Abstract
Clinical manifestations of hereditary spherocytosis (HS) can be abrogated by splenectomy. However, concerns exist regarding exposure of patients to a lifelong risk for overwhelming infections and, to a lesser extent, to vascular complications after total splenectomy. In the search for alternative treatment modalities, we assessed, in a previous pilot study, the potential usefulness of subtotal splenectomy in a small population of patients. During a mean follow-up period of 3.5 years, subtotal splenectomy was shown to be effective in decreasing the hemolytic rate, while maintaining the phagocytic function of the spleen. In the current study, we evaluated the clinical and biologic features of 40 patients with HS who underwent subtotal splenectomy and were monitored for periods ranging from 1 to 14 years. The beneficial effect of subtotal splenectomy included a sustained decrease in hemolytic rate and a continued maintenance of phagocytic function of the splenic remnant. However, mild-to-moderate hemolysis was persistent and accounted for secondary gallstone formation and aplastic crisis in a small subset of patients. Surprisingly, regrowth of the remnant spleen did not seem to have a major impact on the beneficial outcomes of these individuals. Our results suggest that subtotal splenectomy appears to be a reasonable treatment option for management of patients with HS, especially young children.
Introduction
Hereditary spherocytosis (HS) is a relatively common inherited hemolytic anemia, with an estimated incidence in Northern Europe of 1 to 2 in 5000 individuals. Spherocytic red cells, precociously trapped in the spleen, are phagocytosed by splenic macrophages, resulting in chronic hemolysis and an increased propensity for gallstone formation. The clinical expression is heterogeneous, varying from benign chronic hemolysis to severe transfusion-dependent anemia.1 Removal of the spleen results in increased red cell life span, decreased transfusion requirement, and decreased incidence of gallstones. Splenectomy is thus the treatment of choice for moderate-to-severe forms of the disease.2,3 Increased awareness that splenectomized patients face a lifelong risk of overwhelming life-threatening infections has dampened enthusiasm for the routine use of total splenectomy for the management of HS.4-7 The phagocytic activity of splenic macrophages and the synthesis of antipolysaccharide antibodies by splenic B-lymphocytes both are necessary to mount an optimal defense against infections. Infectious complications due to circulating encapsulated bacteria can occur irrespective of age and of the time interval after the surgical procedure.8,9 The risk is particularly high during the first years of life. Although vaccinations and systematic oral penicillin prophylaxis have decreased the overall risk,10concerns still exist about serotypes that are not represented in vaccines, penicillin-resistant pneumococcal strains, and lifelong compliance.9 Recent studies have also raised additional concerns regarding long-term complications after splenectomy, such as atherosclerotic events and pulmonary hypertension,11-13reinforcing the need for alternative treatment strategies. In a previous study, we assessed the potential usefulness of subtotal splenectomy in managing a small population of patients with HS.14 During a mean follow-up period of 3.5 years, subtotal splenectomy was shown to be effective in decreasing the hemolytic rate, while maintaining the phagocytic function of the spleen. Although these findings were encouraging, a number of issues remained unanswered, including whether the clinical benefit can be sustained over a long period and what are the clinical implications of the regrowth of the remnant spleen. This study attempts to address these issues. We evaluated the clinical and biologic features of 40 patients with HS who underwent subtotal splenectomy and were monitored for periods ranging from 1 to 14 years. The beneficial effect of subtotal splenectomy included a sustained decrease in the hemolytic rate that transformed a moderately severe-to-severe hemolytic anemia to mild chronic hemolytic anemia and the continued phagocytic function of the splenic remnant. It did not, however, abolish the risk of aplastic crisis or the formation of gallstones. Interestingly, the regrowth of the remnant spleen did not have a major impact on the beneficial outcome. Subtotal splenectomy thus seems to be a reasonable option for the management of patients with HS, especially young children.
Patients, materials, and methods
Subjects
Our hematology clinic is one of the referral centers for red blood cell diseases in France. Of the cases referred to us between 1985-1998, splenectomy was recommended for 40 patients. After extensive discussions regarding the potential benefits and risks and of obtaining informed consent, subtotal splenectomy was performed. The clinical and hematologic features of these 40 patients with HS who underwent subtotal splenectomy were monitored until December 1999. The patients were 39 children (17 girls and 22 boys; age range: 1 to 15 years, mean age: 8.1 ± 4.3 years) and one man aged 25 years. A diagnosis of HS was confirmed by documenting the decreased membrane surface area by osmotic gradient ektacytometry and by the analysis of red cell indices from an automated hematology analyzer (Bayer Diagnostics, France) as previously described.15 The mode of inheritance of HS was dominant for 20 subjects, recessive for 10 subjects, and indeterminate for 10 others. In 12 individuals, the indication for subtotal splenectomy was severe anemia with a recurrent need for transfusions. For the other 28 individuals, the decision was based on careful clinical monitoring over time of a number of variables, including the degree of chronic anemia, propensity for chronic fatigue, documentation of marked splenomegaly, and presence of gallstones or cholelithiasis (10 subjects).
Splenic necrosis was documented in one child after subtotal splenectomy. Five other children were lost to follow-up during the first year after surgery. For the remaining 34 patients, total duration of follow-up after subtotal splenectomy was 203 patient-years. The mean duration of follow-up for individual patients was 6 ± 3.7 years (range of 1 to 14 years). Seventeen of these subjects were followed for a minimum of 5 years.
Surgical procedure
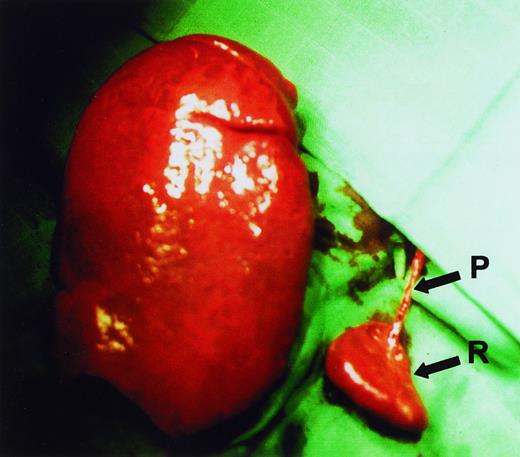
Subtotal splenectomy was performed using the surgical procedure that we described earlier.14 A subtotal resection of 80% to 90% of the enlarged spleen was performed after primary ligation of all pedicles supplying the part of the spleen to be resected (Figure1). The need for a large resection of the spleen, while preventing necrosis of the small splenic remnant, demands that the procedure be carefully and meticulously performed by an experienced surgeon. Half of the patients also underwent a simultaneous cholecystectomy.
Spleen sizes.
Relative sizes of the remnant spleen (R) and the enlarged excised spleen in a HS patient during surgery. It can be seen that a single vascular pedicle (P) provides blood supply to the remnant spleen.
Spleen sizes.
Relative sizes of the remnant spleen (R) and the enlarged excised spleen in a HS patient during surgery. It can be seen that a single vascular pedicle (P) provides blood supply to the remnant spleen.
Assessment of hemolytic rate and of erythropoietic compensation
Mean values of hemoglobin, reticulocyte counts, and bilirubin levels during preoperative and postoperative follow-up periods were compared. Results obtained on blood samples drawn within 2 months after a transfusion were not included in the analysis. In our earlier study,14 we also quantitated red blood cell life span before and after surgery using 51Cr-labeled autologous erythrocytes in 10 patients.
Assessment of the phagocytic function of the spleen
The phagocytic function of the splenic remnant was assessed by sequential evaluation of the number of circulating pitted red cells by interference contrast phase microscopy with Nomarski optics,16 and by the study of the splenic uptake of technetium99-labeled heat-damaged erythrocytes with radionuclide scanning.17
Growth of the splenic remnant
Postoperative growth of the splenic remnant was quantitated over time in a subset of subjects by repeated isotopic splenic scans. Before 1996, multiple planar views of the spleen were acquired by using a parallel collimator, and splenic volume was calculated by using a spheroid geometric model. Since 1996, abdominal single photon emission computed tomography (SPECT) acquisition was performed, and splenic volume was calculated by using a threshold algorithm previously validated on a phantom study. A very good correlation between splenic volume determined with planar or SPECT technique was noted (r = 0.97). Calculated values for the splenic volume were compared with the expected normal splenic volume value by using the algorithm: splenic volume (mL) = 6.516 × body weight (kg) (0.797).18
Postsurgical growth pattern and quality of life
Height growth was assessed by using standard growth curves. The quality of life was evaluated by interviewing both the parents and the affected child regarding the child's sleep patterns, the ability to perform schoolwork, and participation in athletics.
Statistical analysis
Mean values of hemoglobin and reticulocyte count at 1 year, 3 to 4 years, 5 to 6, 7 to 9, and 10 years or more were compared with mean values before subtotal splenectomy. Mean values at 7 to 9 years and 10 years or more were also compared with mean value at 1 year after surgery. Equality of means were compared by Wilcoxon matched-pairs signed-ranks test. A Bonferroni correction was applied to take into account multiple comparison tests (multiplication by 7 applied to theP-values). Correlation between the isotopic splenic volume and the hemoglobin value or reticulocyte count was analyzed with the Spearman coefficient. The threshold significance was set at 0.05.
Results
Surgical procedure
Subtotal splenectomy was feasible in all 40 patients. The lower part of the spleen was preserved in 39 of the 40 cases, whereas in one case, the upper part of the spleen had to be preserved because of an unusual anatomic presentation. The mean duration of surgery was 1 hour 30 minutes ± 30 minutes for subtotal splenectomy, and 1 hour 45 minutes ± 20 minutes for subtotal splenectomy in conjunction with cholecystectomy. During the surgical procedure, 85% ± 6% of the splenic tissue was removed from all patients. In one case, an incidental contact of the thermocauter with the vascular pedicle perfusing the remnant resulted in an arterial wound, leading to remnant necrosis.
Intraoperative bleeding was mild in all but one of the patients who had to be transfused postoperatively and reoperated on to achieve local hemostasis. Cholecystectomy was performed on 22 patients because of either symptomatic (7 cases) or asymptomatic cholelithiasis (8 cases), or at the request of parents to prevent the future occurrence of gallstones (7 cases). In 2 of the patients, stones had to be removed from the main bile duct. During the postoperative course, 20 of the 40 patients experienced transient episodes of fever, but all the children were discharged 4 to 7 days after surgery.
Variations in mean hemoglobin values and mean reticulocyte counts over time
Hemoglobin values and reticulocyte counts after subtotal splenectomy are shown in Figure 2. In the children with at least 10 years of follow-up, no significant differences were noted between the one-year and the 10-year postoperative hemoglobin values and reticulocyte counts. After 8 years, hemoglobin values were above 12 g/dL in 6 of the 8 patients. We observed relapse of a well-tolerated, chronic anemia in only one patient at 5 years after surgery. Red cell life-span determination in 10 patients showed that after subtotal splenectomy red cell half-life (T50) increased on the average by 6.5 days (range: 5 to 14.5 days).14 The mean bilirubin values decreased from 50 mM before surgery to 20 mM after surgery.
Improvement of hematologic parameters after subtotal splenectomy during the follow-up period.
Hemoglobin values and reticulocyte counts were assessed for a 3-year period before surgery and for up to 12 years after subtotal splenectomy. Even after a Bonferroni correction, mean hemoglobin increased significantly from a preoperative mean value of 9.2 ± 2.6 g/dL to respectively 12.7 ± 1.2 g/dL (31 children) (P < .001), 12.6 ± 1.9 (15 children) (P < .001), 12.3 ± 1.9 g/dL (8 children) (P < .01), 11.7 ± 2.0 g/dL (10 children) (P < .01) at 6 to 18 months, 3 to 4 years, 5 to 6 years, 7 to 9 years after surgery; at 10 years, increase in hemoglobin was at the limit of significance (11.1 to1.7 g/dL in 4 children;P = .06). The reticulocyte count decreased significantly from 523 ± 442 × 109/L before surgery to 267 ± 130 × 109/L at 6 to 18 months after surgery (31 children) (P < .05). This decrease in reticulocyte count persisted during subsequent years (388 ± 224 × 109/L [12 children] at 3 to 4 years; 425 ± 194 × 109/L [8 children] at 5 to 6 years; 327 ± 246 × 109/L [9 children] at 7 to 9 years; and 268 ± 81 × 109/L [4 children] at 10 years and more), but without statistical significance. The arrow indicates the time of surgery.
Improvement of hematologic parameters after subtotal splenectomy during the follow-up period.
Hemoglobin values and reticulocyte counts were assessed for a 3-year period before surgery and for up to 12 years after subtotal splenectomy. Even after a Bonferroni correction, mean hemoglobin increased significantly from a preoperative mean value of 9.2 ± 2.6 g/dL to respectively 12.7 ± 1.2 g/dL (31 children) (P < .001), 12.6 ± 1.9 (15 children) (P < .001), 12.3 ± 1.9 g/dL (8 children) (P < .01), 11.7 ± 2.0 g/dL (10 children) (P < .01) at 6 to 18 months, 3 to 4 years, 5 to 6 years, 7 to 9 years after surgery; at 10 years, increase in hemoglobin was at the limit of significance (11.1 to1.7 g/dL in 4 children;P = .06). The reticulocyte count decreased significantly from 523 ± 442 × 109/L before surgery to 267 ± 130 × 109/L at 6 to 18 months after surgery (31 children) (P < .05). This decrease in reticulocyte count persisted during subsequent years (388 ± 224 × 109/L [12 children] at 3 to 4 years; 425 ± 194 × 109/L [8 children] at 5 to 6 years; 327 ± 246 × 109/L [9 children] at 7 to 9 years; and 268 ± 81 × 109/L [4 children] at 10 years and more), but without statistical significance. The arrow indicates the time of surgery.
Platelet counts
During the first month after surgery, the mean platelet count in the 40 patients with HS was 610 × 109/L (range: 367 to 850). Thrombocythemia, defined as platelet count greater than 500 × 109/L, was noted in 30 of the 40 patients after surgery. However, platelet counts returned to values of less than 420 × 109/L in all patients within 2 years.
Transfusion requirements
The overall need for blood transfusions in our HS population decreased dramatically after subtotal splenectomy. Patients with HS received, on the average, 0.32 units of blood per year of life before surgery but only 0.02 units of blood per year of life after surgery. During the follow-up period, only 5 patients required blood transfusions. Parvovirus B19 infection in 3 patients and Epstein-Barr virus infection in one patient led to transient acute aregenerative anemia requiring a single transfusion. A fifth patient was transfused twice during a 4-year period to manage recurrence of acute anemia in conjunction with transient increases in the size of the splenic remnant.
Phagocytic function of the spleen
All patients had been vaccinated against Haemophilus influenzae either before splenectomy or since 1993 during infancy. Strepococcus pneumoniae immunization was performed before surgery, and booster vaccinations were given every 5 years. Oral penicillin prophylaxis was prescribed to all individuals for 1 year after surgery and was only discontinued after documentation of the persistence of the filtering ability of the spleen. In very young children, it was prescribed up to 5 years of age.
No overwhelming infection was documented in any of the patients with a splenic remnant during the follow-up period. Howell-Jolly bodies appeared transiently after surgery in 19 of 40 patients. In 16 of these patients, they were noted only during the first week after surgery, but in 3 others, they persisted up to 3.5, 12, and 33 months. The percentage of pitted erythrocytes was in the normal range (less than 2%) in 26 patients during a follow-up period that ranged from 1 to 14 years. In 2 patients, the percentage of pitted erythrocytes transiently increased at 3 and 6 years after surgery but was found to be normal at a subsequent evaluation performed 6 months later. Radionuclide scanning was performed on 31 children at periods ranging from 1 to 14 years after subtotal splenectomy. In 30 of the children, normal uptake of heat-damaged red cells in the splenic remnant could be consistently shown. In contrast, in the one patient who had experienced perioperative splenic necrosis, no uptake of heat-damaged red cells could be demonstrated. In this patient, the absence of splenic function was confirmed by the finding of increased circulating pitted red cells and Howell-Jolly bodies.
Growth of the splenic remnant
The mean splenic volume before surgery was estimated to be 4 to 5 times larger than that of a normal spleen in age-matched controls. Growth of the splenic remnant over time after surgery was assessed by performing 39 radionuclide splenic scans on 31 children. Significant regrowth of the splenic remnant was noted during the first year after surgery. Surprisingly, after this initial spurt, the rate of growth was much reduced and appeared to reach either a plateau value or show a modest increase over the years (Figure3). The remnant regrowth did not correlate with the measured hemoglobin values (P = .09) or reticulocyte counts (P = .2). It should be noted, however, that a marked increase in the splenic remnant volume was found in 2 children assessed at 8 years after surgery.
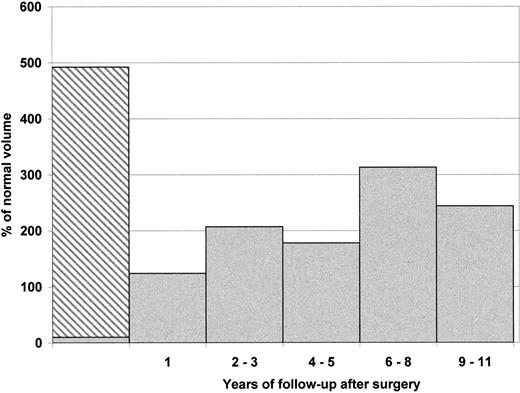
Splenic remnant growth over time in patients with HS after subtotal splenectomy.
Mean volume of the spleen is expressed as percentage (%) of normal volume according to body weight. Mean initial splenic volume before surgery was noted to be 492% for 7 individuals (range: 284% to 820%). The hatched area represents the volume of the spleen excised. After 1 year, the regrowth of the remnant led to a normal splenic volume (mean 124% for 10 children). Mean splenic volume value after 2 to 3 (3 children), 4 to 5 (7 children), 6 to 8 (5 children), and 9 to 11 (3 children) years were respectively 207%, 178%, 313%, and 244%. The large mean splenic volume noted at 6 to 8 years after surgery is skewed because of the inclusion of data from 2 children who exhibited unusually large splenic regrowth (422% and 500% of normal).
Splenic remnant growth over time in patients with HS after subtotal splenectomy.
Mean volume of the spleen is expressed as percentage (%) of normal volume according to body weight. Mean initial splenic volume before surgery was noted to be 492% for 7 individuals (range: 284% to 820%). The hatched area represents the volume of the spleen excised. After 1 year, the regrowth of the remnant led to a normal splenic volume (mean 124% for 10 children). Mean splenic volume value after 2 to 3 (3 children), 4 to 5 (7 children), 6 to 8 (5 children), and 9 to 11 (3 children) years were respectively 207%, 178%, 313%, and 244%. The large mean splenic volume noted at 6 to 8 years after surgery is skewed because of the inclusion of data from 2 children who exhibited unusually large splenic regrowth (422% and 500% of normal).
Postsurgical growth pattern and quality of life
In the 5 prepubertal children who we were able to study, we could document a growth spurt after surgery. The increase was equivalent to 2 standard deviations in normal height growth curves. Improvement in the quality of life was noted in 92% of the cases 1 year after surgery and was sustained. It must be stressed that in some cases such improvement was indeed marked. For example, many patients after surgery were able to effectively sustain physical activities such as cycling or hiking with their unaffected siblings.
Clinical complications and secondary total splenectomies
Three patients experienced complications that led to a secondary total splenectomy. A male patient, who underwent surgery at the age of 26 months, had an acute episode of anemia (hemoglobin value of 7.5 g/dL) 11 months after surgery and an abrupt increase in the remnant spleen size. He was treated with a single red blood cell transfusion. During the following 5 months, the splenic remnant returned to its precrisis size and the hemoglobin value was stable at 10 to 11 g/dL. However, a similar acute episode recurred 2 years later requiring another transfusion and the patient underwent a total splenectomy. Neither the surgical exploration nor the macroscopic and histologic examination of the remnant spleen offered any clues to the cause of these 2 acute episodes. As splenic sequestration crises are observed in sickle cell disease, we looked for but could not document abnormal hemoglobin in this child. Neither could we document clinical or biologic signs of viral-induced splenic enlargement. In 2 other patients, total splenectomy was performed 4 and 6 years after subtotal splenectomy because of the recurrence of icterus, chronic fatigue, and mild anemia. Secondary surgery was uneventful in all 3 cases. Newly formed gallstones, a consequence of the persistent mild hemolysis, were detected by systematic ultrasound echography in 4 of the 18 noncholecystectomized patients 7 to 56 months after surgery.
Discussion
Classical manifestations of HS include anemia, chronic icterus, and cholelithiasis. Total splenectomy, which abrogates hemolysis, is the most attractive treatment option. Yet, it is likely to expose the patient to a lifelong risk for potentially lethal infections.8,9 Chronic hypercoagulable states leading to thrombosis have also been reported in some human heritable hemolytic disorders and in murine HS.19 Secondary atherosclerotic events can occur in human subjects after splenectomy for trauma11 or HS.12 Splenectomized individuals, including patients with HS, are overrepresented in a population with pulmonary hypertension.13 We previously suggested that subtotal splenectomy could potentially be an effective alternative to total splenectomy in HS-affected individuals.14 However, this preliminary study left many questions unanswered. The current study provides data on a larger population of patients, with a substantially longer period of follow-up.
For all 40 patients with HS, the surgery was easily feasible with no perioperative morbidity. In only one case did the splenic remnant necrose, and this occurred after thermal trauma to the pedicle. This highlights the fact that during the surgical procedure the minute vascular pedicle needs to be handled with greatest caution. Sectioning of the parenchyma is better performed with a knife rather than with a thermocautery, so as to prevent electric burns of the single persistent narrow pedicle. In one other case, the abrupt onset of a severe anemia and increase in the size of the remnant spleen led to the surgical removal of the splenic remnant. This complication has previously been reported in individuals with HS who have coexistent hemoglobinopathies20,21 and after partial splenectomy in Gaucher's disease.22
Subtotal splenectomy leads to a decrease in hemolysis, as evidenced by an increase in 51Cr-labeled red blood cell life span, an increase in hemoglobin values, and a decrease in reticulocyte counts. We show here that beneficial clinical effects are sustained over a long period. However, the hematologic improvement after subtotal splenectomy is less spectacular than that observed after total splenectomy.23 A mild hemolytic state is still persistent. The occurrence of severe anemia in 4 patients with virus-related acute red cell aplasia illustrates the fact that the decrease in hemolysis after subtotal splenectomy is not of sufficient magnitude to compensate for a transient arrest in erythropoiesis. Patients and families should be aware of such a possibility when subtotal splenectomy is being considered, especially in the absence of antiparvovirus B19-specific IgG. Similarly, gallstone formation occurred after subtotal splenectomy in the patients in whom cholecystectomy had not been performed, as a result of persistent mild hemolysis.
Various approaches to evaluate the phagocytic function of the remnant spleen shed insight into the postsurgical preservation of this function. During a total observation period of 203 patient- years of life with a splenic remnant, we did not encounter a single case of severe infection. This finding, however, does not allow us to make any definite conclusion because the mortality rate after splenectomy has been estimated to be 0.73 per 1000 years8 and has probably decreased, as a result of the widespread use of prophylactic treatment strategies.10 In contrast, the assessment of pitted red cells and uptake of heat-damaged red cells by the splenic remnant provide strong indirect evidence that the filtering function of the spleen is being sustained. However, it has to be stressed that the ability of such patients to produce antipolysaccharide antibodies that are involved in humoral defense against encapsulated bacteria and are synthesized by a splenic B-lymphocyte subpopulation has still to be evaluated.24 25
Obvious clinical benefits could be documented in patients with HS after subtotal splenectomy. In our experience, chronic discomfort that does not correlate with the intensity of anemia is a frequent finding in untreated patients with HS. Cytokines produced by activated splenic macrophages, the numbers of which are markedly increased in HS,26 could be responsible for chronic discomfort. We suggest that, by decreasing the macrophage mass, subtotal splenectomy may reduce cytokine production and thereby reduce chronic discomfort. The increase in height growth score after surgery provides an additional objective validation of the clinical benefit of subtotal splenectomy.
Several factors are relevant in assessing the potential thrombotic risk after either total or subtotal splenectomy in patients with HS. In the case of total splenectomy, thrombocythemia is often persistent, hemoglobin values are significantly higher than that of normal controls,15 and it is likely that the percentage of circulating abnormal red cells that can potentially activate platelets is increased. In contrast, after subtotal splenectomy, platelet counts returned to normal values, hemoglobin values were found to be in the low normal range, and severely abnormal red cells are still being cleared from circulation. On the basis of these findings, we suggest that subtotal splenectomy is likely to carry a lower risk of thrombotic events than total splenectomy.
If subtotal splenectomy is being contemplated for HS, evaluating the adequate amount of splenic tissue to be removed is an important issue. In a rat model of incremental partial splenic resection, animals could survive intravenous injection of S pneumoniae as long as 25% to 50% of the normal splenic tissue was preserved.27,28 In humans, optimal protection is likely to depend on the residual splenic mass29-31 and on the preservation of adequate blood flow.32 To obtain a splenic remnant accounting for 25% of the volume of a normal spleen required that approximately 90% of the volume of an enlarged spleen be removed. This is achieved by preserving the splenic tissue supplied by a single pedicle. The size of the remnant spleen increased in all cases. Although growth velocity was high during the first postsurgical year, it subsequently slowed, and during subsequent years, the volume of the splenic remnant stabilized. Moreover, we did not find any obvious correlation between the growth velocity of the splenic remnant and the degree of resurgent hemolysis. Should severe hemolysis re-emerge in a subset of patients who underwent subtotal splenectomy during the coming decades, total splenectomy will be considered, and families should be made aware of such a possibility. In our experience, performing a secondary total splenectomy several years after a subtotal splenectomy did not pose any significant surgical difficulties.
Subtotal splenectomy has proven, in our hands, to provide a persistent decrease in the hemolytic rate, while preserving the integrity of splenic phagocytic function. On the basis of our own experience, we favor subtotal splenectomy as the first line of treatment in HS cases in which splenectomy is being considered for transfusion-dependent infants. It should be stated, however, that subtotal splenectomy has to be accompanied by taking all necessary precautions regarding potential sepsis risk in case of secondary necrosis of the remnant. Antipneumococcal and anti-Haemophilus vaccinations must be performed before surgery. Oral penicillin prophylaxis can be discontinued only if normal phagocytic function of the splenic remnant is documented. Hopefully, this alternative management strategy for HS will partly solve the distressing dilemma between the risk of long-term severe infectious complications and the prompt necessity to decrease anemia and provide a better quality of life.
Supported in part by la Direction de la Recherche Clinique Assistance Publique-Hôpitaux de Paris (CRC 96082) and by a National Institutes of Health Grant DK26263.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Narla Mohandas, Life Sciences Division, Berkeley National Laboratory, Mailstop 74-157, 1, Cyclotron Rd, Berkeley, CA 94720; e-mail: mnarla@lbl.gov.

![Fig. 2. Improvement of hematologic parameters after subtotal splenectomy during the follow-up period. / Hemoglobin values and reticulocyte counts were assessed for a 3-year period before surgery and for up to 12 years after subtotal splenectomy. Even after a Bonferroni correction, mean hemoglobin increased significantly from a preoperative mean value of 9.2 ± 2.6 g/dL to respectively 12.7 ± 1.2 g/dL (31 children) (P < .001), 12.6 ± 1.9 (15 children) (P < .001), 12.3 ± 1.9 g/dL (8 children) (P < .01), 11.7 ± 2.0 g/dL (10 children) (P < .01) at 6 to 18 months, 3 to 4 years, 5 to 6 years, 7 to 9 years after surgery; at 10 years, increase in hemoglobin was at the limit of significance (11.1 to1.7 g/dL in 4 children;P = .06). The reticulocyte count decreased significantly from 523 ± 442 × 109/L before surgery to 267 ± 130 × 109/L at 6 to 18 months after surgery (31 children) (P < .05). This decrease in reticulocyte count persisted during subsequent years (388 ± 224 × 109/L [12 children] at 3 to 4 years; 425 ± 194 × 109/L [8 children] at 5 to 6 years; 327 ± 246 × 109/L [9 children] at 7 to 9 years; and 268 ± 81 × 109/L [4 children] at 10 years and more), but without statistical significance. The arrow indicates the time of surgery.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/2/10.1182_blood.v97.2.399/5/m_h80210607002.jpeg?Expires=1765934970&Signature=JM7B7R1kcjqcRHabV0izsVpQwOz~z7acL0dUT7nyCUppNoQj1ChEAyQypRWtKtAcQ6up-KiGwR01uPXnVTtiAx00hz70sD0OutClD3JMSFC4BUfjEPRO1GSyIj3pWhaAna0aC2yGImzJ~KcLxSH8wrV~7o4iWZaY0yKEkEKOD0v~zeH8Y6Do1Ie3h6bMov1efatMRw9-eLFwqd14MZ2-BzmcHWfYS5L2i8JTe8dAZ3lqyW-UEff5KWyoy6Mxn3RSyPyj1cqnD1y1sJGhWEbL1d0eLHEklIkU~obQGF~DmgQqowDIvwNxSIHNKpkWIFDClPoYMZaiHeU8F5qO8WUSlw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal