Abstract
Advanced stage follicular small cleaved and mixed cell lymphoma is characterized by relapse from remission and survival ranging from 6 to 12 years. Because young patients have the greatest compromise in longevity, the efficacy and toxicity of high-dose radiochemotherapy and bone marrow transplantation after conventional chemotherapy was evaluated in a prospective phase II clinical trial. Thirty-seven patients in a minimal disease state after conventional chemotherapy received fractionated total body irradiation and high-dose etoposide and cyclophosphamide, followed by purged autologous bone marrow. A reference sample of 188 patients of similar age, stage, and histology managed at this institution before 1988 was identified for comparison of patient characteristics and outcomes. Compared with reference patients, transplant recipients had a higher tumor burden at diagnosis. With a median follow-up of 6.5 years, the estimated 10-year survival after transplantation was 86%. There was a single lymphoma death yielding a 10-year disease-specific survival of 97%. There were 2 early transplant-related deaths and 2 late acute leukemia deaths. Ten patients relapsed, one with microscopic disease only. High tumor burden at diagnosis and incomplete response to chemotherapy adversely influenced survival in the reference but not in the transplanted patients. The estimated risk of death of 14% and relapse of 30% at 10 years in our transplanted follicular lymphoma patients, the majority of whom had high tumor burdens, compares favorably with our observations in appropriately matched reference patients.
Introduction
Advanced-stage low-grade lymphoma is a relatively indolent disorder with median survivals of 6 to 12 years.1Features of the natural history include a high initial rate of response to chemotherapy and radiotherapy, recurrence of disease, transformation to a more aggressive histology, and occasional spontaneous regression.1-3 Historically, single and multiple drug alkylating agent-based therapy, with or without doxorubicin, has been widely used in primary treatment. Other options have included deferred therapy in selected patients, purine analogues alone or in combination, and interferon as induction or adjuvant therapy.2 4-8 Most studies have failed to demonstrate a survival advantage over historical or conventional controls.
In the 1980s, data emerging from Europe and the United States suggested improved outcomes in selected patients with recurrent non-Hodgkin lymphoma treated with high-dose therapy and autologous bone marrow transplantation (ABMT).9,10 In addition to chemotherapy, ABMT permitted the use of myeloablative doses of total body irradiation. This feature is attractive because follicular lymphomas are exquisitely sensitive to radiotherapy, with sustained remissions in a subset of limited-stage patients treated with moderate-dose regional radiation. The development of techniques to ex vivo purge residual lymphoma from the bone marrow further encouraged research efforts in ABMT.10 These facts provided the rationale to explore the efficacy and safety of high-dose therapy and ABMT in relapsed follicular lymphoma.11-13 Because a major principle of dose effect is intrinsic chemosensitivity of the tumor and tumors acquire drug resistance in response to chemotherapy, the optimal timing for high-dose therapy with curative intent should be primary treatment. On this basis, we conducted a prospective phase II trial of high-dose therapy and ABMT in patients with follicular lymphoma in first partial or complete remission.
Patients, materials, and methods
Patient selection and treatment protocol
Patients 50 years or younger with stage III or IV, previously untreated follicular small cleaved (FSC) or follicular small cleaved and large cell (FM) lymphoma were eligible if they achieved a minimal disease state with conventional chemotherapy, there was no contraindication to the planned treatment, and written, informed consent was obtained. Minimal disease (MD) was defined as no single lymph node more than 2 cm in transverse diameter or a greater than 75% reduction in a confluent lymph node mass and 10% or less involvement by lymphoma on 2 bone marrow biopsies. Among 40 patients evaluated, 37 were eligible. Reasons for ineligibility included severe infection during conventional chemotherapy (n = 1) and failure to achieve MD (n = 2).
The schema for the clinical trial is outlined in Figure1. Whenever possible, a representative lymph node biopsy specimen was obtained to establish a tumor marker for the evaluation of residual disease. Patients initially received conventional chemotherapy cytoreduction. The combination of cyclophosphamide 400 mg/m2 orally 5 times a day, vincristine 1.4 mg/m2 (maximum dose 2 mg) intravenously, and prednisone 100 mg/m2 orally 5 times a day (CVP) was the preferred regimen. Treatment was continued for 2 cycles beyond best response, based on physical examination, computed tomography, residual lymphogram contrast, and bone marrow biopsy. In some cases, treatment was initiated before referral and alternate chemotherapy combinations were used. In other cases, an alternative regimen was used after CVP because MD had not been attained. As detailed in Figure 1, on achievement of MD, patients proceeded to marrow harvest on recovery of the white blood count. However, because of suboptimal yield in some early patients, subsequent harvests were performed after a 60-day recovery period. The marrow was treated with a panel of monoclonal antibodies directed against CD9, CD10, CD19, and CD20 plus rabbit complement as previously described.14 The preparatory regimen consisted of ten 120 cGy fractions of total body irradiation, etoposide (VP16) 60 mg/kg, and cyclophosphamide 100 mg/kg.14
Schema for conventional therapy cytoreduction and autologous bone marrow transplantation.
Schema for conventional therapy cytoreduction and autologous bone marrow transplantation.
Reference population and tumor burden evaluation
For the purposes of comparison, a reference population of 188 patients in whom lymphoma was diagnosed between 1962-1988 was identified from the Stanford Lymphoma Database. These patients were selected on the basis of age 50 years or younger, a diagnosis of advanced stage FSC or FM lymphoma managed with conventional therapy, and availability of medical records for review and follow-up. We verified that overall survival (OS) was not different in the 1962-1978 era (n = 94) compared with the 1979-1988 era (n = 94),P = .36. The tumor burden criteria of the Groupe d'Etudes Lymphomes Folliculare (GELF) were applied to this reference as well as the study population.15 In this system, any one of the following characteristics qualify as a high tumor burden: systemic symptoms, 3 or more lymph node sites greater than 3 cm, a single lymph node site greater than 7 cm, cytopenia (platelets less than 100 000/μL or neutrophils less than 1000/μL) or leukemia (greater than 5000 lymphoma cells/μL), marked splenomegaly, compressive symptoms, and pleural effusion or ascites. For our analysis, circulating lymphoma cells diagnosed on peripheral blood smears were interpreted as evidence of a high tumor burden and splenomegaly was defined as a spleen palpable several finger breadths below the left costal margin.
Polymerase chain reaction analysis
Polymerase chain reaction (PCR) amplification at the major breakpoint regions (MBR) and minor cluster region (mcr) of thebcl-2/IgH rearrangement of the t(14;18) translocation were performed as previously described.16 Diagnostic material (lymph node biopsy) or bone marrow aspirates (if histologically involved) were subjected to PCR analysis to determine a tumor marker for individual patients. Bone marrow samples were analyzed before and after purging and periodically for long-term follow-up.
Follow-up
Recommended follow-up for patients was every 2 months for the first year, every 3 months for the second year, every 4 months for the third and fourth years, every 6 months for the fifth year, and annually thereafter. Chest and abdominal radiographs were recommended with each visit, the latter as long as lymphography contrast remained. Computerized tomography of the abdomen and pelvis and bone marrow biopsy were recommended annually. As previously noted, bone marrow aspirates obtained at Stanford were analyzed for molecular evidence of disease.
Evaluation and statistical methods
OS and freedom from progression (FFP) were calculated from the day of marrow transplantation for study patients and from the date of diagnosis for the reference sample. For the calculation of disease-specific survival, deaths due to other causes (n = 4) were censored. For the endpoint failure-free survival (FFS), the earlier event of relapse or death in remission was considered as the date of failure. Survival was estimated by the method of Kaplan and Meier.17 The log-rank test was used to compare survival curves.18
Results
From August 1988 to April 1994, 37 patients met the eligibility criteria and proceeded to transplantation. Their characteristics are described in Table 1. The median age was 37 years (range 26 to 49). Sixty-two percent were male. Marrow involvement was present in 86%. Twelve patients had more than one extranodal disease site. The median number of Ann Arbor disease sites was 9, ranging from 3 to 13. Despite extensive disease, only one patient was nonambulatory (ECOG performance status 2 or greater) at diagnosis. All patients had one adverse risk factor and one had 4 risk factors according to the International Prognostic Factors Index, but the majority had low- or low-intermediate–risk disease with missing lactate dehydrogenase in 7 patients.13 By eligibility criteria, none of our patients were older than 60 years and, thus, the distribution of IPI scores cannot be compared with the available literature.
Characteristics of 37 study patients
| Characteristic . | N . | % . |
|---|---|---|
| Follicular small cleaved cell | 23 | 62 |
| Follicular mixed small cleaved and large cell | 14 | 38 |
| Male | 23 | 62 |
| Female | 14 | 38 |
| Stage III | 5 | 14 |
| Stage IV | 32 | 86 |
| Bone marrow involvement | 32 | 86 |
| Bone marrow involvement >20% | 14 | 38 |
| B symptoms | 7 | 19 |
| Ann Arbor sites ≥9 | 18 | 49 |
| Extranodal sites ≥2 | 12 | 32 |
| Nonambulatory (ECOG ≥ 2) | 1 | 3 |
| Elevated lactate dehydrogenase | 3* | 10* |
| Characteristic . | N . | % . |
|---|---|---|
| Follicular small cleaved cell | 23 | 62 |
| Follicular mixed small cleaved and large cell | 14 | 38 |
| Male | 23 | 62 |
| Female | 14 | 38 |
| Stage III | 5 | 14 |
| Stage IV | 32 | 86 |
| Bone marrow involvement | 32 | 86 |
| Bone marrow involvement >20% | 14 | 38 |
| B symptoms | 7 | 19 |
| Ann Arbor sites ≥9 | 18 | 49 |
| Extranodal sites ≥2 | 12 | 32 |
| Nonambulatory (ECOG ≥ 2) | 1 | 3 |
| Elevated lactate dehydrogenase | 3* | 10* |
Available in 30 patients.
As seen in Table 2, 73% of patients had a high tumor burden according to the GELF criteria before study entry. About one third of the study patients had bulky disease and 19% had 3 or more lymph node sites greater than 3 cm. The median time from diagnosis to transplantation was 10 months. A single patient was observed initially and treated on disease progression.
Comparison of tumor burden characteristics
| . | Transplanted patients, % . | Reference patients, % . |
|---|---|---|
| n = 37 . | n = 188 . | |
| High tumor burden | 73 | 49 |
| Splenomegaly | 22 | 20 |
| Leukemia/cytopenia | 30 | 6 |
| Bulk > 7 cm | 32 | 18 |
| Effusion/ascites | 5 | 2 |
| Three or more sites > 3 cm | 19 | 36 |
| Constitutional symptoms | 19 | 15 |
| Compression | 3 | 2 |
| . | Transplanted patients, % . | Reference patients, % . |
|---|---|---|
| n = 37 . | n = 188 . | |
| High tumor burden | 73 | 49 |
| Splenomegaly | 22 | 20 |
| Leukemia/cytopenia | 30 | 6 |
| Bulk > 7 cm | 32 | 18 |
| Effusion/ascites | 5 | 2 |
| Three or more sites > 3 cm | 19 | 36 |
| Constitutional symptoms | 19 | 15 |
| Compression | 3 | 2 |
All 37 patients received CVP chemotherapy, exclusively for 6 to 12 cycles in 24 patients, and with other chemotherapy combinations in 13 patients. Other combinations included CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) for 2 to 6 cycles in 6 patients, CMOPP (cyclophosphamide, vincristine, procarbazine, prednisone) for 7 cycles in one patient, CNOP (cyclophosphamide, mitoxantrone, vincristine, prednisone) for 3 to 6 cycles in 2 patients, and ProMACE-MOPP (prednisone, methotrexate, doxorubicin, cyclophosphamide, etoposide, mustard, vincristine, procarbazine, prednisone) for 2 to 6 cycles in 4 patients. The response to treatment was judged to be complete (CR) if no residual disease was found on restaging, which included bilateral marrow biopsies, computed tomographic scans, and physical examination. Eight patients (22%) achieved a CR, whereas the remaining 29 patients met the criteria for MD as previously defined. In 9 patients, the evidence for complete remission was deemed equivocal (CRe), usually the result of continued radiographic abnormalities of uncertain significance. Additional information on the status of the bone marrow was obtained at harvest, when core biopsy specimens from 6 sites were examined microscopically. Although these data were not included in the official restaging, just 28% were scored as negative. The remainder had residual lymphoma (34%), lymphoid aggregates (25%), or were scored as suspicious (13%).
During the course of the study, granulocyte- (G-CSF) and granulocyte-macrophage (GM-CSF)–stimulating factors were introduced into practice. Some patients participated in a published double-blind study of GM-CSF.19 The median time to absolute neutrophil recovery to 500/μL was 13 days and platelet recovery to greater than 20 000/μL was 29 days. The initial patient treated on the study did not receive a purged product. Two patients received a “boost” of unpurged cells at days 34 and 44 because of persistent pancytopenia. With the exception of 2 early deaths, all patients reconstituted successfully. The median number of hospital days was 26. Standard antimicrobial protocols for neutropenic patients and blood product transfusion were followed. The 2 early transplant-related deaths involved a 24-year-old woman with prolonged neutropenia and fungal endocarditis and a 28-year-old man who had an overwhelming Epstein-Barr–related lymphoproliferative disorder develop as published previously.20
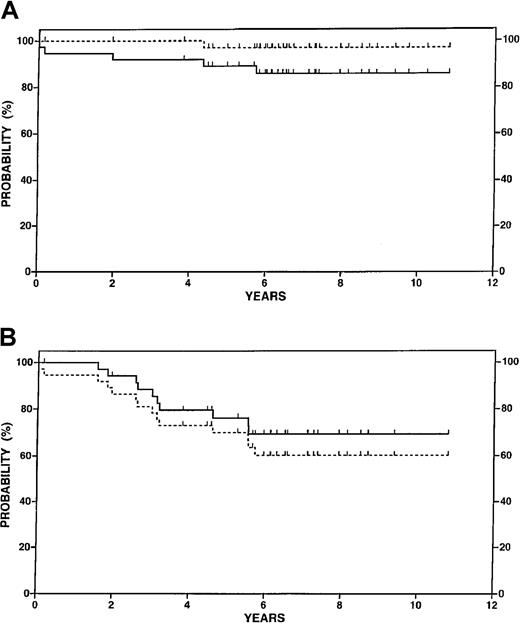
With a follow-up period after transplant ranging from 4 to 12 years (median 6.5), the OS was estimated at 92% at 5 years and 86% at 10 years (Figure 2A). Recurrent lymphoma was associated with a single death. In addition to the 2 early deaths described previously, 2 patients had acute myelogenous leukemia (AML) develop at 23 and 69 months. The estimated disease-specific survival, which considers deaths due to other causes as censored observations, was 100% at 5 years and 97% at 10 years (Figure 2A). Ten patients relapsed after transplantation with lymph node involvement as the site of first relapse in 9 cases. The remaining patient had microscopic disease on a screening marrow biopsy at 3 years; however, the marrow biopsy specimen was negative the following year and the patient has had no further evidence of lymphoma in the 3 years since relapse. One patient relapsed with follicular large cell lymphoma. Second-line treatments were selected according to physician and patient preference. At least 3 patients were successfully treated with the anti-CD20 antibody, rituximab. The estimated FFP at 5 and 10 years after transplant was 76% and 70%, respectively (Figure 2B). FFS was estimated at 70% at 5 years and 60% at 10 years (Figure 2B). The majority of patients continuing in remission have been in compliance with follow-up, although enthusiasm of both physicians and patients for annual bone marrow biopsies waned after 5 to 8 years.
Survival data for 37 patients in the transplantation study.
(A) Overall survival (solid line) and disease-specific survival (dotted line). (B) Freedom from progression (solid line) and failure-free survival (dotted line).
Survival data for 37 patients in the transplantation study.
(A) Overall survival (solid line) and disease-specific survival (dotted line). (B) Freedom from progression (solid line) and failure-free survival (dotted line).
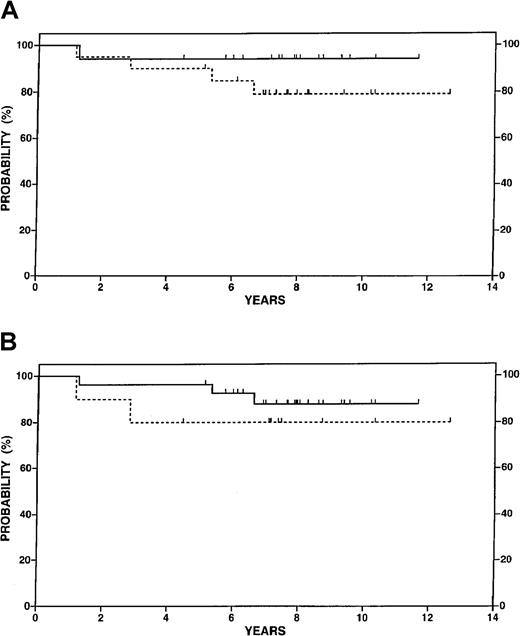
Figure 3A illustrates OS according to response to conventional chemotherapy in the study population. No difference between patients achieving a CR or CRe and those with a very good partial response was seen. Similarly, as shown in Figure 3B, survival differences between high and low tumor burden patients were not apparent although only 10 patients had low tumor burden disease.
Overall survival data for 37 patients in the study according to selected features.
(A) Complete or equivocal complete response to chemotherapy before transplant (n = 17, solid line) versus partial response (n = 20, dotted line), P = NS. (B) High tumor burden at diagnosis (n = 27, solid line) versus low tumor burden (n = 10, dotted line), P= NS.
Overall survival data for 37 patients in the study according to selected features.
(A) Complete or equivocal complete response to chemotherapy before transplant (n = 17, solid line) versus partial response (n = 20, dotted line), P = NS. (B) High tumor burden at diagnosis (n = 27, solid line) versus low tumor burden (n = 10, dotted line), P= NS.
The t(14;18) PCR analysis was performed on 25 patients before and after in vitro purging. Ten (40%) patients were found to be positive. Of those 10 patients, 7 (70%) were converted to PCR-negative, whereas 3 remained persistently positive.
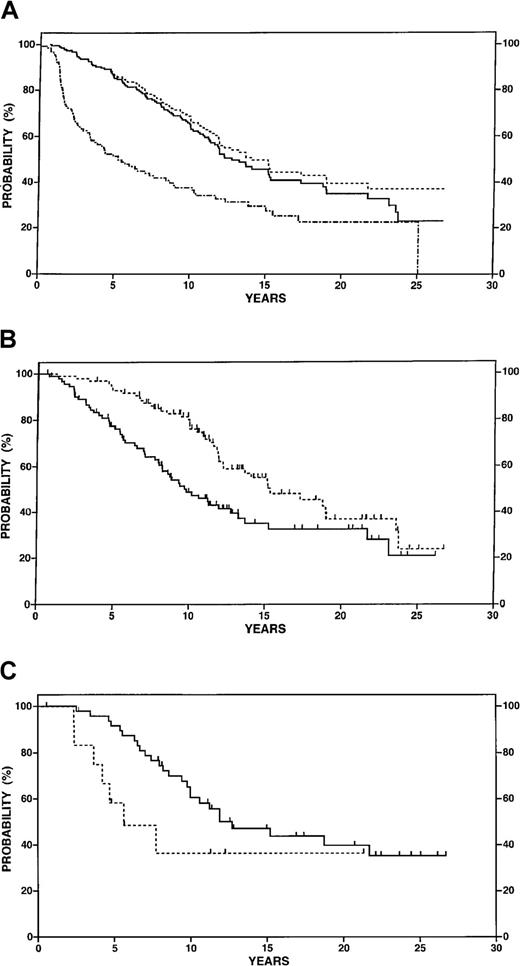
Among reference patients, 52% were male, 70% had stage IV disease, and 72% had FSC histology. Eighty-six percent of the cases that could be scored according to the international prognostic factors index had low or low-intermediate risk.13 The estimated FFP at 5 and 10 years were 52% and 30% for this reference sample and the OS at 5 and 10 years were 88% and 62% (Figure4A). Tumor burden characteristics of the study patients were compared with the reference patients as detailed in Table 2. Seventy-three percent of transplant recipients had high tumor burden disease compared with 49% of the reference sample, and the latter proportion did not change over the interval from 1962 to 1988. FFP may be a misleading endpoint because of potential differences in the methods and frequency of disease surveillance, and significant differences in FFP may not translate into a survival benefit. Therefore, survival was adopted as the major endpoint for further analyses.
Survival data in reference patients 50 years or younger with follicular small cleaved or mixed lymphoma managed at Stanford University.
(A) Overall survival (n = 188, solid line), disease-specific survival (n = 188, dotted line) and freedom from progression (n = 138 with exclusion of no initial therapy patients, dotted and dashed line). (B) Overall survival for high tumor burden at diagnosis (n = 92, solid line) versus low tumor burden (n = 96, dotted line), P = .001. (C) Overall survival for 61 patients treated with CVP who achieved a complete or equivocal complete response (n = 48, solid line) versus partial response (n = 13, dotted line),P = .03.
Survival data in reference patients 50 years or younger with follicular small cleaved or mixed lymphoma managed at Stanford University.
(A) Overall survival (n = 188, solid line), disease-specific survival (n = 188, dotted line) and freedom from progression (n = 138 with exclusion of no initial therapy patients, dotted and dashed line). (B) Overall survival for high tumor burden at diagnosis (n = 92, solid line) versus low tumor burden (n = 96, dotted line), P = .001. (C) Overall survival for 61 patients treated with CVP who achieved a complete or equivocal complete response (n = 48, solid line) versus partial response (n = 13, dotted line),P = .03.
As shown in Figure 4B and in contrast to the transplanted patients, application of the GELF criteria stratified low and high tumor burden reference patients into prognostic subgroups: 92% versus 70% OS at 5 years, P = .0001. A potentially important difference between the transplanted and reference patients is the selection of the former on the basis of response to conventional chemotherapy. Among reference patients treated immediately after diagnosis, 74% achieved a CR or CRe compared with 49% of the study population. Response to induction chemotherapy was of prognostic significance in the reference population overall and in the subset of patients treated with CVP chemotherapy. OS at 10 years was 36% for patients with partial response compared with 59% for patients with CR or CRe after CVP (P = .03) (Figure 4C).
As previously stated, 2 patients had AML develop at 23 and 69 months after transplantation. The former patient had normal marrow cytogenetics at 12 months and a complex karyotype on the diagnosis of AML with deletions of chromosomes 7 and 5. The latter patient had 6 prior cytogenetic analyses of the bone marrow performed. At 3 years, a clonal abnormality was noted that included a chromosome 7 deletion but this clone was not present on 3 subsequent samples. The malignant clone was not related to that seen earlier but did contain a chromosome 7 deletion as well as a 5q deletion.
Discussion
Multiple investigators have reported long remissions with myeloablative therapy and ABMT relative to conventional chemotherapy, albeit with continued relapses.11-13 Whereas possible survival benefit has been suggested in these uncontrolled trials, this positive effect has not been demonstrated definitively.21Freedman et al22 reported 89% OS and 63% disease-free survival without evident plateau at 3 years among 87 patients who underwent ABMT in first remission. Only 36% of patients achieved a CR with CHOP chemotherapy before transplantation in this series in which the high-dose regimen had consisted of cyclophosphamide and total body irradiation. These investigators determined that the presence of minimal residual disease in the reinfused marrow was the most significant prognostic factor for relapse among these patients, concordant with their observations among patients in second or subsequent remission.21-23 Although the number evaluated was small, more than half of our patients had no molecular evidence of lymphoma in the marrow before purging and just 3 had minimal residual disease after purging. Thus, no correlation could be made with outcome in the current series. Differences in these findings may relate to the purging technique, PCR methodology, or variation in cytoreductive therapy before transplantation.
Description of the characteristics of our study population and comparison to results among like patients treated with conventional therapy is essential to the interpretation of outcomes. Thus, an important and unique aspect of the current report is the provision of outcome data among patients of similar age treated without transplantation at the same institution. Longer survival was recorded for transplant recipients relative to reference patients, particularly those with a high tumor burden. Proportionally more patients had high tumor burden among the transplant recipients. However, the study patients were selected on the basis of achieving MD with conventional chemotherapy and response to chemotherapy (CR or CRe versus PR) was highly prognostic in the reference population. In fact, the 59% 10-year OS among CR patients in the CVP-treated reference group was not significantly different (P = .074) from the 86% 10-year OS among the study patients. Although fewer transplanted patients achieved a CR or CRe relative to reference patients, this may be related to greater sophistication in diagnostic techniques and acumen over time rather than a reflection of chemosensitivity. These comparisons indicate that, among patients treated with conventional therapy, OS was influenced by tumor burden and response to therapy, whereas these variables had little or no influence in the transplanted population.
The observation of a single death due to lymphoma in the study population is a remarkable finding given the duration of follow-up. However, the occurrence of 4 deaths unrelated to relapse is of concern. The early deaths were unusual and might not be expected with advances in transplantation such as the use of peripheral blood stem cells and G-CSF, both of which reduce the time to engraftment and regimen-related complications. Secondary MDS and AML, however, continue to be a major concern for long-term survivors of autologous transplantation.22 In a recent series of 552 autologous transplant recipients, the estimated incidence of myelodysplasia at 10 years was 19.8% although others have reported a lower incidence of this complication, which has been related to cumulative doses of conventional cytotoxic drugs, preparatory regimen, and dose of cells reinfused at transplantation.24-26
The major cause of failure in the current study was recurrent disease, which was estimated at 30% at 10 years. Although this observation compares very favorably with historical references, it also indicates the need for further improvement. The use of immunomodulatory and targeted therapies after transplantation when the patient is in a minimal residual disease state is an attractive strategy, although the suppressive effects of high-dose therapy on effector cells and antigen-presenting cells present potential obstacles to this approach.27-29 Enhancement of the preparatory regimen through the use of radioimmunoconjugates or rituximab combined with cytotoxic therapy or the use of rituximab as a means of in vivo purging represent other approaches.30,31 Allogeneic transplantation in follicular lymphoma has been associated with a lower relapse rate and anecdotal evidence of response to donor lymphocyte infusions, suggesting a graft-versus-lymphoma effect.32Newer strategies incorporating nonmyeloablative preparatory regimens may provide this effect with less morbidity and mortality.33
The long natural history of follicular lymphoma requires mature follow-up of clinical trials such as ours. Even then, interpretation of data are subject to error because of potential bias in patient selection. Clinicians appreciate that the course of disease is highly variable and a subset of patients remains healthy, without symptoms and without treatment, at 10 or more years. The estimated probability of death of 14% and relapse of 30% at 10 years in our transplanted patients, the majority of whom had high tumor burden disease, is significantly lower than our reference sample. However, treatments with greater potential for serious early and late effects must be carefully scrutinized and ideally reserved for patients at greatest risk for disease-related morbidity and mortality. We conclude that further investigations of transplantation with the objectives of reducing toxicity and disease recurrence are warranted as are efforts to describe prognostic factors in follicular lymphoma patients.
Supported in part by National Institutes of Health grants CA 49605 and CA 34233.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Sandra J. Horning, 1000 Welch Rd, Suite 202, Palo Alto, CA 94304; e-mail: sandra.horning@stanford.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal