Abstract
Most inhibitory antibodies to human factor VIII (fVIII) bind to epitopes in the A2, ap-A3, or C2 domains. The anticoagulant action of antibodies to the C2 domain is due to inhibition of binding of fVIII to phospholipid. The x-ray structure of the human fVIII C2 domain shows a putative hydrophobic, 3-prong, phospholipid membrane-binding site consisting of Met2199/Phe2200, Val2223, and Leu2251/Leu2252. Additionally, Lys2227, near Val2223, is part of a ring of positively charged residues that may contribute to electrostatic interaction of fVIII with negatively charged phosphatidylserine. In this study, 8 active mutants of human fVIII (Met2199Ile, Leu2252Phe, Phe2200Leu, Val2223Ala, Lys2227Glu, Met2199Ile/Phe2200Leu, Val2223Ala/Lys2227Glu, and Met2199Ile/Phe2200Leu/Val2223Ala/Lys2227Glu), which were constructed on the basis of differences between human, porcine, murine, and canine fVIII at proposed phospholipid binding sites, were expressed. The antigenicity of the mutants toward 5 C2-specific polyclonal human antibodies was measured by using the Bethesda assay. A human monoclonal anti-C2 antibody, BO2C11, and a murine C2-specific monoclonal antibody, NMC VIII-5, were also included in the analysis. In comparison with wild-type, B-domainless fVIII, the Met2199Ile, Phe2200Leu, and Leu2252 single mutants had lower antigenicity toward most of the inhibitors. In contrast, the Val2223Ala and Lys2227Glu mutants usually showed increased antigenicity. These results suggest that C2 inhibitors frequently target the Met2199/Phe2200 and Leu2251/Leu2252 β-hairpins and are consistent with the hypothesis that these residues participate in binding to phospholipid membranes. In contrast, Val2223 and Lys2227 may oppose antibody binding sterically or through stabilization of a low-affinity membrane-binding conformation of the C2 domain.
Introduction
Inhibitory antibodies (inhibitors) to factor VIII (fVIII) develop either as alloantibodies in patients with hemophilia A after infusions of fVIII or as autoantibodies in people without hemophilia.1 Antibodies to epitopes in the A2,ap-A3, and C2 domains in the A1-A2-B-ap-A3-C1-C2 fVIII molecule are responsible for all anticoagulant activity in most inhibitor plasma samples.2,3 The 18-kd C2 domain, defined as residues Ser2173 to Tyr2332 in single-chain human fVIII, contains a phospholipid membrane-binding site that is necessary for the normal procoagulant function of fVIII. Human C2-specific anti-fVIII antibodies inhibit this interaction.4 Consistent with this activity, phospholipid protects fVIII from inactivation by fVIII inhibitors.4,5 The C2 domain also contains part of the von Willebrand factor (vWf) binding site. Some inhibitors may act by interfering with this interaction.8-10
Recently, a 1.5-Å x-ray structure of the human fVIII C2 domain was described.11 Examination of this structure revealed 3 solvent-exposed hydrophobic “feet” consisting of Met2199/Phe2200, Val2223, and Leu2251/Leu2252. A ring of positively charged residues, including Lys2227, which is near Val2223, surrounds these residues. This motif suggested that membrane binding consists of insertion of the hydrophobic feet into the membrane bilayer and is stabilized by electrostatic interaction with negatively charged phospholipid.
Most fVIII inhibitors have been found to cross-react poorly with porcine fVIII. This observation led to the mapping of a major determinant of the C2 epitope to residues Glu2181 to Val2243 by means of a series of constructs that contained porcine substitutions in the human fVIII C2 domain.12 In the current study, we used residues in porcine, murine, or canine fVIII that are homologous to residues Met2199, Phe2200, Val2223, Lys2227, Leu2252, or a combination of these in human fVIII to create a series of recombinant hybrid fVIII molecules. A significant reduction in antigenicity was usually observed in association with mutations at Met2199, Phe2200, and Leu2252, indicating that these residues participate in binding of fVIII to phospholipid membranes and often to inhibitory antibodies.
Materials and methods
Materials
Citrated plasma from patients with hemophilia A and normal pooled human plasma were purchased from George King Biomedical, Inc, Overland Park, KS. Heparin-Sepharose was purchased from Sigma Chemical Co, St Louis, MO. Fetal-calf serum (FCS), geneticin, penicillin, streptomycin, Dulbecco modified Eagle medium (DMEM)-F12, and AIM-V medium were purchased from Life Technologies, Inc, Rockville, MD. Pfu DNA polymerase and the phagemid pBlueScript II KS− were purchased from Stratagene, La Jolla, CA. Murine antihuman fVIII monoclonal antibodies ESH4 and ESH8 were purchased from American Diagnostica, Greenwich, CT. The murine fVIII C2-specific inhibitory monoclonal antibody NMC VIII-5 was a generous gift from Dr Midori Shima, Nara Medical College, Japan. A human fVIII C2-specific IgG4 monoclonal antibody BO2C11, which was cloned from a transformed B-cell line from a patient with hemophilia, was prepared as described previously.13 Citrated human plasma samples from 5 patients with inhibitors (AA, AJ, HR, LK, and RvR) were generous gifts from Dr Dorothea Scandella. They were used either without further purification (HR, RvR, and AJ) or as IgG preparations (LK and AA). Inhibitor IgG was prepared as described previously.14 The inhibitors in HR, LK, AA, and RvR antibodies were specific for the C2 domain on antibody neutralization assays.2 AJ was identified as C2 specific by using a panel of recombinant hybrid human/porcine fVIII molecules.3
Albumin-free recombinant full-length fVIII was a gift from the Hyland-Immuno Division of Baxter Healthcare, Duarte, CA. Synthetic oligonucleotides were purchased from Life Technologies. Restriction enzymes were purchased from New England Biolabs, Beverly, MA, or Promega, Madison, WI. A cell line derived from baby hamster kidney cells was a generous gift from Dr R.T.A. Macgillivray.15 A B-domainless fVIII expression vector, designated HB−/ReNeo, which contains a NotI site 2 bases 3′ to the stop codon and ampicillin and geneticin resistance genes, was prepared as described previously.12 HSQ/ReNeo, a human B-domainless fVIII molecule containing a 14-amino acid segment (SerPheSerGlnAsnProProValLeuLysArgHisGlnArg) in place of the B domain in human fVIII16 was constructed by splicing-by-overlap extension (SOE) mutagenesis17 using HB−/ReNeo as template, essentially as described previously for the corresponding porcine molecule.12 HP20, a B-domainless hybrid human/porcine fVIII molecule containing human A1, A2, ap-A3, and C1 domains and the porcine C2 domain, was prepared as described previously.12
Plasmid DNA was purified by using a Qiagen Plasmid Maxi Kit (Qiagen, Inc, Valencia, CA). Polymerase chain reactions (PCRs) were done with an OmniGene thermocycler (Hybaid, Woodbridge, NJ) usingPfu DNA polymerase. PCR products were gel purified, precipitated with ethanol, and ligated into plasmid DNA by using T4 DNA ligase (Rapid DNA Ligation Kit; Boehringer Mannheim, Indianapolis, IN). Insert-containing plasmids were used to transform Escherichia coli Epicurean XL1-Blue cells. All novel fVIII DNA sequences generated by PCR were confirmed by dideoxy sequencing using an Applied Biosystems (Foster City, CA) 373a automated DNA sequencer and the PRISM dye terminator kit.
Construction of fVIII mutant complementary DNAs
Mutations were made in HSQ codons by SOE mutagenesis to produce the following proteins: Met2199Ile (human to porcine), ATG to ATC; Phe2200Leu (human to canine), TTT to TTG; Val2223Ala (human to canine), GTG to GCC; Lys2227Glu (human to porcine), AAA to GAG; Leu2252Phe (human to murine), CTT to TTC; Met2199Ile/Phe2200Leu, ATG to ATC and TTT to TTG; Val2223Ala/Lys2227Glu, GTG to GCC and AAA to GAG; and Met2199Ile/Phe2200Leu/Val2223Ala/Lys2227Glu, ATG to ATC, TTT to TTG, GTG to GCC, and AAA to GAG.
HSQ/ReNeo was used as the template in the PCRs. The first PCR used the human C1 primer, 5′-GTG GAT TCA TCT GGG ATA AAA CAC-3′, designated H3763+ and corresponding to nucleotides 3763 to 3786 in the HSQ sequence, as the sense primer. The following primers were used as antisense primers: Met2199Ile, 5′-AGG AGA CCA GGT GGC AAA GAT ATT GGT AAA GTA GGA TGA-3′; Phe2200Leu, 5′-TGA AGG AGA CCA GGT GGC CAA CAT ATT GGT AAA GTA GGA-3′; Val2223Ala, 5′-CCA CTC TTT TGG ATT ATT GGC CTG AGG TCT CCA GGC ATT-3′; Lys2227Glu, 5′-GTC CAC TTG CAG CCA CTC CTC TGG ATT ATT CAC CTG AGG-3′; Leu2252Phe, 5′-CTT CAC ATA CAT GCT GGT GAA CAG AGA TTT TAC TCC CTG-3′; Met2199Ile/Phe2200Leu, 5′-AGG AGA CCA GGT GGC CAA GAT ATT GGT AAA GTA GGA TGA-3′; and Val2223Ala/Lys2227Glu, 5′-CAC TTG CAG CCA CTC CTC TGG ATT ATT GGC CTG AGG TCT CCA GGC-3′.
The second PCR used the ReNeo primer, 5′-AGT TTT TCT ACA ACA GAG GAA GTG-3′, designated RE1110− and located 3′ to the C2 domain, as antisense primer. The following primers were used as sense primers: Met2199Ile, 5′-TCA TCC TAC TTT ACC AAT ATC TTT GCC ACC TGG TCT CCT-3′; Phe2200Leu, 5′-TCC TAC TTT ACC AAT ATG TTG GCC ACC TGG TCT CCT TCA-3′; Val2223Ala, 5′-AAT GCC TGG AGA CCT CAG GCC AAT AAT CCA AAA GAG TGG-3′; Lys2227Glu, 5′-CCT CAG GTG AAT AAT CCA GAG GAG TGG CTG CAA GTG GAC-3′; Leu2252Phe, 5′-CAG GGA GTA AAA TCT CTG TTC ACC AGC ATG TAT GTG AAG-3′; Met2199Ile/Phe2200Leu, 5′-TCA TCC TAC TTT ACC AAT ATC TTG GCC ACC TGG TCT CCT-3′; and Val2223Ala/ Lys2227Glu, 5′-GCC TGG AGA CCT CAG GCC AAT AAT CCA GAG GAG TGG CTG CAA GTG-3′.
The SOE reaction used fragments from the PCRs as templates and H3763+ and RE1110 as primers. The SOE product and HSQ/RENeo ligation fragments were generated by using SwaI andNotI.
Met2199Ile/Phe2200Leu/Val2223Ala/Lys2227Glu cDNA was constructed as follows. Met2199Ile/Phe2200Leu cDNA was moved into pBluescript II KS− and digested with Bsu36I. Val2223Ala/Lys2227Glu cDNA was also digested with Bsu36I, and the appropriate fragments were ligated. The resulting Met2199Ile/Phe2200Leu/Val2223Ala/Lys2227Glu cDNA was moved into ReNeo by means of digestion with SwaI and NotI.
Expression of recombinant fVIII molecules
Transfected cell lines were maintained in DMEM-F12 containing 10% FCS, 50 U/mL penicillin, and 50 μg/mL streptomycin. FCS was heat inactivated for 1 hour at 56°C before use. Mutant cDNAs in ReNeo were stably transfected into BHK cells, selected for geneticin resistance, switched to serum-free AIM-V medium for expression, and partly purified by heparin-Sepharose chromatography as described previously.12
fVIII and fVIII inhibitor assays
The activity of recombinant fVIII proteins was measured by a one-stage clotting assay.18 One unit of fVIII was defined as the activity in 1 mL normal citrated human plasma. fVIII inhibitor titers were measured by using the following modification of the Bethesda assay.19 Recombinant fVIII was added to plasma from patients with hemophilia A to a final concentration of 0.8 to 1.2 U/mL and incubated with various concentrations of inhibitor for 2 hours at 37°C. To determine the 50% inhibition point that defines the Bethesda unit, dilutions of inhibitor were made that produced residual activities that spanned at least the range of 35% to 65%. In some cases, replicate dilutions were made and the average value was used. An average of 10 dilutions was made to determine each Bethesda titer.
Semilogarithmic plots of the percentage of residual activity compared with the log of the reciprocal of the inhibitor dilution appeared to be linear in all cases. The data were fitted by nonlinear regression using the Marquardt algorithm (SigmaPlot 5.0; SPSS, Chicago, IL) to the following equation: percentage of residual activity = m (log x − log x50) + 50, where the fitted parameter x50 is the reciprocal dilution that produces 50% inhibition; the fitted parameter m, the slope of the semilog line; and the independent variable x, the reciprocal dilution of the inhibitor sample. The SE of the estimate (average deviation of data points from the regression line) for 62 Bethesda assays was 10.0 ± 4.0 (mean ± SD), indicative of the relatively low precision that is inherent in the assay.
The Bethesda titer equals x50−1. The estimate of the SE of the Bethesda titer was calculated by multiplying the Bethesda titer by the coefficient of variation of x50. The Bethesda titers of fVIII mutants and HB− were compared using the Student t test.
The mass concentration of fVIII in partly purified preparations was determined by a sandwich enzyme-linked immunosorbent assay (ELISA) using ESH4 as the capture antibody and biotinylated ESH8 as the detection antibody as described previously.20 Full-length recombinant fVIII was used as the standard, and values were corrected for the difference in mass between full-length and B-domainless forms of fVIII. Samples were assayed in quadruplicate. The average coefficient of variation was 9.0%. The specific activity of fVIII molecules was calculated by dividing the coagulant activity by the concentration determined by ELISA. The following values were obtained: HB−, 7800 U/mg; Met2199Ile, 12 800 U/mg; Phe2200Leu, 10 200 U/mg; Val2223Ala, 19 600 U/mg; Lys2227Glu, 36 200 U/mg; Leu2252Phe, 10 100 U/mg; Met2199Ile/Phe2200Leu, 10 000 U/mg; Val2223Ala/Lys2227Glu, 33 200 U/mg; and Met2199Ile/Phe2200Leu/Val2223Ala/Lys2227Glu, 14 200 U/mg. The apparent specific activity of some of the mutants was higher than that of HB−. This may have been due to a relatively small decreased ability of the mutants to bind either the capture or the detection antibody compared with HB−, leading to an underestimate of fVIII mass and an overestimate of specific activity.
Results
Hydrophobic residues Met2199, Phe2200, Val2223, Leu2251, and Leu2252 in the C2 domain of fVIII have been proposed to participate in phospholipid membrane binding.11 Additionally, 4 basic residues, Arg2215, Arg2220, Lys2227, and Lys2249, which lie underneath these residues, may stabilize binding to negatively charged phosphatidylserine (PS) head groups. We reasoned that replacement of putative phospholipid binding residues with nonidentical homologous residues from porcine, murine, or canine fVIII would likely produce active molecules that could be evaluated with respect to their antigenicity toward C2-specific inhibitors.
Figure 1 shows the alignment of the human, porcine, murine, and canine fVIII C2 domains. At 4 of the 5 proposed hydrophobic phospholipid binding residues, there was 1 species that differs from human fVIII; these were Met2199 (Ile; porcine), Phe2200 (Leu; canine), Val2223 (Ala; canine), and Leu2252 (Phe; murine). There was a species difference in only 1 of the 4 proposed basic residues, ie, Lys2227 (Glu; porcine). Accordingly, we made Met2199Ile, Phe2200Leu, Val2223Ala, Leu2252Phe, and Lys2227Glu single mutants in human B-domainless fVIII. Additionally, we made 2 double mutants, Met2199Ile/Phe2200Leu (designated C2 D1) and Val2223Ala/Leu2252Phe (C2 D2), and a quadruple mutant, Met2199Ile/Phe2200Leu/Val2223Ala/Leu2252Phe (C2 Q). The locations of the mutated residues in the x-ray structure of fVIII are shown in Figure 2. Met2199/Phe2200 and Leu2251/Leu2252 project from 2 β-hairpin loops. Val2223 projects from an adjacent loop and is near Lys2227.
Mutated sites in the human fVIII C2 domain.
(A) Ribbon diagram showing hydrophobic residues proposed to be involved in phospholipid membrane binding and Lys2227, 1 of the 4 putative positively charged binding residues.11 Met2199, Phe2200, Val2223, Lys2227, and Leu2252 were mutated in this study. Leu2251, which is conserved in human, porcine, murine, and canine fVIII, was not mutated. (B) Space-filling model rotated, as if looking up from the membrane.
Mutated sites in the human fVIII C2 domain.
(A) Ribbon diagram showing hydrophobic residues proposed to be involved in phospholipid membrane binding and Lys2227, 1 of the 4 putative positively charged binding residues.11 Met2199, Phe2200, Val2223, Lys2227, and Leu2252 were mutated in this study. Leu2251, which is conserved in human, porcine, murine, and canine fVIII, was not mutated. (B) Space-filling model rotated, as if looking up from the membrane.
The mutants were stably expressed in serum-free medium from a cell line derived from baby hamster kidney and were then partly purified. The specific coagulant activities of the hybrids on ELISA were equal to or greater than the activity of HB−, indicating that the hybrids were suitable for antigenicity studies. The interaction of the mutants with C2-specific fVIII inhibitors was measured by using the Bethesda assay. The results were compared with those obtained with human B-domainless fVIII (HB−) and a hybrid human/porcine fVIII molecule, HP20, which is human except for substitution of the entire porcine C2 domain.12
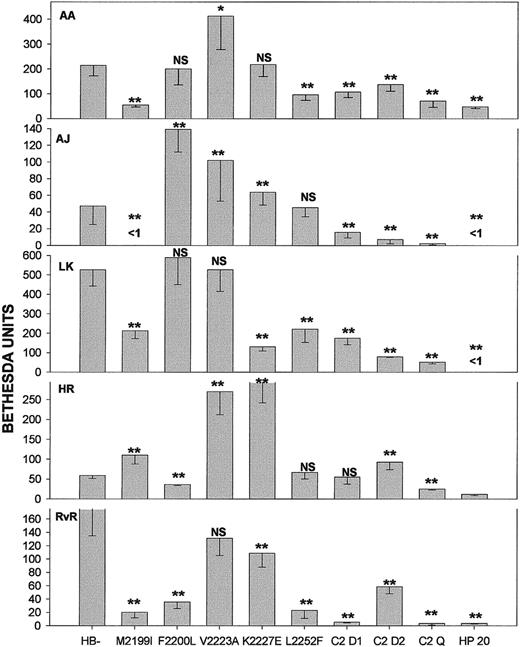
Most fVIII inhibitors are polyclonal IgG populations directed against epitopes both within and outside the C2 domain.2,21However, some inhibitors are C2 specific and are useful for evaluating the effects of substituting nonhuman sequences in the C2 domain.12 We studied C2-specific polyclonal inhibitors from 5 patients (AA, AJ, HR, LK, and RvR; Figure3). A reduction in antigenicity due to mutations at Met2199, Phe2200, Leu2252, or all 3 was always observed, although individual inhibitors varied with respect to the residues they recognized. Surprisingly, there was frequently a significant increase in Bethesda titer, most notably with the Val2223Ala mutant. The double mutant Met2199Ile/Phe2200Leu had low antigenicity toward all 5 antibodies, consistent with the fact that the antigenicity of Met2199Ile or Phe2200Leu was always reduced. Paradoxically, the double mutant Val2223Ala/Lys2227Glu showed a reduction in antigenicity toward all 5 polyclonal antibodies, even though, in 3 cases (AA, AJ, and HR), the corresponding individual mutants had unchanged or increased antigenicity. The antigenicity of the quadruple mutant Met2199Ile/Phe2200Leu/Val2223Ala/Lys2227Glu was equal to or lower than that of the single or double mutants. The antigenicity of HP20 was the lowest of all the mutants, a result consistent with the existence of antigenic residues in addition to Met2199, Phe2200, and Leu2252 that were not mutated in this study.
Bethesda titers of patient polyclonal anti-fVIII antibodies.
Recombinant fVIII was diluted in plasma from patients with hemophilia A, and Bethesda titers of antibodies AA, AJ, HR, LK, and RvR were determined. Shown are means and SDs determined by nonlinear least squares regression analysis. C2 D1 is the Met2199Ile/Phe2200Leu double mutant; C2 D2, the Val2223Ala/Lys2227Glu double mutant; and C2 Q, the Met2199Ile/Phe2200Leu/Val2223Ala/Lys2227Glu quadruple mutant. HP20 is a B-domainless hybrid human/porcine fVIII molecule containing human A1, A2, ap-A3, and C1 domains and the porcine C2 domain. Confidence levels for differences between the mutants and HB− are indicated at the 99.9% level (2 asterisks) and the 99% level (1 asterisk); NS indicates not significant.
Bethesda titers of patient polyclonal anti-fVIII antibodies.
Recombinant fVIII was diluted in plasma from patients with hemophilia A, and Bethesda titers of antibodies AA, AJ, HR, LK, and RvR were determined. Shown are means and SDs determined by nonlinear least squares regression analysis. C2 D1 is the Met2199Ile/Phe2200Leu double mutant; C2 D2, the Val2223Ala/Lys2227Glu double mutant; and C2 Q, the Met2199Ile/Phe2200Leu/Val2223Ala/Lys2227Glu quadruple mutant. HP20 is a B-domainless hybrid human/porcine fVIII molecule containing human A1, A2, ap-A3, and C1 domains and the porcine C2 domain. Confidence levels for differences between the mutants and HB− are indicated at the 99.9% level (2 asterisks) and the 99% level (1 asterisk); NS indicates not significant.
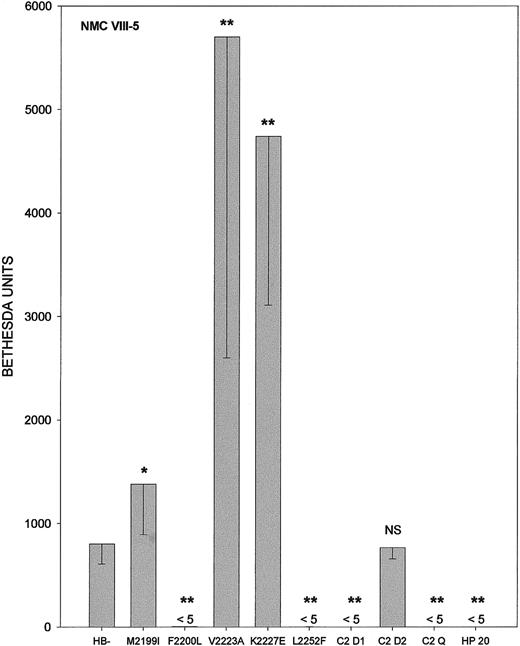
The Bethesda titers of antibodies BO2C1113 and NMC VIII-522 toward HB− and the mutant fVIII molecules are shown in Figure 4 and Figure5, respectively. BO2C11 is a C2-specific human IgG4κ monoclonal antibody derived from transformed B cells from a patient with hemophilia A and inhibitor. It is the only C2-specific human antibody that has been cloned to date. BO2C11 and NMC VIII-5 both recognize the C2 domain of fVIII and inhibit the binding of fVIII to vWF and phospholipid. NMC VIII-5 can compete for the binding of human polyclonal inhibitors to fVIII. The results with BO2C11 and NMC VIII-5 were similar to those obtained with polyclonal antibody HR (Figure 3). In all 3 antibodies, Phe2200 was antigenic, whereas Val2223 and Lys2227 appeared to reduce antigenicity.
Bethesda titers of patient monoclonal antibody BO2C11.
Abbreviations and notations are as described in the legend for Figure 3.
Bethesda titers of patient monoclonal antibody BO2C11.
Abbreviations and notations are as described in the legend for Figure 3.
Bethesda titers of murine monoclonal antibody NMC VIII-5.
Abbreviations and notations are as described in the legend for Figure 3.
Bethesda titers of murine monoclonal antibody NMC VIII-5.
Abbreviations and notations are as described in the legend for Figure 3.
Discussion
Although the x-ray structure of the human fVIII C2 domain was obtained in the absence of phospholipid, 3 hydrophobic feet, consisting of the side chains of Met2199/Phe2200, Val2223, and Leu2251/Leu2252, are present and were postulated to penetrate the membrane bilayer.11 Several positively charged residues, including Lys2227, which may stabilize membrane binding by means of electrostatic interactions with negatively charged PS head groups, surround these residues. Human C2-specific fVIII inhibitors exert their anticoagulant effect by blocking the binding of fVIII to PS.4 Therefore, epitope mapping of these inhibitors is an independent way to identify putative membrane-binding sites.
We prepared a series of recombinant hybrid fVIII molecules in which Met2199, Phe2200, Val2223, Lys2227, Leu2252, or a combination of these were replaced by homologous residues in porcine, murine, or canine fVIII. As anticipated, these conservative replacements did not lead to loss of fVIII coagulant activity. This is important because loss of antigenicity could otherwise be due to protein misfolding, which would not necessarily indicate that mutated residues are part of the antibody epitope. Mutations at Met2199, Phe2200, Leu2252, or a combination of these were associated with a decrease in antigenicity in most of the 7 antibodies tested (Table 1) and the decrease was frequently pronounced (Figures 3-5). This finding is consistent with the hypothesis that the Met2199/Phe2200 and Leu2251/Leu2252 loops participate in membrane binding. Even though all 7 inhibitors recognized the M2199/Phe2200 loop, the effects of mutations at M2199 and PHE2200 often differed. For example, Met2199Ile showed decreased antigenicity and Phe2200Leu had increased antigenicity toward antibody AJ, whereas the opposite was true for BO2C11. Thus, the amino acid specificity of AJ and BO2C11 varied, although both recognized the Met2199/Phe2200 loop.
Antigenicity of fVIII C2 mutants toward C2-specific inhibitory antibodies compared with human fVIII
| Mutant . | Antigenicity* . | ||
|---|---|---|---|
| Less . | Equal . | More . | |
| Met2199Ile | 4/7 | 0/7 | 3/7 |
| Phe2200Leu | 4/7 | 2/7 | 1/7 |
| Val2223Ala | 0/7 | 2/7 | 5/7 |
| Lys2227Glu | 2/7 | 1/7 | 4/7 |
| Leu2252Phe | 4/7 | 3/7 | 0/7 |
| Met2199Ile/Phe2200Leu | 6/7 | 1/7 | 0/7 |
| Val2223Ala/Lys2227Glu | 4/7 | 1/7 | 2/7 |
| Met2199Ile/Phe2200Leu Val2223Ala/Lys2227Glu | 7/7 | 0/7 | 0/7 |
| HP20 | 7/7 | 0/7 | 0/7 |
| Mutant . | Antigenicity* . | ||
|---|---|---|---|
| Less . | Equal . | More . | |
| Met2199Ile | 4/7 | 0/7 | 3/7 |
| Phe2200Leu | 4/7 | 2/7 | 1/7 |
| Val2223Ala | 0/7 | 2/7 | 5/7 |
| Lys2227Glu | 2/7 | 1/7 | 4/7 |
| Leu2252Phe | 4/7 | 3/7 | 0/7 |
| Met2199Ile/Phe2200Leu | 6/7 | 1/7 | 0/7 |
| Val2223Ala/Lys2227Glu | 4/7 | 1/7 | 2/7 |
| Met2199Ile/Phe2200Leu Val2223Ala/Lys2227Glu | 7/7 | 0/7 | 0/7 |
| HP20 | 7/7 | 0/7 | 0/7 |
Significantly different at the 99% confidence level.
Previously, we used a series of recombinant hybrid human/porcine fVIII molecules to map a major determinant of the C2 epitope or epitopes to a segment bounded by residues Glu2181 to Val2243.12 The Met2199/Phe2200 loop is in this region. The Leu2251/Leu2252 loop was neither included nor excluded by this analysis because porcine fVIII also contains leucines at residues 2251 and 2252. Substitution of the entire porcine C2 domain into human fVIII, which produces a molecule designated HP20, was associated with antigenicity lower than that with the more limited substitutions made in the current study (Figures 3-5). This indicates that there are residues outside the Met2199/Phe2200 and Leu2251/Leu2252 loops that contribute to binding by C2 inhibitors.
Recently, x-ray structures of 2 conformations of the factor V (fV) C2 domain in the absence of phospholipid were described.23The researchers proposed a model for phospholipid membrane binding that involves a loop containing tryptophans at positions 26 and 27 (human fV C2-domain numbering), which are homologous to Met2199 and Phe2200 in fVIII. A considerable amount of evidence supports the involvement of this loop in phospholipid membrane binding. An inhibitory monoclonal antibody, HV-1, which blocks the binding of fV to PS, maps to this loop.24-26 Substitution of alanine for residues equivalent to Trp26 and Trp27 in fVa is associated with decreased binding to PS and loss of coagulant activity.24
Additionally, a loop containing Leu79, which is homologous to Leu2251 in fVIII, and a loop containing residues Asn39 to Asn45 were also proposed to participate in phospholipid membrane penetration because of proximity to the Trp26/Trp27 loop.23 The fVIII segment that is homologous to the Asn39 to Asn45 loop, His211 to Asn217, has not been proposed as a hydrophobic phospholipid membrane-binding site.11 Conversely, the loop in fV that is homologous to the Val2223 loop in fVIII was not proposed to participate in phospholipid membrane penetration. In the current study, Val2223Ala and Lys2227Glu mutations were usually associated with an increase in antigenicity (Table 1). Thus, our results do not support the hypothesis that these residues participate in phospholipid membrane binding. However, it is possible that they bind phospholipid but are not often targeted by inhibitory antibodies.
The 2 fV C2 structures have different conformations, designated “open” and “closed,” which are associated with major movements of Trp26 and Trp27 at the phospholipid membrane-binding site.23 It was proposed that these states switch phospholipid membrane binding on and off, respectively.23The reduction in antigenicity associated with Val2223 and Lys2227 may occur because these residues stabilize a similar closed conformational state in fVIII that is associated with low-affinity membrane and antibody binding. Relaxation of this state by the Val2223Ala and Lys2227Glu mutations would then lead to high-affinity antibody binding. Alternatively, Val2223 and Lys2227 may simply interfere with an antigen-antibody lock-and-key interaction that involves high-affinity contacts with other fVIII residues (eg, Met2199, Phe2200, and so forth).
It is important to compare the human C2-specific monoclonal antibody, BO2C11, with polyclonal inhibitors because of the heterogeneity that may confound analysis of the inhibitors. The functional properties of BO2C11 are similar to those of the murine monoclonal antibody NMC VIII-5. Both antibodies inhibit the binding of fVIII to PS and vWf and promote dissociation of the fVIII-vWf complex.13 22 Our results indicate that Phe2200, but not Met2199, is an important part of the epitope recognized by both antibodies (Figures 4 and 5). However, NMC VIII-5 recognized Leu2252, whereas BO2C11 did not. Val2223 and Lys2227 reduced antigenicity with respect to both antibodies. Thus, BO2C11 and NMC VIII-5 appear to recognize overlapping but not identical epitopes.
The RvR antibody was obtained from a patient with hemophilia A who was part of an inhibitor “epidemic” that resulted from exposure to a heat-pasteurized fVIII product, CPS-A.27 In 1990 and 1991, C2-specific antibodies developed promptly after exposure to this product in several previously treated patients without inhibitors in The Netherlands and Belgium. RvR antibodies block the binding of fVIII to both PS and vWf.27 The RvR epitope maps to the N-terminal, Glu2181 to Val2243 region of the fVIII C2 domain recognized by most C2 inhibitors.12 The high-resolution mapping in the current study indicated that RvR is a typical C2 inhibitor that primarily recognizes the Met2199/Phe2200 and Leu2251/Leu2252 loops. Thus, the immunogenicity associated with CPS-A appears to be due to enhanced immune recognition of a normal immunodominant epitope rather than to development of a neoepitope.
Supported by a grant from the National Institutes of Health (R01-HL46215) to P.L.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Pete Lollar, 1639 Pierce Dr, Rm 1003, Woodruff Memorial Bldg, Emory University, Atlanta, GA 30322; e-mail:jlollar@emory.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal