To describe the clinical and biologic features of pediatric acute megakaryoblastic leukemia (AMKL) and to identify prognostic factors, experience at St Jude Children's Research Hospital was reviewed. Of 281 patients with acute myeloid leukemia treated over a 14-year period, 41 (14.6%) had a diagnosis of AMKL. Six patients had Down syndrome and AMKL, 6 had secondary AMKL, and 29 had de novo AMKL. The median age of the 22 boys and 19 girls was 23.9 months (range, 6.7-208.9 months). The rate of remission induction was 60.5%, with a 48% rate of subsequent relapse. Patients with Down syndrome had a significantly higher 2-year event-free survival (EFS) estimate (83%) than did other patients with de novo AMKL (14%) or with secondary AMKL (20%;P ≤ .038). Among patients who had de novo AMKL without Down syndrome, 2-year EFS was significantly higher after allogeneic bone marrow transplantation (26%) than after chemotherapy alone (0%;P = .019) and significantly higher when performed during remission (46%) than when performed during persistent disease (0%;P = .019). The 5-year survival estimates were significantly lower for de novo AMKL (10%) than for other forms of de novo AML (42%; P < .001). Treatment outcome is very poor for patients with AMKL in the absence of Down syndrome. Remission induction is the most important prognostic factor. Allogeneic transplantation during remission offers the best chance of cure; in the absence of remission, transplantation offers no advantage over chemotherapy alone.
Introduction
Acute megakaryoblastic leukemia (AMKL) is a biologically heterogeneous form of acute myeloid leukemia (AML) and is recognized as the seventh subtype of AML (M7) within the French-American-British (FAB) cooperative group classification system.1 AMKL has a bimodal age distribution that peaks in early childhood (age < 3 years) and adulthood.2-6Between 1% and 10% of cases of AML in adults are identified as AMKL.2,7 Although initially thought to be rare among children, AMKL has been diagnosed with increasing frequency in this age group, largely because of improvements in immunophenotyping.5 Contemporary cooperative group studies have found that 3.1% to 10% of all cases of childhood AML are AMKL.8-11 However, the exact incidence of AMKL in children remains undetermined.
Acute megakaryoblastic leukemia is the most common form of AML in children with Down syndrome (DS), and its prognosis is excellent in this group of patients.6,8,12-14 AMKL in other children appears to be more heterogeneous, and its prognostic factors have not been well defined.5 6 Since 1984, our institution has used systematic criteria to establish the diagnosis of AMKL. We reviewed the clinical and biologic characteristics and the outcomes of all 41 cases of childhood AMKL diagnosed at our institution since that time.
Patients, materials, and methods
Patients
All cases of AMKL diagnosed between January 1985 and December 1998 at St Jude Children's Research Hospital were reviewed. Details of clinical presentation, laboratory findings at the time of diagnosis, therapy received, and outcome were collected from patients' medical records, pathology records, and the hospital's central database. The diagnosis of AMKL was established on the basis of the FAB criteria by studies of cell morphology and cytochemistry1and was confirmed by immunophenotyping.5 In the absence of immunophenotyping, the diagnosis was confirmed by electron microscopic identification of platelet peroxidase activity or factor VIII expression, or both in malignant cells. Cytogenetic studies were performed on bone marrow (BM) or peripheral blood samples taken at the time of diagnosis of primary or secondary leukemia; samples were processed and analyzed by standard methods.15,16 During the study period, 2 hematopathologists (F.G.B. and D.R.H.) reviewed the BM smears and biopsy sections and the cytochemical, immunophenotyping, and electron microscopy studies of all patients who had a diagnosis of acute leukemia, and classified cases of AML as M0 to M7 by standard criteria.1 17
For this study, cases of AMKL were retrospectively divided into 3 groups: AMKL associated with DS (DS-AMKL), AMKL occurring as a secondary malignancy (secondary AMKL), and primary AMKL occurring in the absence of DS (de novo AMKL). We identified 41 patients who had AMKL; 2 elected not to be treated at St Jude and 39 received treatment on various institutional protocols.18-23
Statistical analysis
Kruskal-Wallis nonparametric one-way ANOVA was used to compare clinical and biologic features at the time of diagnosis across the 3 groups of patients with AMKL. Survival distributions were estimated by using the method of Kaplan and Meier24 and were compared by using the log-rank test. All estimates of outcomes are reported with 95% confidence intervals. The duration of overall survival was defined as the time period between the date of diagnosis and the date of death from any cause or the date of the last follow-up examination. The duration of event-free survival (EFS) was defined as the time period between the date of diagnosis and the date of an adverse event (relapse, evidence of disease progression, or death from any cause) or the most recent follow-up examination. Early death or remission induction failure was recorded as an event at zero time, with an EFS value of zero. The 2 patients who did not receive treatment at St Jude were excluded from the survival analysis. SAS release 6.12 software (SAS Institute, Cary, NC) was used to perform the statistical analysis.
Results
Patients
During the study period, 1417 cases of acute leukemia were diagnosed; 281 of these were AML and 41 were AMKL. Cases of AMKL composed 2.9% of the cases of acute leukemia and 14.6% of the cases of AML. The median age at the time of diagnosis was 23.9 months (range, 6.7-208.9 months), and the ratio of girls to boys was 0.86:1. Twenty-eight patients were white, 7 were black, and 6 were Hispanic. Twenty-nine patients (70.7%) had de novo AMKL, 6 (14.6%) had DS-AMKL, and 6 (14.6%) had secondary AMKL that occurred subsequent to treatment for B-lineage acute lymphoblastic leukemia. Two patients had completed therapy for acute lymphoblastic leukemia and 4 were receiving maintenance therapy when secondary AMKL was diagnosed. The average time between the diagnoses of ALL and secondary AMKL was 32 months (range, 18.0-64.5 months).
Clinical and laboratory features
Table 1 shows the clinical and laboratory features of the study group as a whole, and those of the 3 groups of patients, at the time of diagnosis. No features identified at the time of diagnosis, other than age and lactic dehydrogenase (LDH) activity, were found to differ significantly between the groups. Patients with secondary AMKL were older than those with DS-AMKL and de novo AMKL (P = .002). There was no difference between the 3 AMKL groups in microscopic BM findings. The mean proportion of BM blast cells in the study group was 51% (SD, 24%). There was no difference between groups in the proportion of blasts in the BM. Aspirated BM samples showed more than 30% blast cells in 83% of patients and 3% to 29% blast cells in 17%; the latter group underwent BM biopsy, which confirmed the presence of more than 30% blast cells and the diagnosis of acute leukemia. Aspirated BM samples were frequently hypocellular. The malignant blast cells in the BM showed cytoplasmic surface blebs in all cases; these were prominent in 86% of cases. In 46% of cases, the blast cells tended to clump. Frequent binucleate blasts were seen in 22% of cases. None of the patients had a prior clinical history consistent with myelodysplastic syndrome. The BM of 6 patients (2 with DS-AMKL, 3 with de novo AMKL, and 1 with secondary AMKL) showed dysmorphic features of at least one hematopoietic lineage.
Clinical and laboratory features of the study group at the time of diagnosis
| Feature . | Total (n = 41) . | DS-AMKL (n = 6) . | Secondary AMKL (n = 6) . | De novo AMKL (n = 29) . |
|---|---|---|---|---|
| Median age*(mo) | 23.9 | 25.8 | 113.3 | 21.6 |
| Sex (M:F) | 22:19 | 1:5 | 3:3 | 18:11 |
| Fever | 8 (19.5%) | 0 | 0 | 8 (27.6%) |
| Lymphadenopathy | 12 (29%) | 1 (16.6%) | 0 | 11 (38%) |
| Hepatomegaly | 20 (49%) | 4 (66%) | 0 | 16 (55%) |
| Splenomegaly | 16 (39%) | 2 (33%) | 0 | 14 (48.3) |
| Hemoglobin (g/dL) (median, range) | 9 (4.0-13.8) | 9.2 (4.6-11.4) | 10.0 (4.0-13.8) | 8.8 (5.9-12.8) |
| WBCs (× 109/L) (median, range) | 11.3 (1.1-59.2) | 14.7 (3.7-57.9) | 2.7 (1.1-6.9) | 12.1 (3.3-59.2) |
| Circulating blast cells (%) (median, range) | 15.0 (0-73) | 5.5 (1-66) | 11.5 (0-43) | 16.0 (0-73) |
| Bone marrow blast cells (%) (median, range) | 50.0 (3-100) | 43.5 (16-72) | 41.75 (30-55) | 52.0 (3-100) |
| Platelets (× 109/L) (median, range) | 40.0 (3-528) | 23.5 (6-127) | 184.0 (6-378) | 40.0 (3-528) |
| Serum LDH† (U/L) (median, range) | 1872 (222-9917) | 2857 (510-9917) | 606 (222-996) | 2030 (459-9606) |
| Feature . | Total (n = 41) . | DS-AMKL (n = 6) . | Secondary AMKL (n = 6) . | De novo AMKL (n = 29) . |
|---|---|---|---|---|
| Median age*(mo) | 23.9 | 25.8 | 113.3 | 21.6 |
| Sex (M:F) | 22:19 | 1:5 | 3:3 | 18:11 |
| Fever | 8 (19.5%) | 0 | 0 | 8 (27.6%) |
| Lymphadenopathy | 12 (29%) | 1 (16.6%) | 0 | 11 (38%) |
| Hepatomegaly | 20 (49%) | 4 (66%) | 0 | 16 (55%) |
| Splenomegaly | 16 (39%) | 2 (33%) | 0 | 14 (48.3) |
| Hemoglobin (g/dL) (median, range) | 9 (4.0-13.8) | 9.2 (4.6-11.4) | 10.0 (4.0-13.8) | 8.8 (5.9-12.8) |
| WBCs (× 109/L) (median, range) | 11.3 (1.1-59.2) | 14.7 (3.7-57.9) | 2.7 (1.1-6.9) | 12.1 (3.3-59.2) |
| Circulating blast cells (%) (median, range) | 15.0 (0-73) | 5.5 (1-66) | 11.5 (0-43) | 16.0 (0-73) |
| Bone marrow blast cells (%) (median, range) | 50.0 (3-100) | 43.5 (16-72) | 41.75 (30-55) | 52.0 (3-100) |
| Platelets (× 109/L) (median, range) | 40.0 (3-528) | 23.5 (6-127) | 184.0 (6-378) | 40.0 (3-528) |
| Serum LDH† (U/L) (median, range) | 1872 (222-9917) | 2857 (510-9917) | 606 (222-996) | 2030 (459-9606) |
DS-AMKL indicates Down syndrome–associated acute megakaryoblastic leukemia; WBCs, white blood cells; LDH, lactic dehydrogenase.
P = .002.
P = .02.
There was no difference between the 3 AMKL groups in the pattern of cytochemical reactivity. Leukemic cells were consistently negative for myeloperoxidase, Sudan black B, and chloroacetate esterase staining. Twelve of 20 cases studied had positive periodic acid-Schiff staining, which was usually weak with a fine granular pattern. Four of 36 cases studied had weak alpha naphthyl butyrate esterase activity. The leukemic cells of all 36 patients tested showed alpha naphthyl acetate esterase activity with a characteristic multifocal punctate cytoplasmic staining pattern; the activity was strong in 33 cases, and 3 cases showed accentuation of staining in the Golgi region. Alpha naphthyl acetate esterase activity was completely inhibited by sodium fluoride in only 2 of the 36 cases and was partially inhibited in the remainder.
There was no difference between the DS-AMKL and de novo AMKL groups in the frequency of myelofibrosis. BM biopsy sections of 21 patients (5 with DS-AMKL, 16 with de novo AMKL) were stained for reticulin. Four patients with de novo AMKL had negative reticulin staining. Of 17 patients with positive reticulin staining, 7 (2 DS-AMKL, 5 de novo AMKL) had a mild increase, 6 (3 DS-AMKL, 3 de novo AMKL) had a moderate increase, and 4 (all with de novo AMKL) had a marked increase in reticulin fibers. No patients with secondary AMKL had reticulin staining studies. Aspirated BM samples showed a rough correspondence between reticulin fibrosis and both the presence of spicules and the overall cellularity of the sample. Absent or mild reticulin fibrosis was roughly correlated with normal or increased cellularity (9 of 11 cases) and moderate or marked reticulin fibrosis with decreased overall cellularity (6 of 10 cases).
Immunophenotyping studies
Thirty-nine patients had immunophenotyping studies; the BM samples of 2 patients (one with secondary AMKL and one with de novo AMKL) were insufficient. All 39 cases were negative for T-cell markers (CD3, CD5), B-cell markers (CD19, CD79a, CD10, CD20), and the monocyte marker CD14. The CD33 antigen was expressed by leukemic blast cells in 91% of cases. Atypical expression of lymphoid-associated antigens CD2 and CD7 was detected in 23% and 50% of cases, respectively; there was no difference in expression between de novo and secondary cases of AMKL. Glycophorin A was detected on the leukemic cells of 18% of cases, again with no difference between de novo and secondary cases. The leukemic cells of 33 patients expressed at least one platelet-associated antigen (CD36, CD41a, CD41b, or CD 61); in the remaining 6 cases, the malignant cell lineage could not be conclusively determined by immunophenotyping. There was no difference among the 3 groups in the expression of the surface antigens tested.
For the 8 patients in whom immunophenotyping was not done or was inconclusive, the diagnosis of AMKL was confirmed by detection of cytoplasmic factor VIII expression (one patient), platelet peroxidase activity (5 patients), or both (one patient). The remaining case showed the translocation t(1;22), which is characteristic of AMKL.25
Cytogenetic features
Cytogenetic studies were performed for 39 patients; for the remaining 2 (one with secondary AMKL and one with de novo AMKL), the quantity of BM sample was insufficient. Seven patients (18%), all of whom had de novo AMKL, had multiple complex karyotypic anomalies. None of the patients with DS-AMKL or secondary AMKL had abnormalities of this type. Four patients with DS-AMKL had numerical chromosomal anomalies in the leukemic clone in addition to constitutional trisomy 21; 3 had an additional chromosome 21, and 3 had an additional chromosome 8. Twelve of the 28 patients with de novo AMKL (43%) but none of the patients with secondary AMKL had trisomy 21 in the leukemic clone. In all, 10 patients with de novo AMKL had trisomy 8 (4 patients), trisomy 19 (5 patients), or both (one patient). The specific AMKL-associated translocation, t(1;22), was noted in only one patient, an infant with de novo AMKL. Cytogenetic anomalies involving chromosome 3, which are common in adult AMKL,7 were noted in 5 patients with de novo AMKL. None of the patients with DS-AMKL or secondary AMKL had involvement of chromosome 3. Chromosome band 11q23 aberrations were identified in 11 patients (4 of 5 with secondary AMKL and 7 of 28 with de novo AMKL). The original ALL clone was not detected in any of the 5 secondary AMKL cases studied; in this group, the 11q23 abnormality was the sole genetic abnormality in 4 of the 5 patients studied, and the remaining patient had monosomy 7.
Treatment
Thirty-two of the 39 patients who received therapy at St Jude were treated on one of 4 institutional AML studies (5 on AML-83, 6 on AML-87, 12 on AML-91, and 9 on AML-97).18-23 The remaining 6 patients received chemotherapy containing high-dose cytarabine (ara-C) and daunorubicin. One patient with secondary AMKL received allogeneic hematopoietic stem cell transplantation (HSCT) as primary therapy, without prior remission induction therapy for AMKL. Twenty-three patients (1 with DS-AMKL, 4 with secondary AMKL, and 18 with de novo AMKL) underwent HSCT. Twenty-two of these transplants were allogeneic (9 matched related, 9 matched unrelated, and 4 mismatched related or unrelated donors) and one (in a patient with de novo AMKL) was autologous.
The indications for HSCT varied over the study period and were defined by individual protocol guidelines. AML-83 and AML-87 did not call for HSCT for patients with AMKL in first complete remission (CR), but some patients with an HLA-matched sibling donor underwent allogeneic HSCT in first CR at the discretion of their primary physicians. Among these was one patient with DS. In the AML-91 protocol, patients with de novo AMKL who had an HLA-matched related donor were considered for allogeneic HSCT in first CR. Those who had no matched related donor or who refused allogeneic HSCT were eligible for autologous HSCT during CR. Patients with secondary AMKL who achieved CR were considered for allogeneic HSCT from an HLA-matched related or unrelated donor. In AML-97, patients with de novo or secondary AMKL in first CR were considered for allogeneic HSCT from suitably HLA-matched related or unrelated donors. Allogeneic HSCT was also used as a salvage therapy for patients who did not achieve CR or for those who relapsed. In the latter 2 studies, patients with DS were not considered for allogeneic HSCT.
Outcome
Thirty-nine patients were included in the survival analysis. Two patients who did not receive treatment at St Jude Children's Research Hospital (one with secondary AMKL and one with de novo AMKL) were excluded. Induction chemotherapy failed to induce remission in 15 patients (1 with DS-AMKL, 2 with secondary AMKL, and 12 with de novo AMKL); except for the patient with DS, all died of progressive disease. Relapses occurred in 6 of 14 patients after HSCT and 5 of 9 patients after intensive chemotherapy. None of the 5 patients with DS-AMKL had relapses. After relapse, 6 patients were treated with chemotherapy, 3 with donor T-cell infusions, and one with allogeneic HSCT (some patients received multiple forms of treatment); 3 received no additional treatment. None of these patients survived beyond 12 months.
The overall mortality rate was 69.2%. The mortality rate was 16.6% for patients with DS-AMKL (1 of 6), 80% for patients with secondary AMKL (4 of 5), and 78% for patients with de novo AMKL (22 of 28). The single adverse event in the DS group was a fatal episode of sepsis during induction therapy.
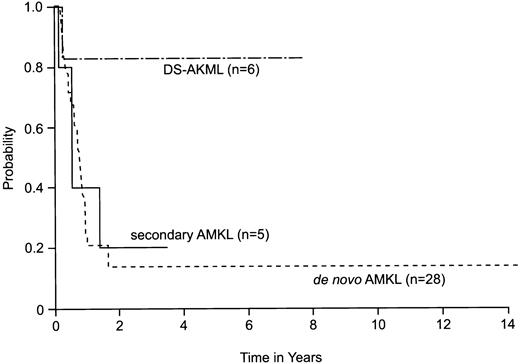
With a median follow-up of 9.5 months (range, 1.8-175 months), the estimate of 2-year survival for all 39 patients was 24% (95% CI = 8%-40%). Patients with DS-AMKL had an excellent prognosis, with an estimated 2-year survival of 83% (95% CI = 43%-100%). In contrast, estimated 2-year survival was only 14% (95% CI = 0%-28%) for patients with de novo AMKL and 20% (95% CI = 0%-46%) for patients with secondary AMKL (P = .08) (Figure 1). Similarly, the 2-year estimate of EFS was significantly higher among patients with DS-AMKL (83%; 95% CI = 43%-100%) than among patients with secondary AMKL (20%; 95% CI = 0%-46%) and de novo AMKL (14%; 95% CI = 0%-28%) (P = .038). Because CR after induction was an important factor in outcome, even among those who underwent allogeneic HSCT, we compared the rates of CR obtained in the 4 protocols for patients who had AMKL in the absence of DS (80% in AML-83, 67% in AML-87, 33% in AML-91, and 89% in AML-97;P = .05). Interestingly, AML 91 had the lowest ara-C dose intensity during the induction remission phase.
Estimated probability of overall survival for 39 patients with AMKL diagnosed and treated at St Jude Children's Research Hospital.
The method of Kaplan and Meier was used to derive the curves;P values were derived by log-rank analysis. Estimated 2-year survival was 83% (95% CI = 43%-100%) for patients with DS-AMKL, 20% (95% CI = 0%-46%) for patients with secondary AMKL, and 14% (95% CI = 0%-28%) for patients with de novo AMKL (P = .08). DS-AMKL indicates Down syndrome–associated AMKL.
Estimated probability of overall survival for 39 patients with AMKL diagnosed and treated at St Jude Children's Research Hospital.
The method of Kaplan and Meier was used to derive the curves;P values were derived by log-rank analysis. Estimated 2-year survival was 83% (95% CI = 43%-100%) for patients with DS-AMKL, 20% (95% CI = 0%-46%) for patients with secondary AMKL, and 14% (95% CI = 0%-28%) for patients with de novo AMKL (P = .08). DS-AMKL indicates Down syndrome–associated AMKL.
Analysis of prognostic factors
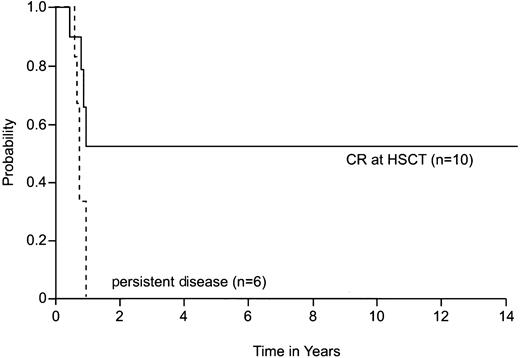
Analysis of prognostic factors included only patients with de novo AMKL. Patients with DS-AMKL were excluded because of their known excellent prognosis and those with secondary AMKL were excluded because of their small number. One patient with de novo AMKL who was not treated at St Jude was also excluded. Among patients with de novo AMKL, the median white blood cell (WBC) count at the time of diagnosis was 12.1 × 109/L (range, 3.3-59.2 × 109/L). The outcomes of patients with WBC counts below 12.1 × 109/L (n = 14) and above 12.1 × 109/L (n = 14) did not differ significantly (P = .07). We also found no significant association between outcome and hemoglobin concentration (≤ 8.0 versus > 8.0 g/dL, P = .08), platelet count (≤ 50 × 109/L versus > 50 × 109/L,P = .18), or the presence or absence of trisomy 21 in the leukemic clone (P = .84) at the time of diagnosis. Survival estimates were, however, significantly influenced by the success of remission induction therapy. Patients who entered CR after initial chemotherapy (n = 16) had significantly higher estimates of 2-year survival (25%; 95% CI = 0%-51%) than did others (n = 12; 0%; P < .001). Also, patients who underwent allogeneic HSCT (n = 16) rather than chemotherapy alone (n = 11) had significantly higher estimates of 2-year survival (30%; 95% CI = 2%-58% versus 0%; P = .007) and EFS (26%; 95% CI = 0%-52% versus 0%; P = .019). In addition, patients who were in CR at the time of allogeneic HSCT (n = 10) had significantly higher estimates of 2-year survival (53%; 95% CI = 11%-95%; (P = .028) and EFS (46%; 95%CI = 8%-84%; P = .019) than did patients who had persistent disease at the time of HSCT (n = 6; both overall survival and EFS = 0%) (Figure 2).
Effect of disease status at the time of transplantation in children with de novo AMKL who do not have DS.
The 2-year survival estimate was 53% (95% CI = 11%-95%) for patients who were in CR at the time of HSCT and 0% for patients who had persistent disease at the time of HSCT (P = .028). HSCT indicates hematopoietic stem cell transplantation.
Effect of disease status at the time of transplantation in children with de novo AMKL who do not have DS.
The 2-year survival estimate was 53% (95% CI = 11%-95%) for patients who were in CR at the time of HSCT and 0% for patients who had persistent disease at the time of HSCT (P = .028). HSCT indicates hematopoietic stem cell transplantation.
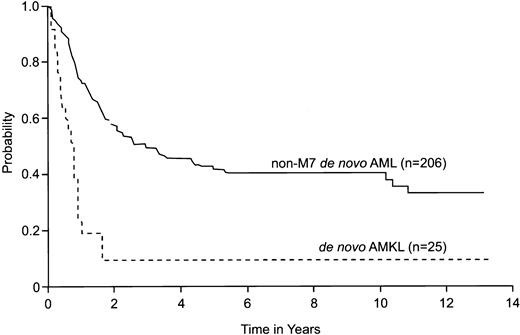
We compared the 5-year survival estimate of patients with de novo AMKL with that of patients with other FAB subtypes (M0-M6) of de novo AML, treated on the 4 frontline protocols during the study period. The 25 patients with de novo AMKL (3 patients who were not enrolled on a protocol were excluded) had a significantly lower 5-year survival estimate (10%; 95% CI = 0%-22%) than did the 206 patients with non-M7 de novo AML (42%; 95% CI = 34%-50%;P < .001) (Figure 3).
Comparison of estimated survival of patients with de novo AMKL (FAB subtype M7) without DS with that of patients with other subtypes of de novo AML (FAB M0-M6).
All patients were treated on 4 St Jude Children's Research Hospital frontline AML protocols (AML-83, AML-87, AML-91, AML-97) between January 1985 and December 1998. Estimated 5-year survival was 10% (95% CI = 0%-22%) for de novo AMKL and 42% (95% CI = 34%-50%) for non-M7 de novo AML (P < .001).
Comparison of estimated survival of patients with de novo AMKL (FAB subtype M7) without DS with that of patients with other subtypes of de novo AML (FAB M0-M6).
All patients were treated on 4 St Jude Children's Research Hospital frontline AML protocols (AML-83, AML-87, AML-91, AML-97) between January 1985 and December 1998. Estimated 5-year survival was 10% (95% CI = 0%-22%) for de novo AMKL and 42% (95% CI = 34%-50%) for non-M7 de novo AML (P < .001).
Discussion
In this large series of patients from a single institution, 2.9% of all cases of childhood acute leukemia and 14.6% of all cases of AML were identified as AMKL. This incidence is higher than that previously reported.5,6,8,26 It is possible that AMKL has been underrepresented in recent clinical trials because of its complex clinical presentation27-31 and the difficulty of obtaining and interpreting BM samples for diagnostic testing.6,8 26Thus, many cases of AMKL may have been classified as undifferentiated leukemia, myelodysplasia, myelofibrosis, or other diseases.
With the available combination of morphologic, cytochemical, cytogenetic, and immunophenotypic methods, AMKL can now be reliably diagnosed.32 We have found a combination of 2 findings to be highly suggestive of AMKL: typical morphologic features of leukemic cells isolated from the BM, such as surface blebs, cell clumping, and binucleation, together with multifocal punctate cytoplasmic alpha naphthyl acetate esterase cytochemical staining that is incompletely inhibited by sodium fluoride. The percentage of blast cells in an aspirated BM specimen is not reliable as a single diagnostic indicator of acute leukemia, as shown by the initial finding of less than 30% leukemic cells in 20% of our cases. For this reason, BM biopsy is indicated for patients with suspected AMKL. The diagnosis should always be confirmed by immunophenotyping or immunohistochemistry. The availability of several antiplatelet monoclonal antibodies and advanced flow cytometry techniques has contributed to improved diagnostic capability in recent years. Detection of the t(1;22) in infants is also helpful in suggesting the presence of AMKL. The approximately 15% incidence of AMKL we found in 281 consecutively diagnosed cases of AML reflects this improved diagnostic accuracy.
Treatment with topoisomerase II inhibitors is a known cause of secondary AML.33-38 In this study, the clinical and biologic characteristics of the patients with secondary AMKL, including age, sex, type of previous leukemia, intensity of exposure to topoisomerase II inhibitors, latency period, presence of cytogenetic abnormalities, and prognosis, were similar to those reported for pediatric patients with other FAB subtypes of secondary AML.33,38 39
Acute megakaryoblastic leukemia occurs about 400 times more frequently in children with DS than in other children.6,14,40 Several collaborative pediatric group studies have found that approximately half of all pediatric cases of AMKL occur in patients with DS.8,13,40 In contrast, only 17% of our patients with de novo AMKL had DS. This disparity may reflect a low rate of referral of patients with DS to our institution. Only 2.6% of the patients diagnosed at our institution during the study period as having AML had DS, whereas the proportion at other institutions is comparable to the 9.8% reported by the Children's Cancer Group (CCG).6,8 13 Alternatively, because the diagnosis of non-DS AMKL is difficult in many cases, patients in this category may be more likely to be referred to a research institution; such referrals would reduce the relative percentage of DS-AMKL cases.
The prognosis of children who have de novo AMKL in the absence of DS has been difficult to determine. Findings increasingly suggest that these patients have a poorer prognosis than do patients with other FAB subtypes of AML. At our institution, patients with de novo AMKL have significantly lower 5-year survival estimates than do patients treated on similar protocols for other FAB subtypes of de novo AML. CCG investigators recently reported that the estimated rate of 4-year disease-free survival in this group of children was only 21%.6 The Berlin-Frankfurt-Münster (BFM) group recently reported that AMKL independently predicts poor prognosis in children with AML.41
Our findings and those of others suggest that standard therapies used for AML are not always optimal for patients with AMKL.6,10,11,42 Appropriate treatment for patients with DS, who have a good prognosis and a relatively low tolerance for high-dose chemotherapy, has been described previously.8,43For patients with secondary AMKL, the outcome of contemporary treatment appears to be as poor as that for other types of secondary AML.38 Patients with de novo AMKL in the absence of DS have a more than 70% probability of achieving remission with regimens containing dose-intensive ara-C, but they have a very high rate of relapse if therapy after remission consists only of intensive chemotherapy. Thus, allogeneic HSCT should be considered for patients who enter remission. In our study, patients who underwent allogeneic HSCT had significantly higher estimated survival and EFS rates than did children who received chemotherapy alone. However, allogeneic transplantation during active disease was not more advantageous than chemotherapy alone.
In conclusion, we have characterized the clinical and biologic features and outcomes of a relatively large series of pediatric patients with AMKL. De novo AMKL has a particularly poor prognosis if treated with intensive chemotherapy alone. These findings support the design of individualized therapeutic approaches adapted to specific subgroups of pediatric AML.
We thank Sharon H. Naron for editorial assistance in the preparation of this manuscript.
Supported in part by National Cancer Institute Cancer Center core grants P30-CA-21765 and PO1-CA-20180, by a Centre of Excellence grant from the state of Tennessee, and by the American Lebanese Syrian Associated Charities (ALSAC).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Raul C. Ribeiro, 332 N Lauderdale, Memphis, TN 38105-2794; e-mail: raul.ribeiro@stjude.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal