAT protein were immunoprecipitated in each lane, as confirmed by stripping and reprobing the membrane with anti-LAT–specific antibody (Figure 2B, upper panel). Of note, the tyrosine phosphorylated form of LAT protein is less efficiently recognized by the specific antibody, as has been previously observed.10
A 36- to 38-kd protein is tyrosine phosphorylated on either CD2 or CD3 stimulation of Jurkat T cells.
Wild-type Jurkat T cells were either left unstimulated, stimulated with the mitogenic pair of anti-CD2 mAbs T112 and T113, or stimulated with anti-CD3ε mAb OKT3 at 37°C for 5 minutes. Cells were then lysed in 1% Brij97 lysis buffer, and clarified PNLs (0.3 × 106 cell equivalents) were resolved on SDS-PAGE, transferred to PVDF membranes, and immunoblotted with the antiphosphotyrosine antibody 4G10. Tyrosine phosphorylation of a 36- to 38-kd protein is shown at the arrow.
A 36- to 38-kd protein is tyrosine phosphorylated on either CD2 or CD3 stimulation of Jurkat T cells.
Wild-type Jurkat T cells were either left unstimulated, stimulated with the mitogenic pair of anti-CD2 mAbs T112 and T113, or stimulated with anti-CD3ε mAb OKT3 at 37°C for 5 minutes. Cells were then lysed in 1% Brij97 lysis buffer, and clarified PNLs (0.3 × 106 cell equivalents) were resolved on SDS-PAGE, transferred to PVDF membranes, and immunoblotted with the antiphosphotyrosine antibody 4G10. Tyrosine phosphorylation of a 36- to 38-kd protein is shown at the arrow.
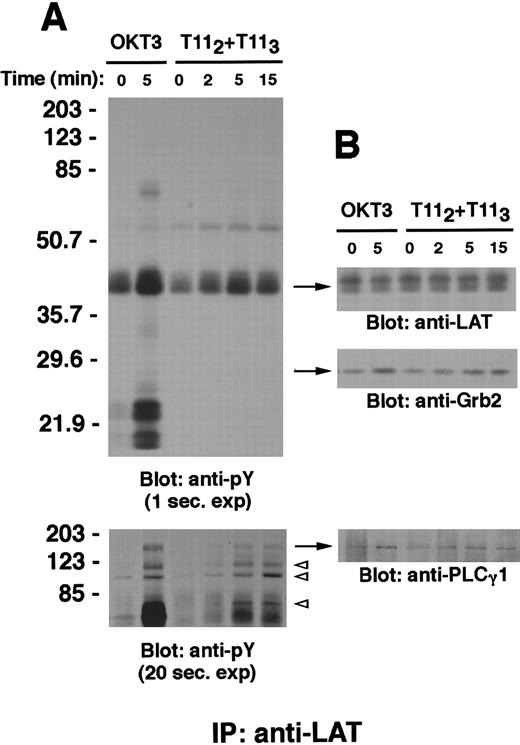
LAT is tyrosine phosphorylated and associates with PLCγ-1, Grb-2, and other tyrosine phosphorylated proteins on CD2 stimulation.
Wild-type Jurkat T cells (107 cells) were stimulated with either the mitogenic pair of anti-CD2 mAbs T112 and T113 or anti-CD3ε mAb OKT3 at 37°C for the indicated periods of time. PNLs were prepared as described in “Materials and methods,” either subjected to immunoprecipitation with the rabbit polyclonal antibody anti-LAT or incubated with protein A–agarose alone (not shown), separated by SDS-PAGE, and immunoblotted with the antiphosphotyrosine antibody 4G10 (A, upper panel). A longer exposure of the upper part of the same membrane is shown to demonstrate bands corresponding to phosphotyrosine-containing proteins of molecular weight between 70 and 203 kd (A, lower panel); in particular, phosphotyrosyl bands of approximate molecular weight of 120, 97, and 76 kd are indicated by open arrowheads. The membrane was stripped and reblotted with anti-LAT (B, upper panel), anti-Grb-2 (B, middle panel) and anti-PLCγ-1 antibody (B, lower panel). Results are representative of 3 independent experiments.
LAT is tyrosine phosphorylated and associates with PLCγ-1, Grb-2, and other tyrosine phosphorylated proteins on CD2 stimulation.
Wild-type Jurkat T cells (107 cells) were stimulated with either the mitogenic pair of anti-CD2 mAbs T112 and T113 or anti-CD3ε mAb OKT3 at 37°C for the indicated periods of time. PNLs were prepared as described in “Materials and methods,” either subjected to immunoprecipitation with the rabbit polyclonal antibody anti-LAT or incubated with protein A–agarose alone (not shown), separated by SDS-PAGE, and immunoblotted with the antiphosphotyrosine antibody 4G10 (A, upper panel). A longer exposure of the upper part of the same membrane is shown to demonstrate bands corresponding to phosphotyrosine-containing proteins of molecular weight between 70 and 203 kd (A, lower panel); in particular, phosphotyrosyl bands of approximate molecular weight of 120, 97, and 76 kd are indicated by open arrowheads. The membrane was stripped and reblotted with anti-LAT (B, upper panel), anti-Grb-2 (B, middle panel) and anti-PLCγ-1 antibody (B, lower panel). Results are representative of 3 independent experiments.
LAT was found to coprecipitate with additional proteins following stimulation. Longer exposures of LAT immunoprecipitation membrane revealed the association of LAT with a number of other phosphotyrosine-containing proteins, including proteins of approximately 135, 120, 97, and 76 kd molecular weight (Figure 2A, lower panel); proteins of similar size have previously been shown to coprecipitate with LAT on TCR-CD3 engagement.10 Control precipitations using protein A–agarose alone, in the absence of anti-LAT antisera, did not reveal any of these phosphorylated proteins (not shown). The membrane was stripped and reprobed with specific antibodies; this led to the identification of the 135-kd protein as PLCγ-1 (Figure 2B, lower panel) and the 120-, 97-, and 76-kd proteins as Cbl, Vav, and SLP-76, respectively, similar to the LAT coprecipitation following TCR signaling (data not shown; also Zhang et al10). Furthermore, increased amounts of Grb-2 were found to coprecipitate with tyrosine phosphorylated LAT following either CD2 or CD3 stimulation compared with the unstimulated control (Figure 2B, middle panel). The precipitation of phosphorylated CD3 and ζ chains (∼18- to 25-kd bands) is observed following CD3, but not CD2, stimulation (Figure 2A and data not shown).
To further explore the kinetics of LAT tyrosine phosphorylation on CD2 stimulation, wild-type Jurkat T cells were either left unstimulated or stimulated via either CD2 or the TCR-CD3 complex for different times, and the pattern of tyrosine phosphorylated proteins in clarified postnuclear lysates was analyzed (Figure3, left panel). Following CD2 stimulation, LAT phosphorylation returned to basal levels after approximately 30 minutes but was still detectable at 120 minutes. The time course of apparent LAT dephosphorylation was similar following CD2 as CD3 stimulation (Figure 3, left panel).
Kinetics of LAT tyrosine phosphorylation in Jurkat T cells.
Wild-type Jurkat T cells (1 × 106 cells/test) were either left unstimulated, stimulated with the mitogenic pair of anti-CD2 mAbs T112 and T113, or stimulated with anti-CD3ε mAb OKT3 at 37°C for the indicated periods of time. Cells were then lysed in 1% Brij97 lysis buffer, and 30 μL of clarified postnuclear lysates (PNL) (0.3 × 106 cell equivalents) were resolved on SDS-PAGE, transferred to PVDF membranes, and immunoblotted with the antiphosphotyrosine antibody 4G10 (left panel). Note the kinetics of pp36-38 phosphorylation, a phosphoprotein identified as LAT by stripping the membrane and reblotting with anti-LAT–specific antibody (right panel, lower). Because the anti-LAT antibody less efficiently recognizes the phosphorylated form of the LAT protein, anti-PLCγ-1 and anti-Vav antibodies were used to confirm loading of equivalent amounts of proteins at different time points following stimulation (right panel, upper).
Kinetics of LAT tyrosine phosphorylation in Jurkat T cells.
Wild-type Jurkat T cells (1 × 106 cells/test) were either left unstimulated, stimulated with the mitogenic pair of anti-CD2 mAbs T112 and T113, or stimulated with anti-CD3ε mAb OKT3 at 37°C for the indicated periods of time. Cells were then lysed in 1% Brij97 lysis buffer, and 30 μL of clarified postnuclear lysates (PNL) (0.3 × 106 cell equivalents) were resolved on SDS-PAGE, transferred to PVDF membranes, and immunoblotted with the antiphosphotyrosine antibody 4G10 (left panel). Note the kinetics of pp36-38 phosphorylation, a phosphoprotein identified as LAT by stripping the membrane and reblotting with anti-LAT–specific antibody (right panel, lower). Because the anti-LAT antibody less efficiently recognizes the phosphorylated form of the LAT protein, anti-PLCγ-1 and anti-Vav antibodies were used to confirm loading of equivalent amounts of proteins at different time points following stimulation (right panel, upper).
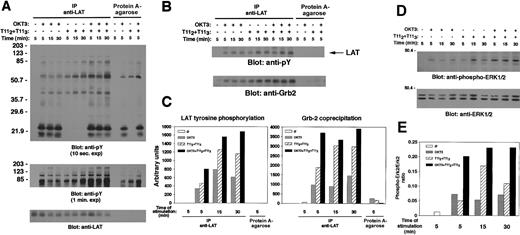
To determine whether either CD2 or TCR-CD3 engagement resulted in LAT tyrosine phosphorylation in peripheral human T cells, human PBLs were either left unstimulated or stimulated for different time points with either anti-CD3 mAb, mitogenic pairs of anti-CD2 mAbs, or a combination of anti-CD3 and pairs of anti-CD2 mAbs to evaluate additivity of costimulation. Cell lysates were prepared and subjected to LAT immunoprecipitation or incubated with protein A-agarose alone, as indicated (Figure 4). As observed in the Jurkat human T-cell line, either TCR-CD3 or CD2 engagement of resting human PBLs induced the time-dependent tyrosine phosphorylation of LAT (Figure 4A, upper panel). LAT phosphorylation was observed at 5 minutes, increased slightly at 15 minutes, and was still present at 30 minutes. Furthermore, costimulation via the CD2 and the CD3 receptors marginally increased LAT tyrosine phosphorylation. LAT phosphorylation was quantified by densitometry (Figure 4C).
Involvement of LAT in CD2 signaling of human peripheral blood T cells.
(A) LAT is tyrosine phosphorylated and associates with a number of tyrosine phosphorylated proteins on CD2 stimulation of natural human T cells. Human PBLs were isolated, as described in “Materials and methods,” and either left unstimulated or stimulated for the indicated periods of time with either OKT3 1 μg, T112plus T113 (1 μL each), or a combination of anti-CD3 and anti-CD2 mAbs. PNLs were either subjected to immunoprecipitation with the rabbit polyclonal anti-LAT antibody or incubated with protein A–agarose alone as indicated, separated by SDS-PAGE, and immunoblotted with 4G10 (upper panel). Precipitation of protein A–agarose alone was included to control for carryover of the stimulating antibody. Note the coprecipitation with LAT of other tyrosine phosphorylated proteins; tyrosylphosphorylated bands in the range of 70 to 203 kd are revealed in a longer exposure of the membrane (middle panel). The membrane was stripped and reblotted with anti-LAT antibody (lower panel). (B and C) CD2 induces association of tyrosine phosphorylated LAT with Grb-2 in human PBLs. A segment of the membrane in panel A is shown here to demonstrate LAT tyrosine phosphorylation (upper panel) and Grb-2 association. Grb-2 coprecipitation was demonstrated by stripping and reblotting the same membrane with anti-Grb-2 antibody (lower panel). In panel C, quantification of LAT tyrosine phosphorylation and Grb-2 coprecipitation was determined by densitometry using the Imagequant software. (D and E) CD2 induces Erk1/2 activation in human PBLs. Thirty μL of postnuclear lysate (0.6 × 106 cell equivalents) from each sample of the experiment described in panel A was set aside prior to immunoprecipitation, separated by SDS-PAGE, and immunoblotted with antiphospho-ERK1/2 antibody (D, upper panel). The membrane was stripped and reblotted with an anti-ERK1/2–specific antibody to control for protein loading (D, lower panel). Quantification of Erk2 (p42 MAPK) phosphorylation was determined by densitometry using the Imagequant software, and expressed as phospho-Erk2/Erk2 ratio (E).
Involvement of LAT in CD2 signaling of human peripheral blood T cells.
(A) LAT is tyrosine phosphorylated and associates with a number of tyrosine phosphorylated proteins on CD2 stimulation of natural human T cells. Human PBLs were isolated, as described in “Materials and methods,” and either left unstimulated or stimulated for the indicated periods of time with either OKT3 1 μg, T112plus T113 (1 μL each), or a combination of anti-CD3 and anti-CD2 mAbs. PNLs were either subjected to immunoprecipitation with the rabbit polyclonal anti-LAT antibody or incubated with protein A–agarose alone as indicated, separated by SDS-PAGE, and immunoblotted with 4G10 (upper panel). Precipitation of protein A–agarose alone was included to control for carryover of the stimulating antibody. Note the coprecipitation with LAT of other tyrosine phosphorylated proteins; tyrosylphosphorylated bands in the range of 70 to 203 kd are revealed in a longer exposure of the membrane (middle panel). The membrane was stripped and reblotted with anti-LAT antibody (lower panel). (B and C) CD2 induces association of tyrosine phosphorylated LAT with Grb-2 in human PBLs. A segment of the membrane in panel A is shown here to demonstrate LAT tyrosine phosphorylation (upper panel) and Grb-2 association. Grb-2 coprecipitation was demonstrated by stripping and reblotting the same membrane with anti-Grb-2 antibody (lower panel). In panel C, quantification of LAT tyrosine phosphorylation and Grb-2 coprecipitation was determined by densitometry using the Imagequant software. (D and E) CD2 induces Erk1/2 activation in human PBLs. Thirty μL of postnuclear lysate (0.6 × 106 cell equivalents) from each sample of the experiment described in panel A was set aside prior to immunoprecipitation, separated by SDS-PAGE, and immunoblotted with antiphospho-ERK1/2 antibody (D, upper panel). The membrane was stripped and reblotted with an anti-ERK1/2–specific antibody to control for protein loading (D, lower panel). Quantification of Erk2 (p42 MAPK) phosphorylation was determined by densitometry using the Imagequant software, and expressed as phospho-Erk2/Erk2 ratio (E).
As with Jurkat T cells, a number of other tyrosine phosphorylated proteins were shown to coprecipitate with LAT on stimulation of peripheral blood T cells; in particular, phosphotyrosine-containing proteins of molecular weight between 70 and 203 kd were revealed in a longer exposure of the membrane (Figure 4A, middle panel). Stimulation-dependent coprecipitation of Grb-2 with tyrosine phosphorylated LAT was also observed (Figure 4B, lower panel). The amount of coprecipitated Grb-2 correlated with the tyrosine phosphorylation of LAT, as quantified by densitometry (Figure 4B,C). Moreover, both TCR-CD3 and CD2 engagement of peripheral blood T cells led to induction of downstream events such as Erk activation, as shown by probing the electrophoresis of PNLs with antiphospho-Erk–specific antibody (Figure 4D). Coligation of both CD2 and TCR-CD3 demonstrated some additivity in the biologic response, even when the specific stimulating antibodies were used at optimal concentrations (Figure4D,E). A correlation between Erk phosphorylation and LAT/Grb-2 association was observed and quantified by densitometry (compare Figure4E with 4C). Taken together, our data suggest a role for the LAT protein as an adapter molecule in signaling via either CD2 or CD3 in peripheral blood human T cells.
On CD2 stimulation, the tyrosine phosphorylation of PLCγ-1 and SLP-76 depends on the LAT adapter molecule
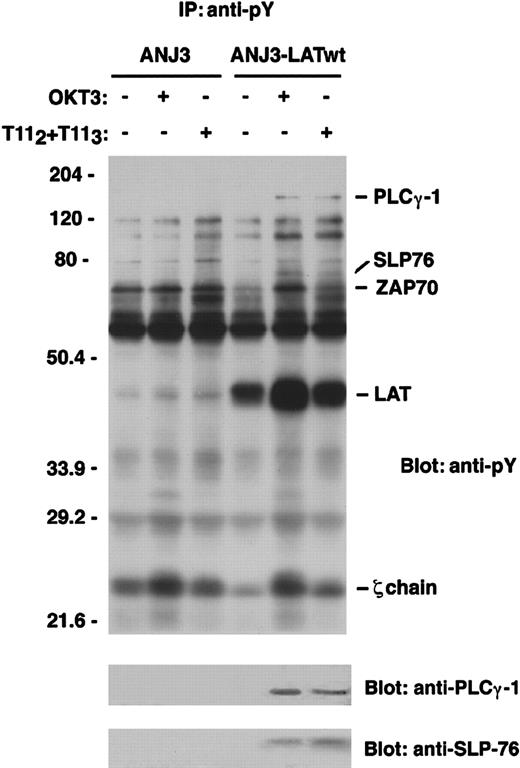
To determine whether LAT functioned as an adapter molecule in CD2-mediated T-cell activation pathways, we used ANJ3, the LAT-deficient mutant of the Jurkat T-cell line, to determine whether CD2-dependent tyrosine phosphorylation of either PLCγ-1 or SLP-76 required LAT, as had been previously shown for CD3 engagement.13 Either ANJ3 or ANJ3 cells reconstituted with a construct expressing the myc-tagged wild-type LAT (ANJ3-LATwt) were left unstimulated or stimulated for the indicated periods of time via either the CD2 or TCR-CD3 molecules, and the pattern of tyrosine phosphorylated proteins was analyzed (Figure5, upper panel). Absent in the p-Tyr immunoprecipitates of the LAT-deficient cell line were proteins of ∼135 and ∼75 kd, both of which are tyrosine phosphorylated on CD2 stimulation in wild-type Jurkat cells (Figure 1 and data not shown) and in the LAT-reconstituted cells (Figure 5). Stripping the membrane and reprobing with specific antibody allowed the identification of the 135-kd protein as PLCγ-1 and the 75-kd protein as SLP-76 (Figure 5, lower panels). These results demonstrate the requirement for LAT in the activation of PLCγ-1 and SLP-76 not only on TCR engagement but also on CD2 stimulation. Notably, the apparent basal phosphorylation of proteins identified as ZAP70 and the TCR ζ chain was increased in cells lacking LAT expression compared with cells reconstituted with the epitope-tagged LAT construct (Figure 5), as has been observed previously.13
LAT is required for tyrosine phosphorylation of PLCγ-1 and SLP-76 on CD2 stimulation of Jurkat T cells.
The LAT-deficient Jurkat T-cell line ANJ3 and the ANJ3 reconstituted with wild-type LAT (ANJ3-LATwt) (107 cells/test) were either left unstimulated or stimulated at 37°C for 5 minutes via either the CD2 or TCR-CD3 molecule, as indicated in Figure 1, and lysed in 1% Brij97 lysis buffer. PNLs were immunoprecipitated with 20 μL of antiphosphotyrosine (PY99) mAb-agarose conjugated, separated by SDS-PAGE, and immunoblotted with 4G10 (upper panel). The same membrane was stripped and blotted with anti-PLCγ-1 and anti-SLP-76 antibody (lower panels). The bands corresponding to PLCγ-1, SLP-76, ZAP70, LAT, and zeta-chain proteins are indicated. Results are representative of 2 independent experiments.
LAT is required for tyrosine phosphorylation of PLCγ-1 and SLP-76 on CD2 stimulation of Jurkat T cells.
The LAT-deficient Jurkat T-cell line ANJ3 and the ANJ3 reconstituted with wild-type LAT (ANJ3-LATwt) (107 cells/test) were either left unstimulated or stimulated at 37°C for 5 minutes via either the CD2 or TCR-CD3 molecule, as indicated in Figure 1, and lysed in 1% Brij97 lysis buffer. PNLs were immunoprecipitated with 20 μL of antiphosphotyrosine (PY99) mAb-agarose conjugated, separated by SDS-PAGE, and immunoblotted with 4G10 (upper panel). The same membrane was stripped and blotted with anti-PLCγ-1 and anti-SLP-76 antibody (lower panels). The bands corresponding to PLCγ-1, SLP-76, ZAP70, LAT, and zeta-chain proteins are indicated. Results are representative of 2 independent experiments.
ZAP70/Syk kinase expression and Src family kinase activity are required for optimal CD2-dependent LAT phosphorylation
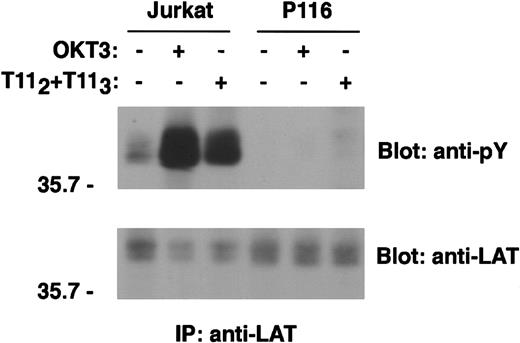
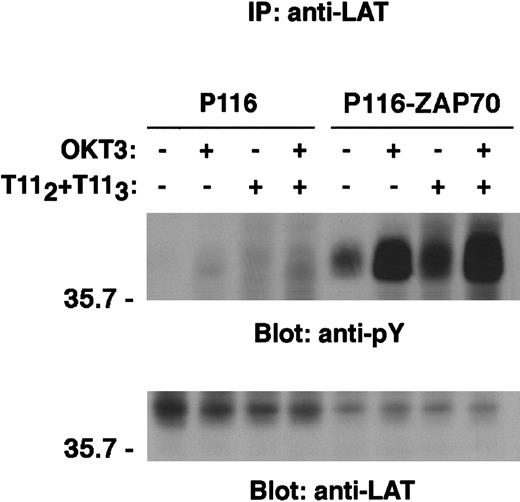
By cotransfection studies, LAT has been shown to be a substrate of the ZAP70 and Syk protein tyrosine kinases,10 a conclusion supported by the lack of LAT tyrosine phosphorylation on TCR engagement in a ZAP70/Syk-deficient Jurkat cell line, P116.26 We wished to determine whether ZAP70/Syk kinases were also required for LAT phosphorylation following CD2 stimulation. In the absence of Syk and ZAP70, minimal but detectable tyrosine phosphorylation of LAT was observed on CD2 stimulation (Figures 6and 7). Despite modest LAT phosphorylation, neither PLCγ-1 nor SLP-76 was observed to be phosphorylated following stimulation via either CD2 or the TCR-CD3 complex (data not shown). Engagement of CD2 and TCR-CD3 concurrently did not bypass the requirement for ZAP70/Syk kinase expression for LAT phosphorylation (Figure 7). In contrast, reconstitution of the ZAP70/Syk-deficient cell line P116 with a construct expressing wild-type ZAP70 restored both CD3- and CD2-mediated LAT phosphorylation (Figure 7).
ZAP70/Syk kinase expression is required for CD2-dependent LAT phosphorylation.
Wild-type Jurkat cells and the ZAP70/Syk-deficient clone P116 (107 cells/test) were left unstimulated or stimulated with either T112+T113 or OKT3 for 5 minutes. PNLs were immunoprecipitated with rabbit anti-LAT antibody or incubated with protein A–agarose alone (data not shown), resolved by SDS-PAGE, and immunoblotted with the antiphosphotyrosine 4G10 mAb. Analysis of LAT tyrosine phosphorylation is shown (upper panel). Immunoprecipitation of comparable amounts of LAT protein was confirmed by stripping the membrane and reblotting with anti-LAT–specific antibody (lower panel). Note that the anti-LAT antibody recognizes the tyrosine phosphorylated form of the protein less efficiently. Results are representative of 4 independent experiments.
ZAP70/Syk kinase expression is required for CD2-dependent LAT phosphorylation.
Wild-type Jurkat cells and the ZAP70/Syk-deficient clone P116 (107 cells/test) were left unstimulated or stimulated with either T112+T113 or OKT3 for 5 minutes. PNLs were immunoprecipitated with rabbit anti-LAT antibody or incubated with protein A–agarose alone (data not shown), resolved by SDS-PAGE, and immunoblotted with the antiphosphotyrosine 4G10 mAb. Analysis of LAT tyrosine phosphorylation is shown (upper panel). Immunoprecipitation of comparable amounts of LAT protein was confirmed by stripping the membrane and reblotting with anti-LAT–specific antibody (lower panel). Note that the anti-LAT antibody recognizes the tyrosine phosphorylated form of the protein less efficiently. Results are representative of 4 independent experiments.
Reconstitution of the ZAP70/Syk-deficient P116 cell line with wild-type ZAP70 restored CD2-mediated induction of LAT phosphorylation.
ZAP70/Syk-deficient Jurkat cells (P116) and the ZAP70-reconstituted clone (P116-ZAP70) were either left unstimulated or stimulated via either CD2 or TCR-CD3 or both receptors for 5 minutes, as described in “Materials and methods.” Immunoprecipitation of LAT was then performed as in Figure 6. Tyrosine phosphorylation of LAT is shown (upper panel). The same membrane was stripped and reblotted with anti-LAT–specific antibody (lower panel). Results are representative of 2 independent experiments.
Reconstitution of the ZAP70/Syk-deficient P116 cell line with wild-type ZAP70 restored CD2-mediated induction of LAT phosphorylation.
ZAP70/Syk-deficient Jurkat cells (P116) and the ZAP70-reconstituted clone (P116-ZAP70) were either left unstimulated or stimulated via either CD2 or TCR-CD3 or both receptors for 5 minutes, as described in “Materials and methods.” Immunoprecipitation of LAT was then performed as in Figure 6. Tyrosine phosphorylation of LAT is shown (upper panel). The same membrane was stripped and reblotted with anti-LAT–specific antibody (lower panel). Results are representative of 2 independent experiments.
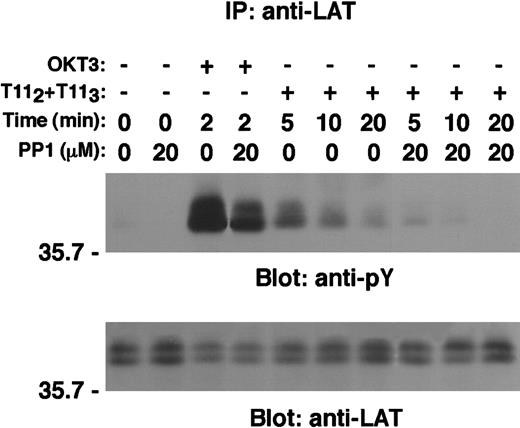
ZAP70 kinase, unlike Syk, requires activation by Src family members, Lck or Fyn.4 To explore the role of Src family members in the CD2-mediated tyrosine phosphorylation of LAT, wild-type Jurkat T cells were either left untreated or treated with the specific Src kinases inhibitor PP127 prior to stimulation via either CD2 or TCR-CD3 molecule. LAT tyrosine phosphorylation was then analyzed on LAT immunoprecipitates (Figure 8, upper panel). A significant, but incomplete, inhibition of CD2- or CD3-dependent LAT tyrosine phosphorylation was observed on treatment with PP1. Coprecipitation of other tyrosine phosphorylated proteins known to associate with phospho-LAT on stimulation was impaired (data not shown). The residual LAT phosphorylation in PP1-treated cells may have been due to incomplete inhibition of Src kinase activity by the concentration (20 μM) of PP1 used in this experiment.
The Src kinases inhibitor PP1 reduced CD2-mediated tyrosine phosphorylation of LAT.
Wild-type Jurkat cells (107 cells/test) were either left untreated or preincubated with the specific Src kinases inhibitor PP1 (20 μmol/L) at 37°C for 30 minutes and then either left unstimulated or stimulated with either OKT3 or T112 plus T113 for the indicated periods of time. Cells were then lysed in 1% Brij97 lysis buffer, and clarified PNLs were subjected to LAT immunoprecipitation as described in “Materials and methods.” Tyrosine phosphorylation of LAT is shown (upper panel). The same membrane was stripped and reblotted with anti-LAT–specific antibody (lower panel).
The Src kinases inhibitor PP1 reduced CD2-mediated tyrosine phosphorylation of LAT.
Wild-type Jurkat cells (107 cells/test) were either left untreated or preincubated with the specific Src kinases inhibitor PP1 (20 μmol/L) at 37°C for 30 minutes and then either left unstimulated or stimulated with either OKT3 or T112 plus T113 for the indicated periods of time. Cells were then lysed in 1% Brij97 lysis buffer, and clarified PNLs were subjected to LAT immunoprecipitation as described in “Materials and methods.” Tyrosine phosphorylation of LAT is shown (upper panel). The same membrane was stripped and reblotted with anti-LAT–specific antibody (lower panel).
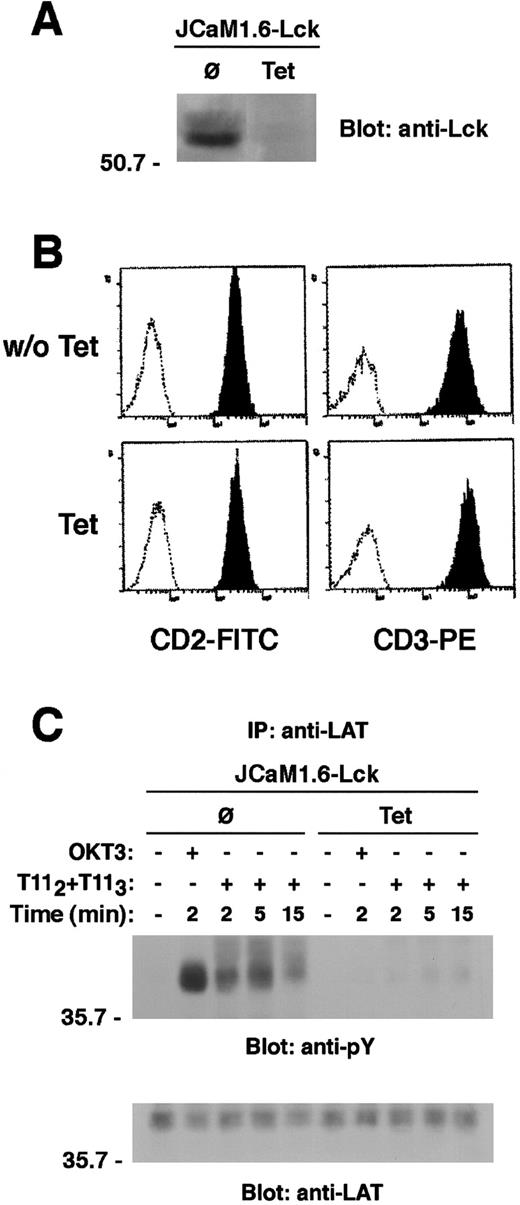
To explore the specific role of Lck in CD2-mediated LAT tyrosine phosphorylation, we used the CD2+ Lck-reconstituted clone of the Lck-deficient Jurkat cell line, JCaM1.6, in which Lck kinase was expressed under the control of a tetracycline-repressible TetR-VP16 fusion protein.18 Treatment of these cells with 1 μg/mL tetracycline for 4 days induced a complete inhibition of Lck expression as shown by an anti-Lck immunoblot of lysates derived from equivalent numbers of either untreated or tetracycline-treated cells (Figure9A). Surface expression of either CD2 or CD3 was not changed by tetracycline treatment (Figure 9B). In contrast to untreated cells, neither CD2 nor CD3 stimulation induced appreciable LAT phosphorylation in the tetracycline-treated cells that lacked Lck expression (Figure 9C). Our data suggest an essential role for Lck in mediating the (ZAP70-dependent) CD2-induced tyrosine phosphorylation of LAT.
Lck kinase expression is required for LAT tyrosine phosphorylation on CD2 stimulation.
(A) The Lck-deficient JCaM 1.6 cells reconstituted with wild-type Lck were either left untreated or treated with tetracycline (Tet) 1 μg/mL for 8 days. Lysates from equivalent cell numbers (0.3 × 106) were immunoblotted for Lck to confirm the tetracycline-regulated Lck expression. (B) Prior to stimulation, the surface expression of both CD3 and CD2 was evaluated in either untreated or Tet-treated cells by direct immunofluorescence. Cells were then either left unstimulated or stimulated via either CD2 or CD3 for the indicated periods of time. Cell lysates were subjected to LAT immunoprecipitation. LAT tyrosine phosphorylation is shown (C, upper panel). The same membrane was stripped and reblotted with anti-LAT antibody (C, lower panel).
Lck kinase expression is required for LAT tyrosine phosphorylation on CD2 stimulation.
(A) The Lck-deficient JCaM 1.6 cells reconstituted with wild-type Lck were either left untreated or treated with tetracycline (Tet) 1 μg/mL for 8 days. Lysates from equivalent cell numbers (0.3 × 106) were immunoblotted for Lck to confirm the tetracycline-regulated Lck expression. (B) Prior to stimulation, the surface expression of both CD3 and CD2 was evaluated in either untreated or Tet-treated cells by direct immunofluorescence. Cells were then either left unstimulated or stimulated via either CD2 or CD3 for the indicated periods of time. Cell lysates were subjected to LAT immunoprecipitation. LAT tyrosine phosphorylation is shown (C, upper panel). The same membrane was stripped and reblotted with anti-LAT antibody (C, lower panel).
LAT is required for Erk1/2 activation on CD2 stimulation
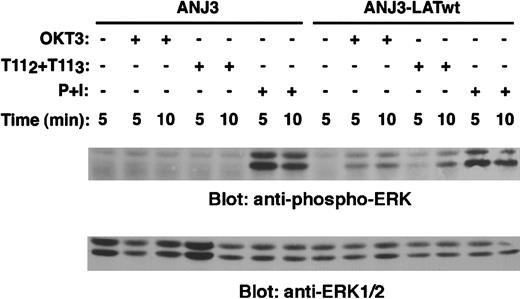
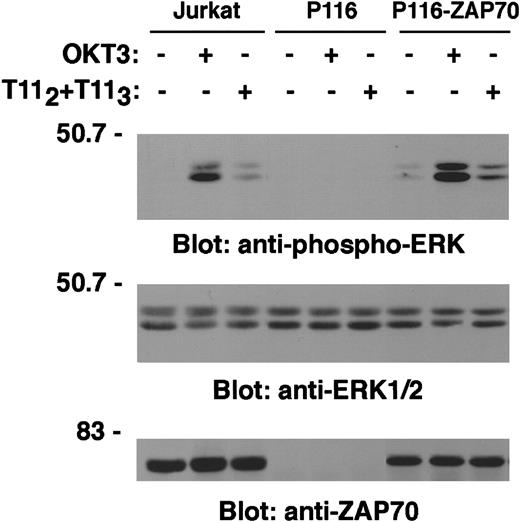
Stimulation of Jurkat T cells through either the TCR-CD3 or the CD2 receptor induces Erk activation.28-30 We wished to determine whether LAT expression was required for Erk activation on CD2 engagement. A specific antiphospho-Erk1/2 mAb was used to probe PNLs derived from either unstimulated, OKT3-, or T112 + T113-stimulated ANJ3 and LAT-reconstituted ANJ3 cells. No Erk activation was observed in ANJ3 cells either on TCR-CD3, as previously shown,13 or CD2 stimulation (ANJ3, Figure 10, upper panel). Reconstitution of ANJ3 with LAT restored both TCR-CD3– and CD2-mediated Erk activation (ANJ3-LATwt, Figure 10, upper panel). Comparable expression of Erk1/2 protein between ANJ3 and LAT-reconstituted ANJ3 cells was confirmed by stripping and reblotting the membrane with an anti-Erk1/2–specific antibody (Figure 10, lower panel). As expected, although no Erk activation was observed in the ZAP70/Syk-deficient P116 cells, reconstitution of ZAP70 expression restored Erk phosphorylation, comparable with that observed in wild-type Jurkat cells, following either TCR-CD3 or CD2 stimulation (Figure 11, upper panel). Erk1/2 expression (Figure 11, middle panel) is comparable in each cell line used, despite differences in ZAP70 expression (Figure 11, lower panel). These data demonstrate that LAT tyrosine phosphorylation by ZAP70/Syk kinases is required for coupling CD2 to Ras pathway activation.
LAT adapter protein is required for coupling CD2 to ERK1/2 activation.
The LAT-deficient Jurkat cells ANJ3 and ANJ3 cells reconstituted with wild-type LAT (ANJ3-LATwt) (106 cells/test) were either left unstimulated, stimulated with T112 + T113, OKT3 or PMA (10 ng/mL) plus ionomycin (1.5 μM) (P + I) at 37°C for the indicated periods of time and lysed in 1% Brij97 lysis buffer. Clarified PNLs (0.3 × 106 cell equivalents) of unstimulated and stimulated cells were resolved by SDS-PAGE, transferred to PVDF membranes, and immunoblotted with an antiphospho-ERK1/2 antibody specific for the dual phosphorylated (Thr202 and Tyr204) activated p44 and p42 MAPK (Erk1 and Erk2) (upper panel). The same membrane was stripped and reblotted with anti-ERK1/2 antibody (lower panel).
LAT adapter protein is required for coupling CD2 to ERK1/2 activation.
The LAT-deficient Jurkat cells ANJ3 and ANJ3 cells reconstituted with wild-type LAT (ANJ3-LATwt) (106 cells/test) were either left unstimulated, stimulated with T112 + T113, OKT3 or PMA (10 ng/mL) plus ionomycin (1.5 μM) (P + I) at 37°C for the indicated periods of time and lysed in 1% Brij97 lysis buffer. Clarified PNLs (0.3 × 106 cell equivalents) of unstimulated and stimulated cells were resolved by SDS-PAGE, transferred to PVDF membranes, and immunoblotted with an antiphospho-ERK1/2 antibody specific for the dual phosphorylated (Thr202 and Tyr204) activated p44 and p42 MAPK (Erk1 and Erk2) (upper panel). The same membrane was stripped and reblotted with anti-ERK1/2 antibody (lower panel).
LAT tyrosine phosphorylation by ZAP70/Syk kinases is required for coupling CD2 to ERK1/2 activation.
Wild-type Jurkat, the ZAP70/Syk-deficient cells P116, and the ZAP70-reconstituted P116 cells (P116-ZAP70) were left unstimulated or stimulated for 5 minutes at 37°C with T112 + T113 or OKT3 and lysed. PNL (0.3 × 106 cell equivalents) were separated by SDS-PAGE and immunoblotted with antiphospho-ERK1/2 antibody as in Figure 10A (upper panel). The same membrane was stripped, cut, and blotted with an anti-ERK1/2 specific antibody (middle panel) and an anti-ZAP70 antibody (lower panel).
LAT tyrosine phosphorylation by ZAP70/Syk kinases is required for coupling CD2 to ERK1/2 activation.
Wild-type Jurkat, the ZAP70/Syk-deficient cells P116, and the ZAP70-reconstituted P116 cells (P116-ZAP70) were left unstimulated or stimulated for 5 minutes at 37°C with T112 + T113 or OKT3 and lysed. PNL (0.3 × 106 cell equivalents) were separated by SDS-PAGE and immunoblotted with antiphospho-ERK1/2 antibody as in Figure 10A (upper panel). The same membrane was stripped, cut, and blotted with an anti-ERK1/2 specific antibody (middle panel) and an anti-ZAP70 antibody (lower panel).
LAT tyrosine phosphorylation couples CD2 stimulation to NFAT and IL-2 transcriptional activation
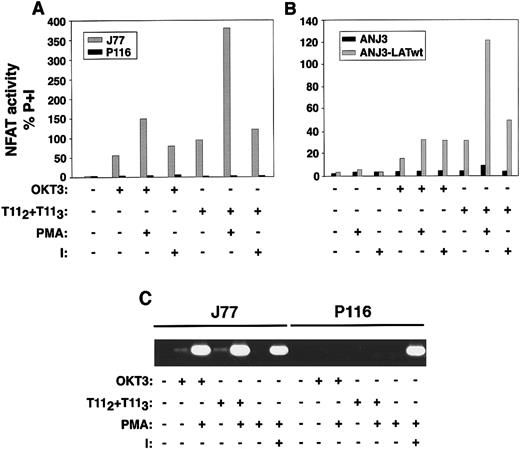
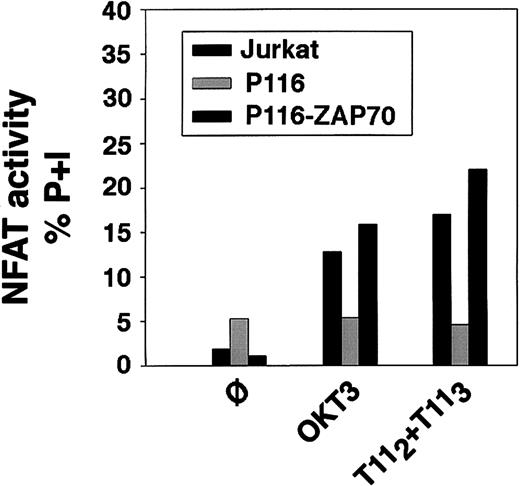
To analyze whether deficient LAT expression or failure of LAT tyrosine phosphorylation translated into a defect in NFAT transcriptional activation, wild-type Jurkat, the ZAP70/Syk kinase-deficient Jurkat cell line P116, the ZAP70-reconstituted P116 cells, the LAT-deficient Jurkat cells ANJ3, and the LAT-reconstituted Jurkat cells ANJ3-LATwt were transiently transfected with a reporterluciferase gene driven by 3 copies of the distal NFAT site derived from the IL-2 promoter.19 Transfected cells were either left unstimulated or stimulated with plate-bound OKT3, mitogenic pairs of anti-CD2 mAbs (T112 + T113), either alone or in combination with either PMA or ionomycin, or PMA plus ionomycin, as indicated. As expected, treatment of each of these cell lines with PMA and ionomycin, agents that bypass surface and proximal signaling events, resulted in equivalent NFAT transcriptional activation (data not shown). However, in the absence of either ZAP70/Syk kinases (P116, Figure 12A) or LAT (ANJ3, Figure 12B), no NFAT transcriptional activation was observed on either CD2 or CD3 stimulation, either alone or in combination with PMA or ionomycin. These latter data demonstrate that activating PKC (with PMA) or modifying internal calcium concentrations (with ionomycin) did not bypass the lack of ZAP70/Syk kinase or LAT expression. Appropriate LAT reconstitution (ANJ3-LATwt, Figure 12B) and ZAP70 reconstitution (P116-ZAP70, Figure13) restored NFAT transcriptional activity on engagement of either CD3 or CD2. Correlation of NFAT transcriptional activation with IL-2 transcription was documented by RT-PCR in appropriately stimulated wild-type and ZAP70/Syk deficient Jurkat cells (Figure 12C).
CD2-mediated NFAT transcriptional activity and IL-2 transcription are dependent on ZAP70/Syk and LAT expression.
(A) Wild-type Jurkat cells (J77) and the ZAP70/Syk-deficient clone P116 were transiently transfected with 10 μg of a reporter plasmid p3xNFAT-luc, carrying the luciferase gene driven by 3 tandem repeats of the distal NFAT sequences derived from the IL-2 promoter, and 1 μg pRL-TK vector, which provides constitutive expression ofRenilla luciferase, as described in “Materials and methods.” Twenty-four hours after transfection, 106cells/test were left unstimulated or stimulated with plate-bound OKT3, T112+T113, each either alone or in combination with 10 ng/mL PMA or 1.5 μmol/L ionomycin (I), or PMA plus ionomycin for 6 hours. Cells were then washed and lysed, and the soluble extract was assayed for luciferase activity. Results are reported as activity of firefly luciferase normalized to that of Renillaluciferase (to correct for transfection efficiency) in the lysate, and expressed as percentage of response obtained with PMA plus ionomycin (P+I). Results are representative of 3 independent experiments. (B) A comparable experiment to that in panel A was performed using the LAT-deficient cells ANJ3 and the ANJ3 cells reconstituted with wild-type LAT (ANJ3-LATwt). (C) CD2-induced IL-2 transcription is dependent on ZAP70/Syk expression. Wild-type Jurkat and the ZAP70/Syk-deficient cells, P116 (1 × 106/test) were either left unstimulated or stimulated with plate-bound OKT3, 1 μL of T112 plus 1 μL of T113, either alone or in combination with PMA, or PMA plus ionomycin for 4 hours. Total RNA was then extracted and subjected to RT-PCR for IL-2 as described in “Materials and methods.” RT-PCR for glyceraldehyde-3-phosphate dehydrogenase was performed as control (not shown).
CD2-mediated NFAT transcriptional activity and IL-2 transcription are dependent on ZAP70/Syk and LAT expression.
(A) Wild-type Jurkat cells (J77) and the ZAP70/Syk-deficient clone P116 were transiently transfected with 10 μg of a reporter plasmid p3xNFAT-luc, carrying the luciferase gene driven by 3 tandem repeats of the distal NFAT sequences derived from the IL-2 promoter, and 1 μg pRL-TK vector, which provides constitutive expression ofRenilla luciferase, as described in “Materials and methods.” Twenty-four hours after transfection, 106cells/test were left unstimulated or stimulated with plate-bound OKT3, T112+T113, each either alone or in combination with 10 ng/mL PMA or 1.5 μmol/L ionomycin (I), or PMA plus ionomycin for 6 hours. Cells were then washed and lysed, and the soluble extract was assayed for luciferase activity. Results are reported as activity of firefly luciferase normalized to that of Renillaluciferase (to correct for transfection efficiency) in the lysate, and expressed as percentage of response obtained with PMA plus ionomycin (P+I). Results are representative of 3 independent experiments. (B) A comparable experiment to that in panel A was performed using the LAT-deficient cells ANJ3 and the ANJ3 cells reconstituted with wild-type LAT (ANJ3-LATwt). (C) CD2-induced IL-2 transcription is dependent on ZAP70/Syk expression. Wild-type Jurkat and the ZAP70/Syk-deficient cells, P116 (1 × 106/test) were either left unstimulated or stimulated with plate-bound OKT3, 1 μL of T112 plus 1 μL of T113, either alone or in combination with PMA, or PMA plus ionomycin for 4 hours. Total RNA was then extracted and subjected to RT-PCR for IL-2 as described in “Materials and methods.” RT-PCR for glyceraldehyde-3-phosphate dehydrogenase was performed as control (not shown).
Reconstitution of the ZAP70/Syk-deficient P116 cell line with wild-type ZAP70 restores both CD3- and CD2-mediated induction of NFAT transcriptional activity.
Wild-type Jurkat cells, the ZAP70/Syk-deficient clone P116 and the ZAP70-reconstituted P116 cells were subjected to transient transfection for evaluation of NFAT transcriptional activity by luciferase assay, as described in Figure 12. Twenty-four hours after transfection, 106 cells/test were then either left unstimulated (ø) or stimulated with plate-bound OKT3, T112+T113, or PMA plus ionomycin for 6 hours. Cells were then washed and lysed, and the soluble extract was assayed for luciferase activity. Results are reported as activity of firefly luciferase normalized to that ofRenilla luciferase (to correct for transfection efficiency) in the lysate, and expressed as percentage of response obtained with PMA plus ionomycin (P+I).
Reconstitution of the ZAP70/Syk-deficient P116 cell line with wild-type ZAP70 restores both CD3- and CD2-mediated induction of NFAT transcriptional activity.
Wild-type Jurkat cells, the ZAP70/Syk-deficient clone P116 and the ZAP70-reconstituted P116 cells were subjected to transient transfection for evaluation of NFAT transcriptional activity by luciferase assay, as described in Figure 12. Twenty-four hours after transfection, 106 cells/test were then either left unstimulated (ø) or stimulated with plate-bound OKT3, T112+T113, or PMA plus ionomycin for 6 hours. Cells were then washed and lysed, and the soluble extract was assayed for luciferase activity. Results are reported as activity of firefly luciferase normalized to that ofRenilla luciferase (to correct for transfection efficiency) in the lysate, and expressed as percentage of response obtained with PMA plus ionomycin (P+I).
Discussion
In this study, we analyzed the role of LAT and Syk family PTKs in T-cell activation induced through ligation of the CD2. We provide biochemical and functional evidence that LAT expression and its tyrosine phosphorylation by ZAP70/Syk kinases are critical, not only in TCR-mediated T-cell activation, but also on CD2 engagement.
Biochemical studies have demonstrated that CD2 stimulation can initiate a multitude of intracellular signaling events, similar to those elicited on TCR engagement. The involvement of Src family PTK in CD2-mediated signaling has been suggested by the demonstration of CD2 coassociation with both Lck and Fyn31-35; CD2 signaling was also shown to be defective in the Lck-deficient Jurkat cell line, JCaM1,24 and in cell lines deficient in the expression of CD45,36-38 a transmembrane protein-tyrosine phosphatase believed to regulate the kinase activities of Src family members.39,40 Here we extend those observations to shown defective CD2-dependent LAT tyrosine phosphorylation in the absence of Lck expression (Figure 9). The expression of Fyn did not bypass the requirement for Lck in CD2-induced LAT phosphorylation, at least in the cell lines used here. However, Lck expression is not an absolute requirement for CD2 signaling as an Lck-independent pathway of CD2 signaling involving Jun kinase has been described.41 The role of ZAP70/Syk family PTKs in CD2 signaling is less clear. The time-dependent activation of ZAP70 kinase has been reported following CD59 cross-linking42; however, other studies24 have failed to demonstrate ZAP70/Syk family activation on CD2 engagement. The overlapping roles of Syk and ZAP70, both of which are capable of expression in T lymphocytes, has confounded the analysis of these kinases. Recently, Ueno and colleagues43 demonstrated that ZAP70 was required for calcium mobilization, but was dispensable for mitogen-activated protein kinase (MAPK) family activation induced by CD2 engagement of human T cells. The analysis was conducted on a human T-cell line derived from a patient with a genetic deficiency of ZAP70 expression. Syk kinase, expressed in these cells, may have compensated for the lack of ZAP70. Our results using the Jurkat P116 cell line support a key role of the ZAP70/Syk kinase family in CD2-mediated T-cell activation. Deficient in expression of both ZAP70 and Syk kinase, the Jurkat P116 cell line displayed severe defects in CD2-induced signaling functions, including protein tyrosine phosphorylation and NFAT-driven and IL-2 transcriptional activation (Figure 12A,C and data not shown). The activation of Erk1/2 MAP kinases, downstream effectors of the Ras pathway, was also defective on CD2 stimulation of the ZAP70/Syk-deficient cell line (Figure 11). Importantly, these defects were overcome by the reconstitution of the P116 cells with ZAP70, demonstrating that the expression of ZAP70 alone, in the absence of Syk, is sufficient to mediate such downstream events. We suggest that, although Syk kinase appears capable of transducing CD2-induced MAPK family activation43 in T cells, ZAP70 kinase is capable of transducing CD2 signaling leading not only to Erk1/2 MAPK activation (Figure 11), but also to calcium mobilization43 and NFAT transcriptional activation (Figure 12). We were unable to address directly the role of Syk kinase in our system because Syk-reconstituted P116 cells are not stable and transient expression of Syk kinase alone conferred NFAT transcriptional activity.44
A deficiency of ZAP70 expression in the P116 cell line did not affect the expression or activation of the Src family members Lck and Fyn on TCR/CD3 stimulation; the tyrosine phosphorylation, however, of certain proteins such as SLP-76 and PLCγ-1 was impaired following either CD3 or CD2 ligation. These defects paralleled the defects in the LAT-deficient ANJ3 cell line (Figures 5, 10B, and 12) suggesting that ZAP70 and LAT are downstream of the Src kinases Lck or Fyn in CD2 as in TCR-CD3 stimulation. Furthermore, the faint tyrosine phosphorylation of LAT (possibly mediated by Lck kinase10) observed following stimulation of P116 cells (Figures 6 and 7) was insufficient for downstream signaling. Optimal phosphorylation by ZAP70 or Syk kinase was required. Taken together these data support the suggestion that LAT, a substrate of ZAP70/Syk kinases, is required to couple CD2 engagement to certain downstream events including tyrosine phosphorylation of SLP-76 and PLCγ-1, Erk activation, and NFAT and IL-2 transcriptional activation.
The mechanism by which LAT is coupled to CD2 remains uncertain. LAT has not been shown to associate directly with the TCR-CD3 complex, but does associate with CD4 and CD8 molecules.45 Thus, the CD4 and CD8 receptors appear to recruit not only the Src family members but also LAT to the TCR-CD3 complex, in proximity, following activation, to ZAP70. Preliminary observations suggest that CD2 and LAT coprecipitate in lysates prepared in Brij97 detergent (Martelli and Bierer, unpublished observations) but there is no evidence to date that the association is direct. These data are consistent with the recent observation that the CD2 molecule is localized to the GEMs of the plasma membrane,46 a domain to which LAT preferentially localizes. The molecular basis of CD2/LAT association is the subject of current investigation.
Recent identification of the CD2-associated molecule CD2AP47 and its human homologous CMS48 has renewed interest in the relationship between CD2 and cytoskeletal elements. The CD2AP interaction with the cytoplasmic domain of CD2 appears to regulate CD2 clustering at the site of the T cell–antigen-presenting cell contact, cytoskeletal rearrangement, and T-cell polarization.47 It is tempting to speculate that the CD2AP/CD2 regulation of T-cell cytoskeletal rearrangements is responsible for the ability of CD2 to lower the threshold of T-cell activation,49 rendering T cells responsive to low antigen densities. Although the signaling intermediates for CD2-dependent cytoskeletal rearrangements have not been defined, CMS has been shown to regulate Rac, a small GTPase that itself regulates cytoskeletal rearrangement. Vav, a GTP exchange factor for Rac, is in turn regulated by SLP76, a protein that we have shown is dependent on LAT following CD2 activation. Taken together, these disparate observations would support a model by which the adapter protein LAT and CD2AP/CMS are both involved in the CD2-dependent regulation of Rac pathways leading to cytoskeletal rearrangement and lymphocyte adhesion.
A number of immune functions, such as IL-2 production and T-cell proliferation, can be elicited via stimulation of either the TCR-CD3 or the CD2 receptor; signaling pathways involving the ZAP70/Syk kinase family and the adapter protein LAT appear to be shared between these cell surface receptors and may be involved in eliciting downstream actions. CD2 has been implicated in a number of specific T-cell functions, such as the regulation of the responsiveness of activated human T cells to IL-1250 and interferon-γ production, that appear not to be regulated nor induced by TCR-CD3 engagement. Exploration of signaling pathways induced by CD2, but not TCR-CD3, ligation, may illuminate the signaling intermediates required for these unique, CD2-dependent functions. The potential existence of such pathways in T cells is supported by the recent observation that TCR partial agonist ligands can selectively activate the Ras-MAPK pathway bypassing the need for activation of Lck and ZAP70, and for LAT phosphorylation.52 We are currently exploring the role of CD2 and LAT in these signaling pathways.
Acknowledgments
We thank Robert Abraham for providing P116 and the ZAP70-reconstituted P116 cells, David E. Straus for providing the Lck-reconstituted JCaM1.6 cells, and Kai Chang for RT-PCR IL-2 experiments.
Supported by a fellowship from Fondazione, Istituto Pasteur—Fondazione Cenci-Bolognetti, University of Rome, La Sapienza, Italy (M.P.M.).
This is a U.S. government work. There are no restrictions on its use.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Barbara E. Bierer, National Heart, Lung, and Blood Institute, Building 10, Room 5D49, 10 Center Dr, Bethesda, MD 20892; e-mail: biererb@nih.gov.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal