Abstract
Sandhoff disease is a lysosomal storage disorder characterized by GM2 ganglioside accumulation in the central nervous system (CNS) and periphery. It results from mutations in the HEXB gene, causing a deficiency in β-hexosaminidase. Bone marrow transplantation (BMT), which augments enzyme levels, and substrate deprivation (using the glycosphingolipid biosynthesis inhibitor N-butyldeoxynojirimycin [NB-DNJ]) independently have been shown to extend life expectancy in a mouse model of Sandhoff disease. The efficacy of combining these 2 therapies was evaluated. Sandhoff disease mice treated with BMT and NB-DNJ survived significantly longer than those treated with BMT or NB-DNJ alone. When the mice were subdivided into 2 groups on the basis of their donor bone marrow–derived CNS enzyme levels, the high enzyme group exhibited a greater degree of synergy (25%) than the group as a whole (13%). Combination therapy may therefore be the strategy of choice for treating the infantile onset disease variants.
Introduction
The GM2 gangliosidoses are progressive, neurodegenerative lysosomal storage diseases.1 The hydrolysis of GM2 is catalyzed by β-hexosaminidase. The HEXA and HEXB genes encode the β-hexosaminidase α and β subunits, respectively.1 Mutations in the α and β subunit genes result in Tay-Sachs and Sandhoff diseases, respectively. Potential therapeutic approaches include enzyme augmentation (enzyme replacement, bone marrow transplantation [BMT], or gene therapy) and substrate deprivation.2,3 In BMT microglial cells of donor origin are thought to repopulate the brain, becoming perivascular macrophages and microglia, and to supply enzyme to neurones by secretion–recapture.4-6 Substrate deprivation utilizes an inhibitor of GSL biosynthesis, such asN-butyldeoxynojirimycin (NB-DNJ).7By partially inhibiting GSL biosynthesis, the residual enzyme activity can hydrolyze the reduced influx of substrate into the lysosome, thus preventing storage. NB-DNJ inhibits the ceramide glucosyltransferase (glucosylceramide synthase, UDP-glucose–N-acylsphingosine D-glucosyltransferase, EC2.4.1.80), which is the first enzyme in the GSL biosynthetic pathway.3,8 In a mouse model of Tay-Sachs disease,NB-DNJ treatment reduced GM2 accumulation in the brain.9 In the symptomatic mouse model of Sandhoff disease, NB-DNJ-treated mice10,11 survived 40% longer than untreated controls.10 BMT of Sandhoff mice extended life expectancy up to 8 months.12 Because the augmentation of enzyme through BMT and substrate deprivation may together show greater efficacy, we have treated Sandhoff mice with BMT and NB-DNJ and find that the 2 therapies act synergistically.
Study design
Animals, treatment procedures, and behavioral tests
Sandhoff mice were bone marrow transplanted at 10 to 16 days of age.12 Drug treatment was carried out as previously described.10,13NB-DNJ was a gift from Searle/Monsanto (St Louis, MO) and Oxford GlycoSciences (Abingdon, United Kingdom). Mice were tested on the bar crossing or inverted screen as previously described.10 The Open Field Test (box 45 × 25 × 12 cm with a 150 cm2 floor grid) was conducted according to published methods.14
Biochemical analysis
Statistical analysis
Survival graphs were analyzed by the log-rank or the Mantel-Haenszel test.15 Log-likelihood test was used for β-hexosaminidase enzyme level correlations. We used the Studentt test to analyze β-hexosaminidase, GSL levels, and locomotion scores. Bar-crossing and inverted-screen test data were analyzed using a nonparametric regression model with a logistic link to the data set. P values were estimated using likelihood ratio. The statistical software used was S-PLUS version 3.4 (MathSoft, Seattle, WA).
Results and discussion
Survival of Sandhoff mice receiving combination therapy
Bone marrow–transplanted Sandhoff (SH) mice were treated withNB-DNJ (600 mg/kg per day) from 10 to 11 weeks of age. Four groups were studied: untreated (SHUT), BMT monotherapy (SHBMT), NB-DNJ monotherapy (SHNB-DNJ), and combination therapy (BMT andNB-DNJ [SHBMT/NB-DNJ]). Mean survival time (Figure 1A) of the SHUTmice was 137 days. Survival was 166 days ± 4 (SHNB-DNJ), 196 days ± 8 (SHBMT), and 239 days ± 20 (SHBMT/NB-DNJ). All treated mice had significantly (P < .001) increased lifespans compared with SHUT mice (Figure 1A). SHBMT/NB-DNJ mice survived significantly (P < .001) longer than SHBMT or SHNB-DNJ mice. The effect of NB-DNJ on BMT was 13% more than additive (P < .089, Figure 1A). When the SHBMT mice were subdivided (50% of the mice into each category) on the basis of central nervous system β-hexosaminidase levels (2.26% ± 0.11% and 3.31% ± 0.94%, low- and high-enzyme groups, respectively; P = .108), the high-enzyme group exhibited 25% synergy (P < .001). The low-enzyme group exhibited an additive effect (P = .71) of NB-DNJ and BMT. Between the SHBMT and SHBMT/NB-DNJ groups, a similar level of β-hexosaminidase enzyme was measured in brain (P = .54) and spinal cord (P = .42) (Figure 1B) and in peripheral tissues (Figure 1C). In SHBMT, there was no correlation between survival and β-hexosaminidase enzyme levels in brain, spinal cord, or peripheral tissues (P < .226). However, in the SHBMT/NB-DNJ group, there was a significant correlation between survival and β-hexosaminidase levels in brain and spinal cord (P < .016, Pearson's correlation analysis) but not in liver, spleen, and kidney.
Survival of treated and untreated Sandhoff mice.
(A) Mice (n = 4-6 per group) were monitored daily, and a humane endpoint was applied when they became moribund and unable to right themselves when laid on their sides. In all the treated groups, the increase in survival relative to SHUT was statistically significant (P < .001). The synergistic effect was estimated using Bootstrap method. Synergy quotient (SQ) was defined asSQ = [(SHBMT − SHUT) + (SHNB − DNJ − SHUT)]/(SHBMT/NB − DNJ − SHUT), where SHUT,SHBMT,SHNB − DNJ, andSHBMT/NB − DNJare the mean survival of the Sandhoff mice untreated, treated with BMT, treated with NB-DNJ, and treated with BMT andNB-DNJ, respectively. If there is synergy, the SQ is less than one. (B) Analysis of β-hexosaminidase activity in nerve tissues and (C) in visceral organs of the Sandhoff mice after BMT treatment. Each organ was assayed in triplicate. The means for the different animals were averaged (± SEM) and expressed as a percentage of wild-type levels. n = 4 to 6 animals per group.
Survival of treated and untreated Sandhoff mice.
(A) Mice (n = 4-6 per group) were monitored daily, and a humane endpoint was applied when they became moribund and unable to right themselves when laid on their sides. In all the treated groups, the increase in survival relative to SHUT was statistically significant (P < .001). The synergistic effect was estimated using Bootstrap method. Synergy quotient (SQ) was defined asSQ = [(SHBMT − SHUT) + (SHNB − DNJ − SHUT)]/(SHBMT/NB − DNJ − SHUT), where SHUT,SHBMT,SHNB − DNJ, andSHBMT/NB − DNJare the mean survival of the Sandhoff mice untreated, treated with BMT, treated with NB-DNJ, and treated with BMT andNB-DNJ, respectively. If there is synergy, the SQ is less than one. (B) Analysis of β-hexosaminidase activity in nerve tissues and (C) in visceral organs of the Sandhoff mice after BMT treatment. Each organ was assayed in triplicate. The means for the different animals were averaged (± SEM) and expressed as a percentage of wild-type levels. n = 4 to 6 animals per group.
Neurologic function
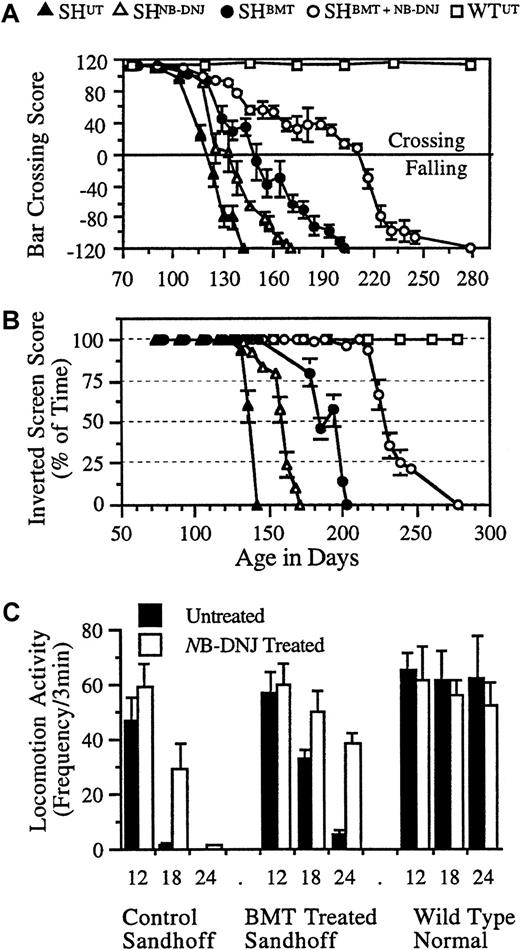
SHBMT, SHNB-DNJ, and SHBMT/NB-DNJ groups differed from the SHUTgroup in both the bar-crossing (P < .01) (Figure2A) and inverted-screen tests (P < .01) (Figure 2B). The SHBMT group performed better in the bar-crossing test than the SHNB-DNJgroup (P < .001), which performed better than the SHUT group (P < .05). The SHBMT/NB-DNJ group performed better than all other treatment groups (P < .023). The rate of decline in the SHBMT/NB-DNJ group was significantly (P < .01) slower than in all other groups. In the inverted-screen test, performance started to decline at approximately 127 days for SHUT but at approximately 140, 175, and 220 days for the SHNB-DNJ, SHBMT, and SHBMT/NB-DNJ groups, respectively. The rates of decline were similar across groups. All groups displayed similar locomotor activity at 12 weeks, comparable to wild-type mice (Figure 2C). However, at 18 and 24 weeks of age, there were significant differences in activity between SHUT and SHNB-DNJ in the BMT (P < .01) and the non-BMT (P < .001) groups (Figure 2C).
Representative neurologic function of treated and untreated Sandhoff mice.
Each animal was tested for its ability to cross a horizontal bar, measuring balance and motor co-ordination (A), and for the amount of time it spent upside down on an inverted screen, measuring muscle strength (B). Data are mean ± 0.4 of the SD. n = 4 to 6 animals per group. Locomotor activity of the NB-DNJ–treated and untreated mice was measured (C). Each animal at 12, 18, and 24 weeks of age was tested for locomotion in an “open-field.” Data are mean ± SD (n = 4-6 animals per group). Between treated and untreated mice, data are statistically significant at 18 weeks (P < .05) and 24 weeks (P < .01) of age in control and BMT-treated Sandhoff mice.
Representative neurologic function of treated and untreated Sandhoff mice.
Each animal was tested for its ability to cross a horizontal bar, measuring balance and motor co-ordination (A), and for the amount of time it spent upside down on an inverted screen, measuring muscle strength (B). Data are mean ± 0.4 of the SD. n = 4 to 6 animals per group. Locomotor activity of the NB-DNJ–treated and untreated mice was measured (C). Each animal at 12, 18, and 24 weeks of age was tested for locomotion in an “open-field.” Data are mean ± SD (n = 4-6 animals per group). Between treated and untreated mice, data are statistically significant at 18 weeks (P < .05) and 24 weeks (P < .01) of age in control and BMT-treated Sandhoff mice.
Glycosphingolipid analysis at end stage
GM2 and GA2 were measured at terminal stage of the disease (Table 1). In the brains of SHNB-DNJ, SHBMT, and SHBMT/NB-DNJ mice, the GA2 levels were comparable to those in SHUT (P ≤ .478), even though they lived longer than the controls. All treated groups had the same GA2 storage burden as the untreated mice, suggesting a slower rate of GA2 accumulation. The brain GM2levels were similar in the SHNB-DNJ, SHBMT, and SHUT mice (P ≤ .614), but the SHBMT/NB-DNJ mice had approximately 30% higher levels than the other groups (P ≤ .017). In spinal cord the GM2 storage levels were comparable to those in brain. However, the spinal cord GA2 levels were significantly lower in the SHBMT (21%, P < .001) and in the SHBMT/NB-DNJ (12%, P < .01) groups than in the SHUT group. This may be due to higher levels of donor enzyme in the spinal cord (approximately 12% of normal; Figure1B). In the SHNB-DNJ group, the liver GM2 level was reduced by approximately 17% (P < .01) whereas the GA2 level was comparable to that of the SHUT(P = .26) at end stage. However, in the SHBMTand SHBMT/NB-DNJ groups, the liver GSL composition approximated that of the wild type because of high donor-enzyme levels (Figure 1C).
Analysis of storage glycosphingolipids GM2 and GA2 at the terminal stage of disease in Sandhoff mice
| Tissue . | Storage GSLs (% untreated control) . | Therapeutic group . | ||
|---|---|---|---|---|
| SHNB-DNJ . | SHBMT . | SHBMT/NB-DNJ . | ||
| Brain | GM2 | 107.2 ± 10.4 | 104.6 ± 15.3 | 133.0 ± 18.7* |
| GA2 | 96.3 ± 7.8 | 94.7 ± 7.1 | 103.4 ± 1.5 | |
| Spinal cord | GM2 | 111.1 ± 3.1 | 98.3 ± 12.1 | 134.7 ± 18.1* |
| GA2 | 107.4 ± 4.1 | 79.9 ± 5.2* | 88.3 ± 8.1* | |
| Liver | GM2 | 82.7 ± 0.5* | 43.6 ± 4.4* | 40.1 ± 4.8* |
| GA2 | 104.7 ± 7.3 | ND | ND | |
| Tissue . | Storage GSLs (% untreated control) . | Therapeutic group . | ||
|---|---|---|---|---|
| SHNB-DNJ . | SHBMT . | SHBMT/NB-DNJ . | ||
| Brain | GM2 | 107.2 ± 10.4 | 104.6 ± 15.3 | 133.0 ± 18.7* |
| GA2 | 96.3 ± 7.8 | 94.7 ± 7.1 | 103.4 ± 1.5 | |
| Spinal cord | GM2 | 111.1 ± 3.1 | 98.3 ± 12.1 | 134.7 ± 18.1* |
| GA2 | 107.4 ± 4.1 | 79.9 ± 5.2* | 88.3 ± 8.1* | |
| Liver | GM2 | 82.7 ± 0.5* | 43.6 ± 4.4* | 40.1 ± 4.8* |
| GA2 | 104.7 ± 7.3 | ND | ND | |
Data are representative of 3 independent analyses of the same samples (n = 4-6 per group); ND corresponds to levels that are undetectable by high performance thin layer chromatography.
Significantly different from the control untreated values (see text for details).
The onset of symptoms and the rates of disease progression were delayed significantly in SHBMT/NB-DNJ relative to the monotherapy groups. By increasing the level of enzyme, the therapeutic response toNB-DNJ was enhanced. In brain, β-hexosaminidase activity from BMT was relatively small (approximately 2% to 5% of normal). The survival increase in the SHBMT/NB-DNJ group was 13% more than the sum of the 2 monotherapy effects, indicating synergy. The survival of the combination group revealed 2 cohorts, those that survived the longest and those that died at an earlier time point. Survival correlated with the enzyme level in the brain. Synergy was approximately 25% for the high enzyme group compared to 13% for the group as a whole. The additional brain enzyme reconstitution needed to achieve approximately 25% synergy was only approximately 1.05% of the wild-type level. Mechanisms for the clinical benefit of BMT may extend beyond augmentation of enzyme levels. This may involve an inflammatory component with activated microglia contributing to neuronal dysfunction or death, as has been proposed for Alzheimer disease. The replacement of diseased microglial cells with wild-type cells by BMT, in combination with direct substrate reduction in neurones byNB-DNJ, may have led to synergy.
Glycosphingolipid analysis at terminal stage showed that for SHBMT/NB-DNJ there was an increased storage burden of GM2 and enhanced survival. This suggests that there is not a simple threshold level of brain GM2 storage, which leads to disease. It supports a more complex mechanism of pathogenesis, as suggested previously.12 Levels of GA2 are similar in all groups at their respective endpoints, suggesting that in the mouse there may be a threshold level of GA2 that elicits disease. It is unclear why there is no elevated storage in the BMT group, despite their enhanced survival. After approximately 4 months it is possible that the rate of synthesis is lowered because of neuronal dysfunction, death, or both or that BMT only impacts the storage levels later in the disease course.
In conclusion, NB-DNJ therapy shows promise in treating GSL storage diseases, which involve neuropathology. These 2 therapeutic approaches act synergistically, provided that a high enough level of enzyme reconstitution can be achieved.
Acknowledgments
We thank Searle/Monsanto and Oxford GlycoSciences forNB-DNJ, Julia McAvoy and David Smith for excellent technical assistance, and Rob Deacon for his advice on behavioral tests.
F.M.P. is a Lister Institute Research Fellow. M.J. is supported by a Biotechnology and Biological Science Research Council studentship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Frances M. Platt, Department of Biochemistry, University of Oxford, South Parks Road, Oxford OX1 3QU, United Kingdom; e-mail: fran@glycob.ox.ac.uk.

![Fig. 1. Survival of treated and untreated Sandhoff mice. / (A) Mice (n = 4-6 per group) were monitored daily, and a humane endpoint was applied when they became moribund and unable to right themselves when laid on their sides. In all the treated groups, the increase in survival relative to SHUT was statistically significant (P < .001). The synergistic effect was estimated using Bootstrap method. Synergy quotient (SQ) was defined asSQ = [(SHBMT − SHUT) + (SHNB − DNJ − SHUT)]/(SHBMT/NB − DNJ − SHUT), where SHUT,SHBMT,SHNB − DNJ, andSHBMT/NB − DNJare the mean survival of the Sandhoff mice untreated, treated with BMT, treated with NB-DNJ, and treated with BMT andNB-DNJ, respectively. If there is synergy, the SQ is less than one. (B) Analysis of β-hexosaminidase activity in nerve tissues and (C) in visceral organs of the Sandhoff mice after BMT treatment. Each organ was assayed in triplicate. The means for the different animals were averaged (± SEM) and expressed as a percentage of wild-type levels. n = 4 to 6 animals per group.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/1/10.1182_blood.v97.1.327/6/m_h80110544001.jpeg?Expires=1769161824&Signature=JY1UacRhkGNIzf3HYRX0k8-0TZfwD8iJHpjZvJ4RXly~GVubyqQW99qMROIwV9Y94q3311Hdt36FGymni3pwEh71ZIqatmdPGk5qf8r3Lp9q2g3XDZmZgRWgUUddkD7-uoSFhmdlHcdwiYEhSVZB7icluNwcP47GQ~7PNhP64iF3Wj25eAtX4v9hmlMD3030ZGFfxviAnHwl7A3-tso6j5IMWt2ni5jRtf-bD7yQPUWoL0KJ7ygaUZ3jz6UexxaJU7DgHKprU5-PGrmL1t5FHXGxSnPdszdcdfCJUlBafkLx35YJdHw2~IzxRIL05jCmTa5r~OtM~FkCnB84VuH8WA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal