Abstract
Shwachman-Diamond syndrome (SDS) is an inherited bone marrow disorder with varying cytopenias and a strong predilection to myelodysplastic syndrome (MDS) and acute myeloid leukemia. Previously, it was found that the percentage of CD34+ cells in bone marrow and the in vitro colony formation from CD34+ cells of patients with SDS were markedly reduced. For these reasons, and because apoptosis is central in the pathogenesis of bone marrow dysfunction in MDS, this study was initiated to delineate the role of apoptosis in the pathogenesis of the marrow failure. Eleven children with SDS were studied. Compared to normal controls, patients' marrow mononuclear cells plated in clonogenic cultures showed a significantly higher tendency to undergo apoptosis. The defect in SDS was found in patients with and without MDS. Patients showed a more prominent decrease in colony formation and increased apoptosis after preincubation with activating anti-Fas antibody. Fas expression on marrow cells from patients was significantly higher than from normal controls. The difference between patients and controls for Fas expression was also significant for the following cell fraction subpopulations: CD34−/CD38−, CD34−/CD38+, and CD34+. In conclusion, SDS hematopoietic progenitors are intrinsically flawed and have faulty proliferative properties and increased apoptosis. Bone marrow failure in SDS appears mediated by increased apoptosis as the central pathogenetic mechanism. This increased propensity for apoptosis is linked to increased expression of the Fas antigen and to hyperactivation of the Fas signaling pathway.
Introduction
Shwachman-Diamond syndrome (SDS) is an inherited multisystemic disorder characterized by varying degrees of bone marrow hypoplasia with cytopenias.1-3 Furthermore, it has been noted recently that patients with the syndrome have a marked propensity for myelodysplastic syndrome (MDS) and acute myeloblastic leukemia (AML); these occur in up to one third of the patients.1-6The genetic or molecular defects of the disease and the factors that predispose patients to these complications are still unknown. We have previously shown that the bone marrow of patients with SDS is characterized by a decreased frequency of CD34+ cells and that marrow CD34+ cells have a reduced ability to form hematopoietic colonies in vitro.7 In the study described herein, we investigated whether these conditions are due to increased programmed cell death (apoptosis).
Recently, apoptosis has surfaced as an important mechanism in the development of several congenital hypoplasia syndromes, such as renal hypoplasia8 and spinal muscular atrophy.9Furthermore, preliminary studies suggested that in some patients with 3 other inherited bone marrow failure syndromes—Diamond-Blackfan anemia,10 Fanconi anemia,11-14 and myelokathexis15—apoptosis plays a role in pathogenesis. In addition, programmed cell death is central in the pathogenesis of MDS, to which patients with SDS are predisposed. Increased apoptosis of bone marrow precursors contributes to ineffective hematopoiesis in a large proportion of adult patients with MDS16-20; the highest rates occur in patients with advanced stages of MDS.
Fas-induced apoptosis has been recognized as a central apoptosis pathway.21 Fas is a membrane glycoprotein member of the tumor necrosis factor receptor family, which contains an integral death domain within its cytoplasmic tail. After binding of the Fas ligand or activation of the anti-Fas antibodies, Fas-associated death domain (FADD) is recruited to the integral death domain of Fas.22 In turn, a death effector domain of FADD binds to procaspase 8.23 Consequently the trimerization of Fas-FADD–procaspase 8 results in the cleavage of procaspase 8, the generation of an active caspase 8, and further activation of the apoptosis pathway.
It has been reported that normal human hematopoietic progenitors do not express Fas.24 However, increased Fas expression on marrow cells from 40% of patients with MDS25,26 was demonstrated, though overexpression of the Fas antigen was not associated with a clear increase of Fas-mediated apoptosis in these patients after the addition of a functional anti-Fas antibody.25 It was also shown that fractional Fas expression by hematopoietic progenitors from patients with aplastic anemia is higher than in hematopoietic progenitors from normal controls27 and that interferon-γ might induce Fas expression on CD34+ cells and apoptosis of CD34+ progenitors.28
For these reasons, we initiated a study to test our hypothesis that apoptosis is central to the pathogenesis of the marrow dysfunction in SDS. We also investigated whether the presumed increased apoptosis in the disease is mediated through the Fas signaling pathway.
Patients, materials, and methods
Patients were included if they fulfilled the diagnostic criteria of our institution.1 In brief, patients were included if they had at least one of the following criteria for exocrine pancreatic dysfunction: abnormal findings on quantitative pancreatic stimulation test, low serum cationic trypsinogen levels, abnormal findings on 72-hour fecal fat analysis plus evidence of pancreatic lipomatosis by ultrasonography or computed tomography, and at least one of these hematologic abnormalities—neutropenia, marrow granulocytic hypoplasia, hypoplastic bone marrow, or myelodysplastic syndrome. Accordingly, 11 patients were included in this study, which was conducted from February 1997 to January 1999 (Table 1). No patient was transfusion dependent or had MDS or AML that had been diagnosed previously. However, during the study, an unsuspected marrow cytogenetic abnormality of clonal isochromosome (7q) was unmasked in one patient (UPN 1).4 Results of patients' marrow studies were compared to those of 11 hematologically healthy bone marrow donors. Consent for the study was obtained from all patients or their parents and from all controls.
Hematologic characteristics of patients with Shwachman-Diamond syndrome at the time of the study
| UPN . | Age (y)/sex . | Blood counts . | Bone marrow cellularity . | Cytogenetics . |
|---|---|---|---|---|
| 1 | 7/M | N, A, T | ⇓ | 46XY,i(7q) |
| 3 | 14/M | N | ⇓ | 46,XY |
| 4 | 16/F | N, T | ⇓ | 46,XX |
| 5 | 13/F | N, A | ⇓ | 46,XX |
| 6 | 2/M | N, A | UA | 46,XY |
| 7 | 14/F | N, A, T | ⇓ | 46,XX |
| 8 | 18/M | N, A, T | ⇓ | 46,XY |
| 11 | 8/M | N, T | ⇓ | 46,XY |
| 12 | 2/F | N | n | 46,XX |
| 14 | 11/M | N, A, T | UA | 46,XY |
| 15 | 2/F | N, A, T | ⇓ | 46,XX |
| UPN . | Age (y)/sex . | Blood counts . | Bone marrow cellularity . | Cytogenetics . |
|---|---|---|---|---|
| 1 | 7/M | N, A, T | ⇓ | 46XY,i(7q) |
| 3 | 14/M | N | ⇓ | 46,XY |
| 4 | 16/F | N, T | ⇓ | 46,XX |
| 5 | 13/F | N, A | ⇓ | 46,XX |
| 6 | 2/M | N, A | UA | 46,XY |
| 7 | 14/F | N, A, T | ⇓ | 46,XX |
| 8 | 18/M | N, A, T | ⇓ | 46,XY |
| 11 | 8/M | N, T | ⇓ | 46,XY |
| 12 | 2/F | N | n | 46,XX |
| 14 | 11/M | N, A, T | UA | 46,XY |
| 15 | 2/F | N, A, T | ⇓ | 46,XX |
UPN indicates unique patient number; N, neutropenia (neutrophil concentration < 1.5 × 109/L); A, anemia (hemoglobin concentration < 2 SD below mean, adjusted for age)29; n, normal; T, thrombocytopenia (platelet count < 150 × 109/L); ⇓, decreased; UA, unavailable.
Bone marrow samples
Bone marrow aspirates were collected from the posterior superior iliac crest to an α medium containing 10 U/mL preservative-free heparin, layered over Percoll or Ficoll-Hypaque (according to the objective of the experiment), and centrifuged for either 10 minutes at 2000 rpm or 25 minutes at 1600 rpm, respectively. Light-density cell fraction was collected and washed twice in Iscove medium. Depending on the experiment (see below), cells were used fresh or were cryopreserved in dimethyl sulfoxide in liquid nitrogen until use.
CD34+ cell purification
Light-density mononuclear cells obtained after Percoll separation underwent CD34+ enrichment by the Mini-MACS immunomagnetic separation system (Miltenyi Biotec, Auburn, CA) as previously described.7 After Percoll separation, mononuclear cells were incubated with biotinylated anti-CD34 antibodies. After they were washed, cells were incubated with Streptavidin Microbeads (Miltenyi Biotec, Auburn, CA), washed again, and transferred through a magnetic column. A purification of approximately 98% was achieved by passing cells through a second magnetic column.
Clonogenic assays of highly enriched marrow CD34+cells or mononuclear cells
After they were thawed, cells were plated in triplicate at a density of 2 × 105 marrow mononuclear cells/1 mL dish or 3 × 103 CD34+ cells/1 mL dish containing 1.9 mL 0.8% methylcellulose (Fluka, Buchs, Switzerland), 0.9 mL fetal calf serum, 10−5 M 2-mercaptoethanol (Sigma, St Louis, MO), 40 U/mL IL-3 (Immunex, Seattle, WA), 10 ng/mL granulocyte-colony stimulating factor (Amgen, Thousand Oaks, CA), 50 ng/mL mast cell growth factor (Immunex), and 2 U/mL erythropoietin (Ortho Biologics, Manati, Puerto Rico). Cultures were incubated at 37°C in a humidified atmosphere (5% CO2 and air). Colonies of 50 cells or more were scored after 13 days under an inverted microscope.
Percentage of apoptotic cells in clonogenic assays of highly enriched marrow CD34+ cells or mononuclear cells
We assessed apoptosis after incubating the cells in cultures for 7 days because we were mainly interested in evaluating CD34+ cell-derived early proliferating progenitors.30 In addition, assaying the patients' cultures at an earlier stage was impossible because of low numbers of proliferating cells, consistent with marrow dysfunction. After 7 days, one of each of the triplicate dishes was analyzed for the percentage of apoptotic cells, as previously described.31 Cultured cells were collected after dissolving the methylcellulose by incubation in the culture dishes with Iscove medium. To a 1 × 105 cell mixture, 10 μL fluorescein isothiocyanate (FITC)-conjugated annexin V (R&D Systems, Minneapolis, MN) and 10 μL propidium iodide (PI; R&D Systems) were added, and cells were incubated in the dark for 15 minutes at room temperature. Subsequently, binding buffer (R&D Systems) was added, and the cells were immediately analyzed by Coulter Epics XL-MCL flow cytometer (Coulter, Hialeah, FL). A control tube of unstained cells was run first, and markers were set on a forward scatter/side scatter cell dot plot, gating out large aggregates and small particles. Then samples stained with annexin V only or PI only were analyzed, and spilling-over signals were subtracted by increasing the corresponding percentage of compensation. Quadrant statistics for annexin V−/annexin V+ and PI−/PI+ populations were then set. The test tube was analyzed last. Events falling outside the negative staining regions identified by the control samples were considered positive staining for either annexin V only (apoptosis) or annexin V and PI (late apoptosis/necrosis).
Effect of anti-Fas antibody on colony formation
When cells were plated, activating mouse antihuman Fas IgM antibody (clone CH-11; Immunotech, Marseilles, France) was added at a concentration of 2 μg/mL to one of the triplicate clonogenic assays of CD34+ cells described above. Cultures with or without the antibody were incubated at 37°C in a humidified atmosphere (5% CO2 and air). CFU-GM colonies (containing 50 cells or more) were scored after 14 days under an inverted microscope. In addition, 500 ng/mL blocking mouse antihuman Fas IgM antibody (clone ZB4; Immunotech) was added to duplicate clonogenic assays of marrow mononuclear cells (2 × 105 cells per culture dish) from a patient (UPN 8, Table 1) to control for the role of Fas ligation in apoptosis.
Effect of activating anti-Fas antibody on apoptosis rate of cultured marrow mononuclear cells
After 13 days in culture, 2 mL Iscove medium with activating mouse antihuman Fas IgM antibody (clone CH-11; Immunotech) was added for 18 hours to one of the triplicate clonogenic assays of marrow mononuclear cells so that the final concentration of the antibody reached 2 μg/mL. One of the triplicate cultures served as a control to which 2 mL Iscove medium was added without the antibody. Eighteen hours later, cells were harvested and assayed for apoptosis as described above.
Fas expression on marrow cell subpopulations
Freshly obtained bone marrow cells were layered over Ficoll and centrifuged for 25 minutes at 1600 rpm. Light-density cell layer was collected and washed in Iscove medium and then washed twice in phosphate-buffered saline. To 1 × 105 cell mixtures the following antibody combinations were added (all purchased from Immunotech): phycoerythrin (PE)-conjugated mouse antihuman Fas IgM monoclonal antibody (clone 7C11, 8 μL/105 cells); FITC-conjugated mouse antihuman CD34 IgG1 monoclonal antibody (clone 581, 4 μL/105 cells); PE–cyanine 5 (PC5)–conjugated mouse antihuman CD38 IgG1 monoclonal antibody (clone LS198, 4 μL/105 cells); PE-conjugated IgM mouse isotype control (clone GC323, 8 μL/105 cells); FITC-conjugated IgG1 mouse isotype control (clone 679.1Mc7, 4 μL/105 cells) and PC5-conjugated IgG1 mouse isotype control (clone 679.1Mc7, 4 μL/105 cells); PE-conjugated IgG1 isotype control (8 μL/105 cells), FITC-conjugated anti-CD34 antibody (4 μL/105 cells), and Cy5-conjugated anti-CD38 antibody (4 μL/105 cells); PE-conjugated anti-Fas antibody (8 μL/105 cells), FITC conjugated anti-CD34 antibody (4 μL/105 cells), and PC5-conjugated anti-CD38 antibody (4 μL/105 cells).
Cells were incubated with the antibody in the dark at 4°C for 30 minutes, then washed twice with phosphate-buffered saline and analyzed by a Coulter Epics XL-MCL flow cytometer. At least 50 000 events were collected.
Statistics
Colony numbers in clonogenic assays and Fas expression on hematopoietic cells from patients and controls were expressed as mean ± SEM. Student t tests were used to determine the statistical significance of differences between apoptotic cell proportions and cells expressing apoptosis-related proteins of patients compared to controls. The correlation between the number of colonies and the proportion of apoptotic cells was calculated using the Fisher exact test. P < .05 was considered significant.
Results
Apoptosis rate of cultured marrow CD34+ cells or mononuclear cells
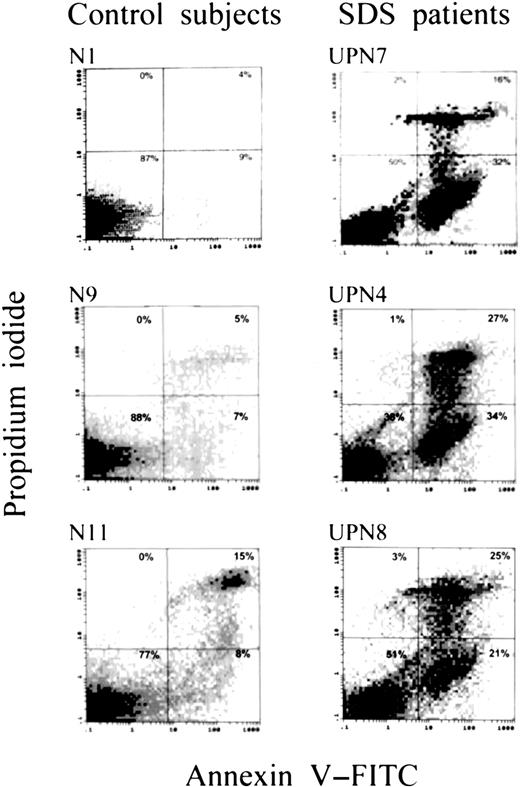
Compared with those of 11 normal controls, bone marrow mononuclear cells plated in methylcellulose cultures from 7 patients showed an increased percentage of apoptotic cells by the annexin V/PI assay (37% ± 3% vs 13% ± 2% in controls; P < .05); a higher percentage of late apoptotic/necrotic cells (18% ± 3.1% vs 6.4% ± 3.1%; P < .01); and a markedly lower percentage of viable cells (53% ± 7.2% vs 79% ± 3.1%;P < .01) (Figures 1,2). Representative dot plots of apoptosis analysis from 3 patients and 3 normal controls are shown in Figure 1, illustrating larger populations of apoptotic cells and lower populations of viable cells in the patients. Furthermore, in one patient with SDS and secondary MDS (UPN 1), the proportion of cells undergoing apoptosis (43%) exceeded the range of the patients with SDS without MDS (mean, 24.1%; range, 10%-38%). Interestingly, there was no correlation between colony numbers and apoptosis rate by the Fisher exact test.
Apoptosis rate of cultured marrow mononuclear cells.
Representative dot plots of apoptosis analysis of marrow cells from 3 patients and 3 control subjects after 7 days of incubation, showing larger populations of apoptotic cells and smaller populations of viable cells in the patients.
Apoptosis rate of cultured marrow mononuclear cells.
Representative dot plots of apoptosis analysis of marrow cells from 3 patients and 3 control subjects after 7 days of incubation, showing larger populations of apoptotic cells and smaller populations of viable cells in the patients.
Apoptosis rate of cultured marrow mononuclear cells.
Apoptosis rates (mean ± SEM) of cultured marrow cells from 7 patients with SDS (▪) and 11 control subjects (■) after 7 days of incubation, obtained using an annexin V/PI method.
Apoptosis rate of cultured marrow mononuclear cells.
Apoptosis rates (mean ± SEM) of cultured marrow cells from 7 patients with SDS (▪) and 11 control subjects (■) after 7 days of incubation, obtained using an annexin V/PI method.
Effect of anti-Fas antibody on colony formation
To evaluate the sensitivity of patients' marrow cells to Fas ligand, the effect of adding activating anti-Fas antibody was investigated on the in vitro colony growth of marrow CD34+cells. The addition of activating anti-Fas antibody to clonogenic assays of CD34+ cells of patients decreased CFU-GM colony formation by 72% ± 8%. In contrast, the decrement in CFU-GM colony formation in normal controls was only 40% ± 12% (P < .05) (Figure 3A). When blocking anti-Fas antibody was added to clonogenic assays of marrow mononuclear cells from a patients with SDS (UPN 8), the BFU-E colony production increased from 1 (±1) per 2 × 105 cultured cells to 27 (±5) per 2 × 105 cells, and the CFU-GM increased from 0 per 2 × 105 cells to 18 (±5) per 2 ± 105 cells.
Effect of activating anti-Fas antibody on colony formation and apoptosis.
Effect of activating anti-Fas antibody in clonogenic assays from patients with SDS and control subjects. (A) Reduction in colony formation when CD34+ cells were plated in cultures containing the antibody compared to cultures without the antibody. (B) Fold increment in apoptosis rates after 18 hours' incubation with the antibody (mean ± SEM).
Effect of activating anti-Fas antibody on colony formation and apoptosis.
Effect of activating anti-Fas antibody in clonogenic assays from patients with SDS and control subjects. (A) Reduction in colony formation when CD34+ cells were plated in cultures containing the antibody compared to cultures without the antibody. (B) Fold increment in apoptosis rates after 18 hours' incubation with the antibody (mean ± SEM).
Effect of activating anti-Fas antibody on apoptosis rate of cultured marrow mononuclear cells
The effect of added activating anti-Fas antibody on the apoptosis rate of cultured marrow mononuclear cells in clonogenic assays was also investigated. Compared to normal controls, patients with SDS showed a more prominent increase in apoptosis (110% ± 40% vs 25% ± 25%; P = .08) after adding anti-Fas antibody for 18 hours before harvest (Figure 3B).
Fas expression on marrow cell subpopulations
Fas expression on post-Ficoll marrow cells from patients was significantly higher than on those of normal controls (28% ± 4% vs 13% ± 2%; P < .05) (Figure4). The difference between patients and controls was also statistically significant when the subpopulation of mononuclear cells was gated and analyzed 27.0% ± 2.6% vs 15% ± 1.8%; P < .01) (Figure 4). Fas expression on the granulocytic cell fractions in patients and controls was relatively low compared to that on mononuclear cells. Although the mean Fas expression on patients' granulocytic cells was double that of normal controls, the difference was not statistically significant (8.1% ± 2.7% vs 4.0% ± 1.6%; P = .17) (Figure4). Variability of Fas expression on neutrophils among the patients was high. Two patients with mild to moderate neutropenia at the time of the study (UPN 3, UPN 4) showed low levels of Fas expression—0.1% and 4.2%, respectively. The 6 other patients showed expression ranging from 12.3% to 37.1%. The range of Fas expression on neutrophils in normal controls was 0% to 13.6%.
Fas expression on marrow granulocytes and mononuclear cells.
Representative histograms of Fas expression on marrow cells from 3 patients with Shwachman-Diamond syndrome and 3 control subjects, showing larger populations especially of mononuclear cells expressing Fas in the patients.
Fas expression on marrow granulocytes and mononuclear cells.
Representative histograms of Fas expression on marrow cells from 3 patients with Shwachman-Diamond syndrome and 3 control subjects, showing larger populations especially of mononuclear cells expressing Fas in the patients.
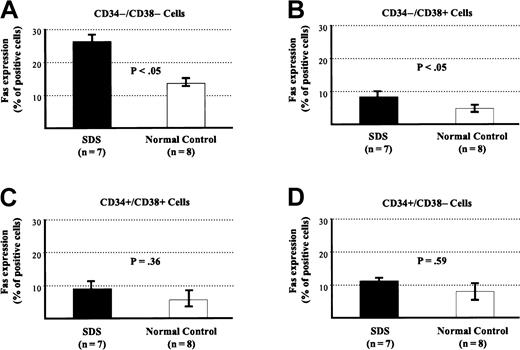
To determine at which stage of development cells acquire Fas antigen, subpopulations of marrow cells were studied for the expression of the hematopoietic markers CD34 and CD38. Cell populations at various maturation stages were first identified on a dual-staining flow cytometric histogram by their staining pattern for each of these 2 antigens. Then each cell population was gated and analyzed for the expression of Fas antigen (Figures 5,6). The difference in Fas expression between patients and controls was significant when mature cells, namely CD34−/CD38− (27% ± 5% vs 13% ± 3%;P < .05) and CD34−/CD38+(8% ± 1.1% vs 4.5% ± 1.2%; P < .05) cells were analyzed. Furthermore, Fas expression on more primitive cells (CD34+ cells) from patients and controls was higher than from normal controls (14.8% ± 2.8% vs 5.9% ± 1.6%;P < .05). When subpopulations of CD34+ cells were evaluated separately, the numbers of events obtained by flow cytometry from these rare populations were low. Differences in Fas expression, though higher, were not statistically significant: CD34+/CD38− (11.3% ± 4% vs 8.6% ± 1.6%; P = .59) and CD34+/CD38+ (8.5% ± 2.1% vs 5.2% ± 3%; P = .36).
Fas expression on marrow CD34+/CD34− cells.
Fas expression marrow cells from patients with SDS and control subjects. (A) After Ficoll light-density cell fraction. (B) CD34+ cells. (C) CD34− cells.
Fas expression on marrow CD34+/CD34− cells.
Fas expression marrow cells from patients with SDS and control subjects. (A) After Ficoll light-density cell fraction. (B) CD34+ cells. (C) CD34− cells.
Fas expression on various marrow cell subpopulations expressing or not expressing CD34/CD38.
Fas expression of various marrow cell subpopulations from patients with SDS and control subjects. (A) CD34−/CD38−. (B) CD34−/CD38+. (C) CD34+/CD38+. (D) CD34+/CD38−.
Fas expression on various marrow cell subpopulations expressing or not expressing CD34/CD38.
Fas expression of various marrow cell subpopulations from patients with SDS and control subjects. (A) CD34−/CD38−. (B) CD34−/CD38+. (C) CD34+/CD38+. (D) CD34+/CD38−.
Because of the hypoplastic nature of the SDS marrow specimens, it was impossible to conduct all the experiments on all patients. However, in those patients from whom sufficient cells were aspirated, several experiments were performed, and no inconsistencies were found between apoptosis rates, Fas expression, or sensitivity to anti-Fas antibody. For example, 3 patients (UPN 1, UPN 4, and UPN 7) underwent apoptosis and Fas expression analysis. In all 3 patients, Fas expression on marrow samples (27%-45%) was higher than the range of normals (4%-19%), and apoptosis rates (32%-43%) were higher than those of normals (3%-27%). Cultured marrow cells from 2 patients (UPN 1, UPN 4) were also studied for the increment in apoptosis after stimulation with activating anti-Fas antibody and were found to have a 194% to 278% increase compared to a 130% to 140% increase in normals. Only one patient (UPN 3) had low Fas expression (16%) on the marrow sample. This patient had only a 139% increment in apoptosis rate after stimulation with activating anti-Fas antibody, which was comparable to that in normals. For the other patients, only the mean values from patients were compared to the means from normals, as described.
Discussion
Various degrees of cytopenia are found virtually in all patients with SDS.1,7 We have previously shown that the cytopenia and the hypocellular bone marrow in the disorder are not related to inhibitory factors but to faulty progenitors and a deficiency of stroma-derived stimulatory activity.7,32 Bone marrow from patients with SDS is characterized by decreased levels of CD34+ cells. Furthermore, marrow CD34+ cells from patients with SDS have a reduced potential to generate hematopoietic colonies of all lineages in vitro.7
This study tested the hypothesis that bone marrow failure in SDS is mediated by increased programmed cell death. We used methylcellulose cultures because they represent an excellent in vitro method for growing hematopoietic progenitors and stem cells. Apoptosis was assessed after incubating the cells in cultures for 7 days because we were mainly interested in evaluating proliferating progenitors at an early stage.30 We found a significant increase in the proportion of cultured marrow cells undergoing apoptosis. This finding suggests that bone marrow failure is mediated by increased apoptosis, probably as a central pathogenetic mechanism. Increased apoptosis may be the primary mechanism contributing to the bone marrow failure in SDS. Less likely, but also possible, are that bone marrow failure is primarily due to defective proliferative mechanisms and that the role of apoptosis is relatively minor. The previously reported increased rate of apoptosis in other inherited hypoplastic syndromes of bone marrow or other tissues suggests that at least some of these disorders are caused by increased apoptosis.
We investigated whether the increased apoptosis rate of the syndrome is mediated by hypersensitivity to activating anti-Fas antibody. Two methods were used: Fas-induced decrease in colony formation and Fas-induced increase in apoptosis. In both techniques, we demonstrated that marrow cells from patients are more sensitive to activating anti-Fas antibody than those from normal controls. In addition, blocking anti-Fas antibody dramatically improved the clonogenic potential of marrow mononuclear cells from a patient with SDS, confirming the key role of Fas in mediating impaired SDS colony growth.
The next question was whether the overexpression of Fas antigen on marrow cells is responsible for the hypersensitivity to Fas. We showed that marrow cells from patients with the syndrome bear higher levels of Fas antigen. Because cytopenia is characteristic of SDS and because of our concomitant findings of hypersensitivity to activating anti-Fas antibody, high Fas expression, and increased apoptosis, it is very likely that the findings are related. It might be, however, that the Fas pathway is not the only apoptotic pathway involved and that other proapoptotic pathways also contribute to the increased programmed cell death in SDS. A major hematologic defect in SDS is neutropenia. In this study, the mean Fas expression on patients' granulocytic cells was double that of normal controls. This difference, however, was not statistically different, which can be attributed to the relatively small number of patients and the large variability in Fas expression on neutrophils among these patients. Given that Fas expression was high in SDS hematopoietic progenitors at all stages of maturation, it is likely that a significant number of neutrophil progenitors underwent apoptosis before they became fully mature. An alternative explanation could be that the neutropenia in SDS is not mediated exclusively by apoptosis through the Fas pathway.
Finally, we investigated at which maturation stage hematopoietic cells express higher levels of Fas antigen. Interestingly, from a relatively early maturation stage (CD34+), marrow cells start to express higher Fas levels than normal. In the very early stages (CD34+/CD38− and CD34+/CD38+), the differences from normal were not statistically significant probably because these cell populations were very small, especially in patients with SDS. What does stimulate Fas expression on SDS hematopoietic progenitors? This can be either a direct or an indirect sequel of the genetic defect. Once the SDS gene is identified and cloned, it remains to be determined whether it functions to directly inhibit the activation state of the Fas pathway.
Because patients with SDS have a marked tendency toward MDS and because increased apoptosis of bone marrow precursors contributes to ineffective hematopoiesis in many patients with MDS, we hypothesized that hematopoietic progenitors from the SDS patient with secondary MDS (UPN 1) would be especially prone to programmed cell death. Indeed, in this patient the proportion of cells undergoing apoptosis was markedly higher than in the other patients. It therefore seems likely that apoptosis plays an important part in the worsening cytopenias of the disease during progression to MDS. Further, we found that, as in MDS, SDS marrow cells overexpress Fas antigen. All these defects were found in SDS patients with and without MDS. These findings provide important insight into the preleukemic nature of this syndrome, raising the possibility that SDS is an inherited MDS from its inception.
As noted before, programmed cell death is thought to play a role in the pathogenesis of bone marrow failure in several inherited bone marrow failure syndromes. The increased tendency of bone marrow cells to undergo apoptosis has been demonstrated in Fanconi anemia.11-14 It has been suggested that the normal Fanconi anemia C gene product serves, in part, to modulate interferon-γ signals, possibly by shortening the kinetics of Stat-1–phosphate decay.12 Hematopoietic progenitors bearing Fanconi anemia C mutations fail to modulate interferon-γ signals normally and, as a result, undergo apoptosis executed through the Fas pathway. Regarding Blackfan-Diamond anemia, the elevation of serum Fas ligand33 and the accelerated apoptosis of marrow cells in response to erythropoietin deprivation10 have been demonstrated. The relevance of these findings to the pathogenesis of Blackfan-Diamond anemia requires further clarification because elevated Fas ligand levels do not necessarily mean increased apoptosis and because in this disorder erythropoietin levels are high. In myelokathexis, accelerated apoptosis of granulocytes and depressed expression of bcl-x in bone marrow–derived granulocyte precursor cells have been shown.15 Taken together with the results of the present study, these findings suggest that marrow failure in several inherited marrow failure syndromes is mediated by increased apoptosis as a central, major pathogenetic pathway. When transformation to MDS occurs, the apoptosis rate may increase. Impaired balance between the activation and inactivation of proapoptotic and antiapoptotic genes and the expression of their respective proteins in a disease-specific pattern seems responsible for this sequence of events.
We thank Wilma Vanek, Tom Grunberger, and Nagel Sharfe for technical assistance.
Supported in part by grants from the Audey Stanley Memorial Leukemia Research Fund, Shwachman-Diamond Support Canada, Aplastic Anemia Association of Canada, Elizabeth Rose Herman Fund, and The Severe Chronic Neutropenia International Registry.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Yigal Dror, Division of Hematology/Oncology, The Hospital for Sick Children, 555 University Ave, Toronto, Ontario M5G 1X8, Canada; e-mail: yigal.dror@sickkids.ca.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal