The effect of a recombinant hybrid human interferon α (IFN-α) (which cross-reacts with murine cells) on C57BL/6 mice infected with Plasmodium yoelii sporozoites or parasitized erythrocytes was determined. IFN-α did not inhibit the development of the parasite in the liver, but it did reduce the blood parasite load and the hepatosplenomegaly induced by the infection in mice injected with blood-stage parasites. The extent of anemia in IFN-α–treated and control mice was similar, despite the lower parasite load in the IFN-α–treated mice. The reduced blood parasite load in IFN-α–treated mice was associated with reduced erythropoiesis and reticulocytosis. As reticulocytes are the preferred target cells for the strain of P yoelii used (P yoelii yoelii 265 BY), it was postulated that the inhibition of reticulocytosis in IFN-α–treated mice was causally related to the observed decreased blood parasite load. This was supported by the finding that IFN-α inhibited a different strain ofP yoelii (17X clone A), which also displays a tropism for reticulocytes, but not a line of Plasmodium vinckei petteri, which infects only mature red blood cells. As human malaria species also display different tropism for reticulocytes, these findings could be relevant for people coinfected with multiple Plasmodium species or strains or coinfected with Plasmodium and virus.
Introduction
Malaria remains a disease that is estimated to kill 1.5 million to 2.7 million people each year.1 The parasite and its mosquito vector have become resistant to several drugs in recent years, and this has stimulated a search for new therapeutic strategies. Recombinant cytokines can confer protection against bacterial, viral, and some intracellular parasitic infection. Some of these cytokines can also inhibit the development of the malaria parasite. Recombinant interferon γ (IFN-γ) inhibits the development of the liver stage of murine,2 simian,3 and human3,4 malaria in vitro and in vivo. Protection against murine and simian malaria is also obtained after treatment with recombinant (r) IL-12.5-7
Type I interferons were originally described as potent antivirus substances that are produced by animal cells infected with virus. However, the role of type I IFNs in the defense against nonvirus pathogen is much less well studied.8 IFN type I (IFN-α or β) inducers, such as Newcastle disease virus, statolon, or polyriboinosinic-polyribocytidylic acid,9-13 and unpurified serum IFN14 protect against Plasmodium berghei infection. However, the action of IFN-α alone is still not known.
In this study, we have analyzed the effect of a recombinant human IFN-α hybrid that cross-reacts with murine cells on the course and the pathology of infection with different species or strains of rodentPlasmodium, initiated by inoculation with either sporozoites or parasitized erythrocytes. We show that inhibition of parasite-induced reticulocytemia by IFN-α influences only parasite species that are restricted to reticulocytes. Our results extend previous observations15 whose interpretation is complicated by the fact that a corticosteroid, which is a potent immunosuppressor, was used to alter reticulocyte production. We discuss our results in the context of the role of cytokines in the pathogenesis of malaria.
Materials and methods
Mice and parasites
Female C57BL/6 mice, 5 to 6 weeks old, were purchased from Charles River Breeding Laboratories (Saint Aubin-Les-Elbeuf, France). Sporozoites of the uncloned line of the 265 BY strain ofPlasmodium yoelii yoelii were obtained from infected salivary glands of Anopheles stephensi mosquitoes, 16 to 21 days after a meal of blood from an infected mouse. The salivary glands were dissected out aseptically, homogenized in an all-glass grinder, and diluted in culture medium or phosphate-buffered saline (PBS). Sporozoites were counted, and mice were injected intravenously (IV) with 4000 or 20 000 sporozoites. The blood parasites used in this study were from the uncloned line (265 BY) or from a cloned line, clone A, of the 17X NL strain16 17 (kindly given by Dr G. Snounou, Imperial School of Medicine, London, England) of the P yoelii yoelii strain. Blood parasites of an uncloned line ofPlasmodium vinckei petteri 106HW were also used. The parasites were suspended in Alsever's buffer and stored as a frozen stabilate in liquid nitrogen. Mice were inoculated intraperitoneally (IP) with 106 parasitized erythrocytes (PEs) in 100 μL Alsever buffer.
IFN-α treatment
A recombinant hybrid IFN-α (BDBB, a hybrid of α8 and α1, CIBA-GEIGY 35269)18 was a gift from Dr Marcus Grütter (Ciba Geigy, Basel, Switzerland). Its specific activity on mouse embryonic fibroblasts was 5.1 × 106U/mg.18 The lyophilized IFN-α was suspended in PBS containing 0.1% (wt/vol) bovine serum albumin (BSA) (fraction V) (Sigma Chemical, St Louis, MO). The control was 0.1% BSA in PBS. Liver-stage studies were done in mice treated daily with IFN-α or the control preparation for 4 days, starting the day before the IV injection of sporozoites. Blood-stage studies were done in mice that were first injected IP with 200 μL IFN-α (5 × 104units [U] per injection) or the control preparation and then injected IP with the parasites 1 hour later. Treatment was continued daily for 25 days.
Quantification of P yoelii ribosomal DNA in the liver of infected mice
Quantification was performed as described.19 Liver biopsies (100 mg, corresponding to one fourth of the right lobe) were removed 42 hours after injection of 20 000 sporozoites, frozen in liquid nitrogen, and stored at −80°C for subsequent DNA isolation. Genomic DNA was extracted by means of the Easy-DNA kit (Invitrogen, San Diego, CA). DNA pellets were air-dried for 5 minutes at room temperature and suspended in 100 μL TE (10 mM Tris-HCl; 1 mM EDTA, pH 8.0; 5% sarcosyl; 200 μg/mL proteinase K) at 37°C for 30 minutes. They were then stored at 4°C before being used as a template in a polymerase chain reaction (PCR) using the following malaria oligonucleotide primers: rPLU3 5′-TTT TTA TAA TAG TAA CTA CGG AAA AGC TGT-3′ and rPLU4 5′-TAC CCG TCA TAG CCA TGT TAG GCC AAT ACC-3′. Template DNA (1 μg DNA sample) was added to a solution containing (final concentrations) 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.01% gelatin, 1% Triton X-100, 200 μM deoxynucleoside triphosphate, 125 nM of each primer, and 0.5 U Taq polymerase (ATGC Biotechnologie, Noisy le Grand, France) in a total volume of 50 μL. The solution was subjected to 45 cycles of amplification with an Omnigene Temperature Cycler (Hybaid, Teddington, England). In each cycle of PCR, the mixture was denatured at 95°C for 45 seconds (5 minutes for the first cycle), annealed at 64°C for 45 seconds, and extended at 72°C for 1 minute. The quality and quantity of the DNA extraction were assessed before amplifying theβ2-microglobulin gene for all samples by means of the following primers: 5′-GGC TCG CTC CGT GAC CCT AGT CTT T-3′ and 5′-TCT GCA GGC GTA TGT ATC AGT CTC A-3′. The conditions of amplification were 60°C for 20 seconds for annealing. PCR products (243 base pairs [bp] for ribosomal DNA or 300 bp for β2-microglobulin) were visualized by gel-electrophoresis and ethidium bromide staining. Plasmodium PCR products were quantitated by high-pressure liquid chromatography. Briefly, 20 μL PCR reaction was passed through an ion exchange column (Gen-Pack FAX column) (Waters, Milford, MA) to separate the PCR product from the other components of the PCR reaction. The elution peak of the amplified fragment was identified, and the peak area was calculated. A reference standard curve was prepared with products obtained after amplification of serial dilutions of DNA extracted from purified erythrocytes infected with P yoelii.
Determination of hematological parameters and blood parasite load
Hemoglobin (Hb) concentrations were determined every 2 days.20 Briefly, 2 μL tail-vein blood was diluted in 500 μL Drabkin's solution (Sigma), and Hb was assayed in 96-well microtiter plates (Costar, Cambridge, MA) in a volume of 100 μL by measuring the absorption at 405 nm (OD405nm) in an enzyme-linked immunosorbent assay reader (Bio-tek Instruments, Winooski, VT). Values were converted to milligrams per milliliter by means of a standard curve of human Hb (Sigma) dissolved in Drabkin's solution. Red blood cells (RBCs) were counted in a Malassez chamber by diluting 1 μL tail blood in 1 mL PBS. For evaluating reticulocyte number, 2 methods were initially compared: Brilliant Cresyl Blue staining21 and Giemsa staining.15,22 Similar results were obtained for both. Giemsa staining was preferred because it was more practical when assessing a large number of mice for extensive periods. Blood smears were fixed with methanol and stained with 10% Giemsa (Merck, Darmstadt, Germany) in Giemsa buffer, and polychromatophilic RBCs were scored as reticulocytes. The parasitemia was determined daily by means of the same thin blood smears by counting PEs for at least 1000 erythrocytes. The percentage of parasitemia was calculated as the number of parasitized cells per 100 erythrocytes. Since infected mice develop anemia, the results were also expressed as the density of the parasites in the blood. This was defined as the number of parasites per microliter of blood (parasite load), calculated from the percentage of parasitemia multiplied by the number of RBCs. Values from parasitemia and parasite load were then transformed by means of the formula x′ = log(x + 1) as described.23
Preparation of spleen and bone marrow cell suspensions
Mice were killed at various times after parasite injection, and their livers and spleens were removed and weighted. The femurs were removed aseptically. Suspensions of spleen cells were prepared by passing dissociated spleens through a sterile fine-wire mesh. RBCs were lysed with ammonium chloride potassium buffer, and the cells were then washed with RPMI 1640 medium (Sigma) containing 10% heat-inactivated fetal calf serum (FCS) (Sigma); 1% penicillin-streptomycin (PS) solution (100 × stock solution) (Gibco BRL, Paisley, Scotland); and L-glutamine (2 mM, Gibco). Bone marrow cells were flushed with 1 mL cold RPMI medium, supplemented as above, by means of a 26-gauge needle attached to a syringe. Cell aggregates were allowed to settle out for 10 minutes. Supernatants were recovered and the cells were washed in culture medium. Nucleated cells were counted; their viability was assessed by trypan blue exclusion; and they were then tested for colony formation in vitro.
Erythropoietic progenitor assays
The number of erythroid precursor cells, burst-forming units–erythroid (BFU-E) and colony-forming units-erythroid (CFU-E), were determined by means of 1 mL methylcellulose cultures.24 Bone marrow cells were plated in duplicate in 35-mm Petri dishes (Costar) at 7.5 × 104 cells/mL, and spleen cells were plated at 15 × 104 cells/mL in methylcellulose medium: 0.8% (wt/vol) methylcellulose (Fluka Chemie, Buchs, Switzerland) prepared as previously described25; 10% FCS; 1% PS; 1 U/mL mouse recombinant erythropoietin (Boehringer Mannheim, Mannheim, Germany); c-kit ligand; and 2-mercaptoethanol (5 × 10−5 M) (Sigma) in Optimem culture medium (Gibco) supplemented with NaHCO3 (2.4 g/L). The c-kit ligand was obtained from the supernatant of a cultured cell line, CHO-mcf (a gift from the Genetics Institute, Boston, MA), and its optimal concentration was previously determined by means of a mast cell line. CFU-E (clusters of 8 or more cells) and BFU-E (hemoglobinized colonies of at least 50 cells) were scored under an inverted light microscope after incubation for 3 days (CFU-E) or 8 days (BFU-E) in a 5% CO2-humidified incubator at 37°C.
Statistical analysis
Differences between mean values were analyzed for statistical significance with GraphPad Prism Software (version 3.0) (San Diego, CA) by means of the nonparametric Mann-Whitney test and withP < .05 used as the level of significance.
Results
Effect of IFN-α on the P yoelii 265 BY liver stage
Daily injections of IFN-α (5 × 104 U) for 4 days, starting on the day before the mice were inoculated with 4000 sporozoites, did not prevent the development of parasitemia (Table1, experiment 1). Morever, IFN-α–treated mice developed parasitemia at the same time as the control mice, and the time courses of parasitemia in the 2 groups were similar (data not shown). These results were confirmed by determining the parasite load in the liver by quantitative PCR. Mice challenged with 20 000 sporozoites and treated with IFN-α as in experiment 1 had amounts of parasite ribosomal DNA in their livers similar to the control mice 42 hours after challenge (Table 1, experiment 2). Thus, IFN-α did not inhibit the liver stage of malaria.
Influence of interferon-α on the P yoeliiliver stage in vivo
| Treatment* . | Infected mice/challenged mice† (experiment 1) . | P yoeliiribosomal DNA in the liver (log pg)‡ (experiment 2) . |
|---|---|---|
| Control preparation | 5/5 | 0.88 ± 0.13 |
| NS | ||
| Interferon α | 5/5 | 0.68 ± 0.13 |
| Treatment* . | Infected mice/challenged mice† (experiment 1) . | P yoeliiribosomal DNA in the liver (log pg)‡ (experiment 2) . |
|---|---|---|
| Control preparation | 5/5 | 0.88 ± 0.13 |
| NS | ||
| Interferon α | 5/5 | 0.68 ± 0.13 |
NS indicates not significant (P = .26).
Mice were given 5 × 104 U interferon-α or control preparation daily for 4 days beginning the day before challenge.
Mice were challenged intravenously with 4000 sporozoites. Mice were considered infected when they developed parasitemia.
Mice were challenged intravenously with 20 000 sporozoites and killed 42 hours later for determination of parasite ribosomal DNA (rDNA) load in their livers. Results are expressed as the mean values of the parasite rDNA (log pg), obtained from 5 mice ± SE.
Effect of IFN-α on blood parasite development
C57BL/6 mice injected with the blood stage of the uncloned line ofP yoelii 265 BY normally develop a moderate blood parasite load, and most of the mice recover from the infection (Figure1), although mortality can occur when parasitemia and anemia develop rapidly. The parasitemia was significantly lower in mice treated with IFN-α than in controls, and parasites were cleared sooner from the blood of treated mice than in controls (Figure 1A). This effect on parasite development was also seen when blood parasite load was evaluated (Figure 1B).
Effect of recombinant human (rh) IFN-α on parasitemia and blood parasite load of C57BL/6 mice infected with P yoelii 265 BY.
Mice were infected with 106 PE on day 0, and the number of parasites was determined every 2 days. The experimental mice were injected IP with IFN-α (5 × 104 U per injection) daily from day 0 to 25; control mice were similarly injected at the same time with PBS/BSA. Results are expressed either as the logarithm-transformed percentage of parasitemia (parasitized cells per erythrocyte number) (panel A) or as the logarithm-transformed blood parasite load (number of parasites per microliter blood) (panel B). Symbols represent the mean ± SE of 5 mice. The experiment was repeated twice with similar results. Each d refers to a dead mouse. *P < .05 (Mann-Whitney test). ○ indicates control; ▪, IFN-α.
Effect of recombinant human (rh) IFN-α on parasitemia and blood parasite load of C57BL/6 mice infected with P yoelii 265 BY.
Mice were infected with 106 PE on day 0, and the number of parasites was determined every 2 days. The experimental mice were injected IP with IFN-α (5 × 104 U per injection) daily from day 0 to 25; control mice were similarly injected at the same time with PBS/BSA. Results are expressed either as the logarithm-transformed percentage of parasitemia (parasitized cells per erythrocyte number) (panel A) or as the logarithm-transformed blood parasite load (number of parasites per microliter blood) (panel B). Symbols represent the mean ± SE of 5 mice. The experiment was repeated twice with similar results. Each d refers to a dead mouse. *P < .05 (Mann-Whitney test). ○ indicates control; ▪, IFN-α.
Influence of IFN-α on hepatosplenomegaly in infected mice
Infection with Plasmodium species causes marked hepatosplenomegaly. As IFN-α inhibited the development of blood parasites, we determined the effect of IFN-α on the liver and spleen weights of mice infected with P yoelii265 at 6, 14, and 30 days after inoculation of the parasite. The splenomegaly in IFN-α–treated and control mice was not different on day 6 (data not shown). But the malaria-associated splenomegaly in IFN-α–treated mice was significantly less pronounced than in controls (P < .05) on day 14 (Table2). This difference was no longer observed 30 days after infection (data not shown). Likewise, IFN-α significantly inhibited (P < .05) hepatomegaly on day 14 (Table 2).
Effect of interferon-α on hepatosplenomegaly and parasitemia
| . | Treatment* . | |||
|---|---|---|---|---|
| BSA . | IFN-α . | P yoelii + BSA . | P yoelii+ IFN-α . | |
| Liver weight, mg | 1010 ± 61 | 802 ± 50 | 1340 ± 25 | 1092 ± 25† |
| Spleen weight, mg | 93 ± 4 | 94 ± 11 | 686 ± 50 | 389 ± 16† |
| Parasitemia, % | — | — | 16.4 ± 9.8 | 0.8 ± 0.5† |
| . | Treatment* . | |||
|---|---|---|---|---|
| BSA . | IFN-α . | P yoelii + BSA . | P yoelii+ IFN-α . | |
| Liver weight, mg | 1010 ± 61 | 802 ± 50 | 1340 ± 25 | 1092 ± 25† |
| Spleen weight, mg | 93 ± 4 | 94 ± 11 | 686 ± 50 | 389 ± 16† |
| Parasitemia, % | — | — | 16.4 ± 9.8 | 0.8 ± 0.5† |
BSA indicates bovine serum albumin; IFN, interferon; P yoelii, Plasmodium yoelii.
Mice, infected or uninfected, were injected daily with 0.1% BSA or recombinant human IFN-α. Samples were taken 14 days afterP yoelii 265 BY inoculation. Results are mean ± SE of 4 mice per group. The experiment was repeated twice with similar results.
Significantly different (P < .05).P values were calculated by comparing IFN-α–treated infected mice with the corresponding infected control mice (Mann-Whitney test).
Autopsies of the livers and spleens of IFN-α–treated parasite-injected mice revealed that they were lighter colored than those of control mice, indicating less parasite pigment in these organs. This was confirmed histologically (data not shown).
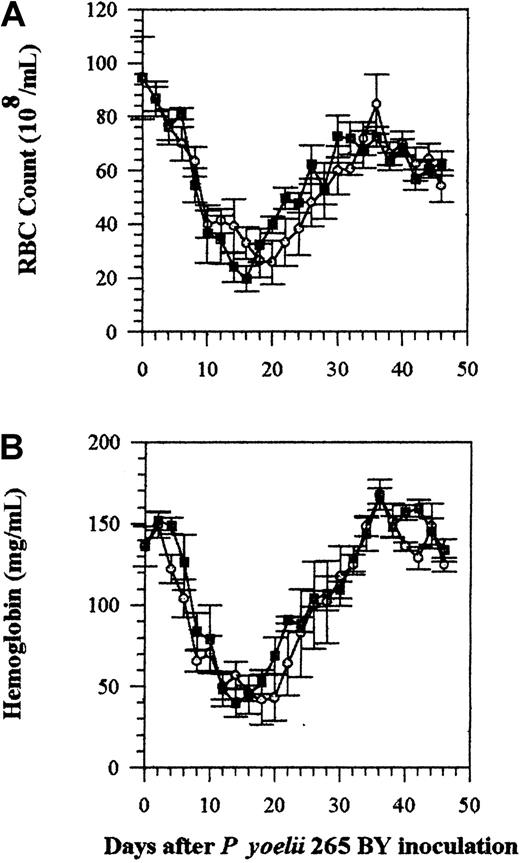
Effect of IFN-α on malaria-induced anemia
Despite the reduction of blood parasite load by IFN-α, the severity of anemia (measured by Hb level and RBC count) was the same in IFN-α–treated and control mice (Figure2). Both groups of mice were markedly anemic after infection with P yoelii 265 BY, with the lowest Hb concentrations and RBC counts on days 12 to 22. There were slight decreases in Hb and RBC counts in IFN-α–treated uninfected mice (data not shown).
Effect of rhIFN-α on anemia.
RBC count (panel A) and Hb concentrations (panel B) were measured in C57BL/6 mice infected with P yoelii 265 BY. Control and IFN-α–treated mice were injected IP daily, until day 25, with 0.1% BSA (vehicle) or rhIFN-α (5 × 104U). Symbols represent the mean ± SE of 5 mice. The experiment was repeated twice with similar results. ○ indicates control; ▪, IFN-α.
Effect of rhIFN-α on anemia.
RBC count (panel A) and Hb concentrations (panel B) were measured in C57BL/6 mice infected with P yoelii 265 BY. Control and IFN-α–treated mice were injected IP daily, until day 25, with 0.1% BSA (vehicle) or rhIFN-α (5 × 104U). Symbols represent the mean ± SE of 5 mice. The experiment was repeated twice with similar results. ○ indicates control; ▪, IFN-α.
Erythropoiesis during IFN-α treatment of malaria infection
As the level of parasitemia is usually directly correlated with the degree of malaria-induced anemia,26 we undertook experiments to understand the dissociation between them in IFN-α–treated mice (ie, reduced blood parasite load with no reduction in the extent of anemia).
The numbers of early (BFU-E) and late erythroid (CFU-E) progenitors in the bone marrow and spleen were measured on day 14, a few days before the peak of reticulocytosis, in mice injected with P yoelii265 BY. The number of BFU-E in the bone marrow was slightly decreased in control infected mice (Figure 3A), whereas the number of CFU-E was significantly increased (Figure 3C). This increase in CFU-E was not so great in IFN-α–treated mice (Figure 3C).
Effect of rhIFN-α on BFU-E and CFU-E colonies.
The effect of rhIFN-α on the number of BFU-E (panels A, B) and CFU-E (panels C, D) in the bone marrow (panels A, C) and spleen (panels B, D) of C57BL/6 mice, 14 days after inoculation with P yoelii 265 BY. Control and IFN-α–treated mice, infected or uninfected, were injected IP daily with 0.1% BSA (vehicle) or rhIFN-α (5 × 104 U). CFU-E colonies were scored on day 3 and BFU-E colonies on day 8 after plating. Bone marrow CFU-E and BFU-E are expressed as the number of colonies per femur. Results are presented as mean numbers of colonies ± SE for duplicate cultures from 2 to 4 mice.
Effect of rhIFN-α on BFU-E and CFU-E colonies.
The effect of rhIFN-α on the number of BFU-E (panels A, B) and CFU-E (panels C, D) in the bone marrow (panels A, C) and spleen (panels B, D) of C57BL/6 mice, 14 days after inoculation with P yoelii 265 BY. Control and IFN-α–treated mice, infected or uninfected, were injected IP daily with 0.1% BSA (vehicle) or rhIFN-α (5 × 104 U). CFU-E colonies were scored on day 3 and BFU-E colonies on day 8 after plating. Bone marrow CFU-E and BFU-E are expressed as the number of colonies per femur. Results are presented as mean numbers of colonies ± SE for duplicate cultures from 2 to 4 mice.
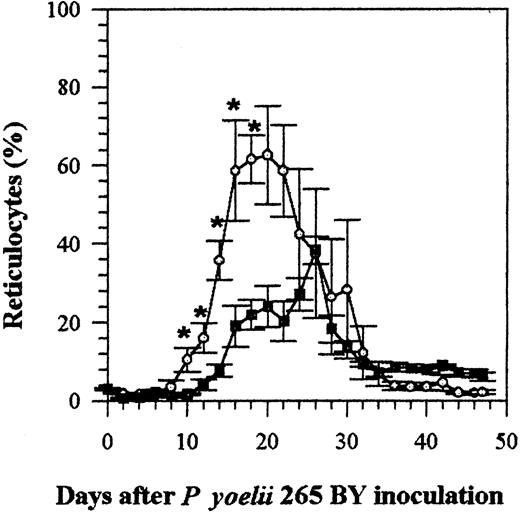
Effect of IFN-α on reticulocytosis induced by malaria infection
The infection of mice with P yoelii causes them to became anemic, which then results in reticulocytosis. The number of reticulocytes in the blood of IFN-α–treated and control infected mice remained within the normal range, less than 5%, until day 8 (Figure 4). The number of reticulocytes then increased in both groups, but reticulocytosis was less marked (P < .05 on days 10 to 18) in mice treated with IFN-α (Figure 4) (the percentage of reticulocytes in uninfected mice remained under 8%). Most (60% to 100%) of the parasitized cells were reticulocytes, demonstrating the selective tropism of the P yoelii strain 265 BY for reticulocytes. This was not changed by IFN-α treatment (data not shown).
Effect of rhIFN-α on reticulocytosis in C57BL/6 mice infected with P yoelii 265 BY.
Control and IFN-α–treated mice were injected IP every day with 0.1% BSA or with rhIFN-α (5 × 104 U). Symbols represent the mean ± SE of 5 mice. The experiment was repeated twice with similar results. *P < .05 (Mann-Whitney test). ○ indicates control; ▪, IFN-α.
Effect of rhIFN-α on reticulocytosis in C57BL/6 mice infected with P yoelii 265 BY.
Control and IFN-α–treated mice were injected IP every day with 0.1% BSA or with rhIFN-α (5 × 104 U). Symbols represent the mean ± SE of 5 mice. The experiment was repeated twice with similar results. *P < .05 (Mann-Whitney test). ○ indicates control; ▪, IFN-α.
Effect of IFN-α on blood parasite loads of other strains or species of rodent Plasmodium
Different strains of Plasmodium have different tropism for mature and immature RBCs. As the effect of IFN-α on parasitemia was associated with the reduction in circulating reticulocytes in aPlasmodium strain with a tropism for reticulocytes, we assessed the effect of the same treatment on other strains or species that had different tropism for RBCs. We used 2 different parasites. One was a cloned line from the P yoelii 17X isolate that also preferentially infects reticulocytes (Fahey and Spitalny21and our observations) and causes a moderate parasitemia in C57BL/6 mice (P yoelii 17X NL, clone A). The other species used (P vinckei petteri 106HW) causes a fulminate infection and shows a tropism for mature cells (our observation).
As for P yoelii 265 BY infected mice, IFN-α also reduced the blood parasite load of mice infected with P yoelii clone A during the first peak of infection, but not during recrudescence, which occurs when IFN-α treatment was stopped (Figure5). However, IFN-α had no effect on the development of the blood parasite load of mice infected with P vinckei: all the mice, both treated and untreated, died 9 to 11 days after infection (Figure 5). Almost 100% of the cells parasitized by this strain were mature RBCs (data not shown).
Effect of rhIFN-α on blood parasite load of C57BL/6 mice infected with P yoelii 17X NL clone A or P vinckei petteri.
IFN-α (5 × 104 U per injection) and control mice were injected IP from days 0 to 25. The blood parasite load is expressed as in Figure 1. Symbols represent the mean ± SE of 6(P yoelii) or 5 and 4 (P vinckei) mice. The experiments were repeated twice with similar results. Each d refers to a dead mouse. *P < .05 (Mann-Whitney test). ○ indicates control; ▪, IFN-α.
Effect of rhIFN-α on blood parasite load of C57BL/6 mice infected with P yoelii 17X NL clone A or P vinckei petteri.
IFN-α (5 × 104 U per injection) and control mice were injected IP from days 0 to 25. The blood parasite load is expressed as in Figure 1. Symbols represent the mean ± SE of 6(P yoelii) or 5 and 4 (P vinckei) mice. The experiments were repeated twice with similar results. Each d refers to a dead mouse. *P < .05 (Mann-Whitney test). ○ indicates control; ▪, IFN-α.
Discussion
We have shown that treatment with recombinant hybrid human IFN-α (BDBB), which is biologically active on murine cells,18reduced blood parasite load in mice infected with parasitized erythrocytes of 2 different parasites with a specific tropism for reticulocytes (P yoelii 265 BY or P yoelii 17X NL clone A), but not in mice infected with a line with tropism for mature RBCs (P vinckei petteri). The reduction in the blood parasite load of IFN-α–treated mice was also accompanied by a less severe hepatosplenomegaly (especially on day 14 after infection).
However, IFN-α did not inhibit the liver-stage infection with P yoelii 265 BY sporozoites (Table 1). These results do not agree with published reports that inducers of type I IFN or a crude serum IFN strongly suppressed the infection of mice with P bergheisporozoites.9-14 Aside from the fact that we used P yoelii rather than P berghei, the major reason may be that we used a pure recombinant IFN-α, whereas previous investigators used IFN inducers, which can induce the production of IFN-α, IFN-β, and even some IFN-γ, as well as other cytokines such as IL-6,27 which can inhibit liver-stage malaria both in vivo and in culture.2,28 29
It is difficult to understand how IFN-α inhibits blood-stage malaria. Schultz et al13 reported that P berghei–parasitized erythrocytes were much less infective or not infective after incubation with crude serum containing IFN. Many years ago, we were unable to confirm these results when we used partially purified cell-culture IFN-α/β (I.G., unpublished observations, 1970), and information obtained during the past 30 years on how IFN acts indicates that IFN is unlikely to act on cells without nuclei (ie, erythrocytes). If IFN-α does not act directly on parasitized cells, it must act in some other manner in malaria-infected animals. C57BL/6 mice infected with P yoelii rapidly became severely anemic. Our examination of the erythropoietic response to malaria infection in untreated mice infected with parasitized erythrocytes showed an increase in CFU-E colonies in the bone marrow and spleen of mice 14 days after infection with P yoelii 265 BY. This increase was inhibited in IFN-α–treated mice. Likewise, more than 60% reticulocytes were observed in malaria-infected control mice, at the peak of infection, whereas the percentage of reticulocytes was much lower (approximately 30%) in IFN-α–treated mice. The reticulocytes were the cellular target for this strain of P yoelii, in both control and IFN-α–treated mice, as 60% to 100% of the parasitized cells were reticulocytes at the peak of parasitemia.
We suggest that the reduction in the blood parasite load in IFN-α–treated mice is due to the fewer reticulocytes available for infection by P yoelii and that the decreased number of reticulocytes is due to the inhibition by IFN-α of splenic CFU-E in infected mice. This was confirmed in experiments showing that IFN-α inhibits P yoelii 17X clone A, which also infects reticulocytes, but not P vinckei petteri, which infects only mature RBCs. It has to be noted that β-methasone, a drug that suppresses erythropoiesis, also selectively inhibits an uncloned line of P yoelii 17X, which infects reticulocytes, but notP vinckei chabaudi, which does not.15
The degree of anemia in malaria is usually correlated with the blood parasite load, especially with rodent malaria parasites.26It was surprising, therefore, to find that the onset and degree of anemia in mice infected with P yoelii 265 BY was the same in both control and IFN-treated mice, despite the reduced blood parasite load. As there was a reduced blood parasite load in IFN-α–treated mice, there was also less lysis of RBCs in these mice. But there were fewer CFU-E in IFN-α–treated mice, which resulted in fewer reticulocytes, and thus it may have contributed to the profound anemia in these mice. IFN-α may have inhibited erythropoiesis directly30-34 or indirectly by inducing the production of other molecules that can inhibit erythropoiesis, such as nitric oxide or IFN-γ.35-37
Despite the extent of parasitemia and anemia, most mice infected withP yoelii 265 BY or 17X NL clone A recovered. For clone A, there were no differences on parasite load during recrudescence in mice treated and not treated with IFN-α. Treatment was stopped at day 25, and this caused a small augmentation of reticulocytes in blood circulation (data not shown). However, this augmentation in the number of reticulocytes was not sufficient to allow a significant increase in parasite development during recrudescence in treated animals as compared with untreated ones.
Here we demonstrated that IFN-α inhibits blood parasite load in mice infected with 2 different strains of P yoelii and that this phenomenon was associated with the inhibition of reticulocytosis. Our interpretation of the effect of IFN-α on blood parasite load is not necessarily applicable to all human parasites, since they show different tropism for reticulocytes. However, the merozoites of certain human malaria species, such as Plasmodium vivax, invade only reticulocytes.38,39 Reticulocytes and young RBCs are also more susceptible to invasion by certain strains of Plasmodium falciparum than are older RBCs.40 It has to be noted that virus infections, such as dengue virus or hepatitis B virus, are very frequent in most areas where malaria is endemic, and these virus infections trigger the production of high concentrations of IFN-α, even for long periods.41 This suggests that P vivax and P falciparum parasites may be susceptible to the effect of IFN-α. Thus, virus infections might influence the course and pathogenesis of malaria infection in virus-infected humans. Moreover, in people infected with more than one species or strain ofPlasmodium, viral infection could lead to a selection of the parasite species or strain with tropism for mature cells. There have been few reports to date that suggest that IFN-α is produced duringfalciparum malaria infection.42-45 As mixed infections of P falciparum and P vivax are frequent in several malaria-endemic regions,46 it is tempting to speculate that in coinfected humans, IFN-α induced byP falciparum infection could block the production of reticulocytes and inhibit the development of P vivax. This clearly deserves further study.
In conclusion, we propose that IFN-α inhibits P yoeliiinfection by blocking reticulocyte production. We also have proposed that mice treated with rhIFN-α could be a good experimental model for studies on the way malaria infection causes anemia and regulates parasite interactions in coinfected individuals.
We thank Dr E. Hulier for her help in quantification of parasite by PCR; Dr David Woodrow for histology; Dr Georges Snounou for carefully reviewing the manuscript; and Dr Owen Parkes for editing the English text.
Supported by a grant from the Institut Electricité et Santé(L.R.) and a fellowship (BM1455/94 and BD9255/96 to A.M.V.) from the Junta Nacional de Investigação Cientifica e Tecnologica, Portugal.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Laurent Rénia, INSERM U445, ICGM, Hôpital Cochin, Bâtiment Gustave Roussy, 27, rue du Fbg Saint Jacques, 75014 Paris, France; e-mail:renia@icgm.cochin.inserm.fr.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal