Abstract
The serglycin proteoglycan is best known as a hematopoietic cell granule proteoglycan. It has been found that serglycin is synthesized by endothelial cells, is localized to cytoplasmic vesicles, and is constitutively secreted. Serglycin messenger RNA in human umbilical vein endothelial cells (HUVECs) and cultured human aortic endothelial cells was detected by reverse transcription–polymerase chain reaction.35S-sulfate–labeled secreted and intracellular proteoglycans were analyzed. It was found that 85% of the proteoglycans synthesized during culture were secreted. A core protein of the appropriate size for serglycin was detected by analysis of the chondroitinase-digested 35S-sulfate–labeled HUVEC proteoglycans. This was the major core protein of the secreted chondroitin sulfate proteoglycans. Recombinant serglycin core protein was used to generate an antibody in chickens. A core protein identified by Western blotting of chondroitinase digests of HUVEC proteoglycans corresponded to the major 35S-sulfate– labeled core protein. Identical results were obtained with 2 hematopoietic cell lines. Cyto-immunofluorescence showed cytoplasmic vesicular and perinuclear labeling in hematopoietic cells and HUVECs. The serglycin-containing vesicles in HUVECs are distinct from the Weibel-Palade bodies, which contain von Willebrand factor. Confocal microscopy showed that tissue plasminogen activator was distributed similarly to serglycin. Serglycin may be important for the function of these vesicles and, once secreted, for the modulation of the activity of their constituents.
Introduction
The serglycin proteoglycan was initially discovered as a secretory and membrane-associated product of rat L2 yolk sac tumor cells,1 and its core protein was the first proteoglycan gene to be cloned.2 L2 cells are thought to originate from parietal endoderm.3 Over a number of years, it was found that several types of hematopoietic cells in several species synthesize a proteoglycan with a very small core protein and a characteristic resistance to trypsin digestion.4-10 The proteoglycan core protein was purified from human platelets,11 and its amino acid sequence and the complementary DNAs (cDNAs), which were cloned from human12-14 and murine15 hematopoietic cells, were found to be highly homologous to the rat L2 serglycin core protein. Messenger RNA (mRNA) for serglycin was subsequently identified in most blood and bone marrow–derived cells by Northern blotting or in situ hybridization.10,16-21 Serglycin has thus come to be known as the hematopoietic proteoglycan. The serglycin proteoglycan is distinguished by the S/G (single-letter amino acid codes) repeat region in the central portion of the molecule, which is the site of attachment of the glycosaminoglycan (GAG) chains and gives the molecule its unique structural characteristics. The proteoglycan has either heparin or chondroitin sulfate GAGs, or it can be a hybrid of chondroitin sulfate and heparan sulfate chains, depending upon the cell source.19-21
The function of the serglycin proteoglycan is not known, but it is likely that it is involved in packaging of proteins into secretory granules and/or directing the secretion of such molecules as cytokines or chymases.22 These modes of regulation might reflect the interactions of the proteoglycan with other granule constituents or could involve osmotic effects that are due to the extensive hydration of the GAG chains. Serglycin is stored in granules for secretion upon activation by some cells and is secreted constitutively by others; in some cases, both mechanisms appear to coexist.7,10,19-21The chondroitin sulfate serglycin from platelets or other hematopoietic cells binds to such diverse molecules as intragranular platelet factor 4, macrophage inhibitory protein–1α, and lysozyme23; to the cell surface proteoglycan CD4424; and to matrix molecules such as fibronectin and collagen.20,25,26 The heparin and the oversulfated chondroitin sulfate serglycins from mast cells appear to bind to specific proteases.22 The contents of the storage granules in which serglycin is found in the different hematopoietic cells (eg, platelet alpha granules, mast cell secretory granules, neutrophil tertiary granules, eosinophil secretory granules) are very different from each other. Thus, this proteoglycan may be very versatile in its functional properties in accordance with the specific structure conferred by the different cells in which it is synthesized.
We have now found that this proteoglycan is expressed in a variety of human endothelial cells. We have characterized the serglycin secreted from human umbilical vein endothelial cells (HUVECs) and found that it is the major secreted chondroitin sulfate proteoglycan of these cells. We have localized the proteoglycan to cytoplasmic vesicles that appear to be different from Weibel-Palade bodies. In contrast, the cytoplasmic distribution of serglycin is similar to that of tissue plasminogen activator (tPA).
Materials and methods
Cells and culture conditions
HUVECs were isolated by established protocols.27All culture media were from Gibco (Grand Island, NY), except for fetal bovine serum from Hyclone (Logan, UT). The cells were cultured in medium 199 with 10% fetal bovine serum, 80 μg/mL endothelial cell growth supplement (ECGS), 50 μg/mL heparin from pig intestinal mucosa (grade I-A) (Sigma Chemical, St Louis, MO), with 100 U/mL penicillin and 100 μg/mL streptomycin, and fungizone on gelatin-coated tissue culture flasks. ECGS was prepared as described.28 Cells were passaged at a 1:4 split ratio from confluent cultures and reached confluency again at about 6 to 7 days. The medium was changed every 3 days. Cells were used up to passage 6. A human aortic endothelial cell (HAEC) culture was kindly donated by Dr Sandor S. Shapiro. A human skin microvascular endothelial cell line (HMVEC) was donated by Dr Jaime Caro. We used 2 hematopoietic tumor cell lines. HL-60 cells (promyelocytic)29 were from the ATCC (Rockville, MD) and were cultured in RPMI 1640 with 10% fetal calf serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 5 mM glutamine. CHRF-288-11 cells (megakaryoblastic)30 were a gift from Dr Michael Lieberman (Cincinnati, OH) and were cultured in Fischers medium with 20% horse serum, 100 U/mL penicillin, and 100 μg/mL streptomycin.
The effect of tumor necrosis factor (TNF)–α on the amount of serglycin in the cells was assessed under the conditions described by Kulseth et al.31 Cells were incubated for 24 hours with 7 μg/mL TNF-α (Sigma). The cells were immunostained with antiserglycin antiserum or with rabbit anti–von Willebrand factor (vWF) as described below and were analyzed by confocal microscopy.
RNA extraction and mRNA analysis
Cells were detached from the culture dishes with trypsin/EDTA and pelleted, and RNA was extracted with Trizol reagent (Gibco). Reverse transcription–polymerase chain reaction (RT-PCR) was performed as described previously.31The forward primer used for serglycin was 5′-51AATGCAGTCGGCTTGTCCTG, and the reverse primer was 5′-323GCCTGATCCAGAGTAGTCCT, to generate a 272–base pair (bp) fragment that bridged the first intron and included about half the S/G repeat region. The numbering was according to Stellrecht and Saunders.14 Primers for actin, used as a control, were forward 5′-558CATGCCATCCTGCGTCTGGACCTG and reverse 5′-1119GCTTGCTGATCCACATCTGCTGG, to give a 562-bp fragment.32 33 The PCR products were electrophoresed on 1% agarose gels with Tris/acetate/EDTA buffer and visualized with ethidium bromide. All putative serglycin bands shown in this report were excised from the gels and subcloned into the TA cloning vector (Invitrogen, Carlsbad, CA) for nucleotide sequencing. Sequencing was performed on an automated sequencer in the Thomas Jefferson University core facility.
Radiolabeling of proteoglycans
HUVECs were grown in the complete medium as described above, with 50 μCi/mL 35S-sulfate (ICN Radiochemicals, Costa Mesa, CA) for sequential 3-day periods during a 9-day culture. The radiolabel was added either at the time the cells were passaged, at day 3, or at day 6, and cells and media were harvested 3 days later. Cells were lysed in 0.2% Triton X-100 in 8 M urea and 50 mM Tris HCl, pH 8.0. The cell lysate and the culture medium were subjected to DEAE-Sephacel (Pharmacia, Piscataway, NJ) chromatography. Nonproteoglycan components were eluted with 0.1 M NaCl and then 0.23 M NaCl in 8 M urea and 50 mM Tris HCl, pH 8.0. Most of the heparin, which had been added as a supplement to the culture medium, was eluted with 0.42 M NaCl, and the35S-sulfate–labeled proteoglycan fraction was eluted with 4 M guanidine HCl in 50 mM sodium acetate, pH 8.0. The proteoglycans were subjected to chromatography on Sepharose CL 6B (Pharmacia) columns. The columns were eluted with 4 M guanidine HCl, 50 mM sodium acetate, and 0.2% Triton X-100, pH 7.0, and approximately 50 fractions were collected between the Vo and the Vt. The radioactivity was quantitated by liquid-scintillation counting in Ecolume scintillation cocktail (ICN Radiochemicals). All enzymes for GAG depolymerization were purchased from Seikagaku America (Ijamsville, MD). Chondroitinase ABC digestion was performed as described by Oike et al34 in 100 mM Tris HCl, 30 mM sodium acetate, 10 mM disodium EDTA, 10 mM N-ethylmaleimide, 5 mM phenylmethylsulfonyl fluoride (PMSF), and 0.36 mM pepstatin, pH 8.0, at 37°C for 2 hours. Chondroitinase ACII digestion was performed in the same buffer at pH 6.0. Heparitinase II digestions were performed in 50 mM Tris HCl, 5 mM CaCl2, 0.5 mg/mL bovine serum albumin, and 1 mM PMSF. For the double digestion, the heparitinase digestion was performed for 1 hour at 42°C, followed by addition of chondroitinase ABC buffer and inhibitors and chondroitinase ABC, and the mixture was incubated for an additional 2 hours at 37°C. The digestion products were monitored by Sepharose CL-6B chromatography as described above, by PD-10 column chromatography (Pharmacia), and by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). GAGs were released from the core protein by digestion with 0.2 N NaOH at 37°C for 2 hours. Proteoglycans were digested with trypsin (Gibco) at 1 mg/mL in calcium- and magnesium-free Hanks balanced salt solution at 37°C for 24 hours. These digests were analyzed by chromatography on Sepharose CL 6B.
Identification of the HUVEC35S-sulfate–labeled serglycin core protein
The 35S-labeled proteoglycans from the culture medium were obtained by chromatography as described above on DEAE-Sephacel (Pharmacia). The samples were digested with the depolymerizing enzymes described above. The digests were electrophoresed on SDS-PAGE with the use of 4% to 20% gradients, and the digestion products were visualized by autoradiography on Fuji film (Fisher Scientific, King of Prussia, PA). All electrophoresis reagents were from Bio-Rad (Richmond, CA).
Preparation of recombinant human serglycin core protein
An insert including all of exon 2 and the coding region plus the 3′-untranslated region of exon 3 up to the EcoRI site was prepared by PCR from the serglycin cDNA, which was kindly donated by Dr Grady F. Saunders.14 The insert was cloned into pGEX-6P-2 (Pharmacia) to generate a serglycin-glutathione sulfotransferase (GST) fusion protein. The plasmid was grown in JM 109 cells at 30°C to an absorbance at 600 nm of 0.6 and was then induced for 2 hours with 1 mM isopropylthio-β-galactoside. The proteins were extracted from the cell pellets by means of B-PER Bacterial Protein Extraction Reagent (Pierce, Rockford, IL). The GST–serglycin fusion protein was purified with the use of Glutathione Sepharose 4B (Pharmacia). The serglycin core protein was removed from the fusion protein by digestion with PreScission Protease (Pharmacia). The digest was then passed through another Glutathione Sepharose 4B column to separate the GST and the protease, which is itself a GST-fusion protein, from the serglycin core protein. The sizes of the recombinant fusion protein and the purified serglycin were determined by SDS-PAGE with the use of 4% to 20% gradient minigels (Bio-Rad). The protein was transferred to a ProBlott (Applied Biosystems, Foster City, CA) membrane in 0.1 M 3-(cyclohexylamino)-1-propanesulfonic acid buffer containing 10% methanol at pH 11; the blot was stained with Coomassie Blue R200; and the first 10 amino acids of the purified serglycin core protein were sequenced on an automated sequencer in the University Protein Core Facility.
Preparation of antibodies against recombinant serglycin
The concentration of protein was determined by means of the Bio-Rad Protein Assay (Bio-Rad). The protein was concentrated to 0.75 mg/mL by means of Centricon-3 centrifuge filters (Millipore, Bedford, MA). The protein was sent to Cocalico Biologicals (Reamstown, PA) for production of antibody in chickens. Western blotting using serum from test bleeds demonstrated the presence of antibodies specific to the recombinant serglycin. Subsequently, total egg yolk immunoglobulin (Ig)–Y, purified at the Cocalico facility, was also shown to have antiserglycin activity. The anti–human serglycin antibody was purified from the IgY by affinity chromatography. We dialyzed 2 mg of recombinant human serglycin core protein against 2 L of 0.1 M phosphate buffer, pH 8.1. Recombinant serglycin was immobilized on CNBr-activated Sepharose 4B (Amersham Pharmacia) as follows: We suspended 0.5 g of CNBr-activated Sepharose 4B in 1 mM HCl and incubated it for 30 minutes at room temperature. The column was then washed with 200 mL of 1 mM HCl. The recombinant serglycin core protein was added to the column and incubated overnight on a shaker at 4°C. The column was then washed with 30 mL phosphate buffer. Glycine (0.2 M) was then added to the column, which was incubated at room temperature for 2 hours. The column was then washed with 3 alternating cycles of 0.1 M acetate buffer, pH 4.0 containing 0.5 M NaCl, followed by a wash with phosphate buffer, pH 8.1. The antibody solution was dialyzed against 20 mM Tris-HCl containing 0.5 M NaCl, pH 7.4. The column was washed with 5 bed volumes of 20 mM Tris-HCl containing 0.5 M NaCl, pH 7.4. The antibody solution was added to the column and incubated on a shaker at room temperature for 2 hours. The column was allowed to drain and washed with 20 mL phosphate buffer. We added 25 μL 1M Tris, pH 11.4, to 10 numbered Eppendorf tubes. We added 5 mL of 0.1 mM glycine, pH 2.8, to the column and collected 0.5 mL fractions in the numbered Eppendorf tubes. Absorbances at 280 nm were taken to determine which fractions contained the purified antibody.
Identification of the HUVEC serglycin core protein by Western blotting with antiserglycin IgY
The core proteins obtained by chondroitinase ABC and heparitinase digests of the culture medium proteoglycans were rechromatographed on DEAE-Sephacel and eluted with a sequential gradient of 0.1 to 0.5 M NaCl, to allow separation of the core protein from the reagents in the digestion mixture. The presence of a sulfated sugar on each of the 4 to 6 hexasaccharide stubs enables this separation. Both conditioned medium from the HUVECs and fresh medium were analyzed in this manner. In one experiment, a chondroitinase ACII digest was prepared with the use of the same buffer used for the chondroitinase ABC digestion but at pH 6.0. The chondroitinase ACII digests were not rechromatographed on DEAE-Sephacel because removal of the terminal sulfated sugars by this enzyme precluded high-affinity binding of the core protein to the column. All fractions were electrophoresed through 4% to 20% Tris-HCl minigels (Bio-Rad), and transferred electrophoretically to nitrocellulose. The transfer was performed in 25 mM Tris, 192 mM glycine, and 20% methanol for 1 hour at 100 V at 4°C. The nitrocellulose blot was blocked with 5% nonfat dry milk and immunostained with the purified chicken antiserglycin antibody. Blots were incubated with chicken anti–human serglycin diluted 1:100 with milk at room temperature for 1 hour with shaking. The blots were rinsed and washed with wash buffer. The secondary antibody was rabbit anti–chicken IgG alkaline phosphatase–conjugate (Sigma) diluted 1:1000. Blots were incubated with the secondary antibody for 1 hour at room temperature with shaking. The blots were rinsed and washed with wash buffer. Blots were developed by adding BCIP/NBT Phosphatase Substrate (Kirkegaard & Perry Laboratories, Gaithersburg, MD). The developing was stopped by rinsing the blot in distilled water. Immunolocalization of the serglycin core protein was also assessed on Western blots of proteoglycans from HL-60 and CHRF 288-11 cells, which are known to synthesize this proteoglycan, by colocalization of the radiolabeled core protein by autoradiography.
Intracellular immunofluorescent staining protocol
HUVECs were grown on 1% gelatin in Chamber Slides (Nalge Nunc, Naperville, IL) until confluent. Hematopoietic cells from suspension cultures (CHRF 288-11 and HL60) were cytocentrifuged onto Colorfrost Plus microscope slides (Fisher Scientific, Pittsburgh, PA). Slides were fixed in 4% paraformaldehyde fixative for 10 minutes. Slides were washed 3 times in 1 × Dulbecco phosphate buffered saline (DPBS; Gibco). Cells were permeabilized in 0.5% Triton in DPBS for 4 minutes. Slides were then washed 3 times with DPBS. Slides were blocked in a solution of 3% bovine serum albumin in DPBS for 5 minutes. For detection of serglycin, the primary antibody was chicken antihuman serglycin. It was diluted 1:20 in DPBS. Slides were incubated with the primary antibody for 1 hour in a humidified chamber at room temperature. Slides were then washed 3 times with DPBS. The secondary antibody was rabbit antichicken IgG heavy and light chains, fluorescein labeled (Pierce) and diluted 1:40 in DPBS. DAPI (Molecular Probes, Eugene, OR) was reconstituted according to the manufacturer's instructions and diluted 1:300 in the secondary antibody. Slides were incubated with the secondary antibody for 1 hour in a humidified chamber. Slides were then washed 3 times in DPBS. A tertiary antibody, swine antirabbit immunoglobulin, fluorescein labeled (Dako, Carpinteria, CA) and diluted 1:40 in DPBS, was used to amplify the fluorescent signal. Slides were incubated for 1 hour in a humidified chamber. Slides were then washed 3 times in DPBS. A drop of 5% n-propyl gallate in glycerol was added, and coverslips were placed onto the slides. For detection of vWF, we used rabbit antihuman vWF (Sigma), diluted 1:200 in DPBS. The secondary antibody was swine antirabbit immunoglobulin fluorescein conjugate (Dako), diluted 1:40 in DPBS. The staining procedure was as described for serglycin. Photographs were taken with P1600 Kodak (Rochester, NY) film under epifluorescence by means of a Nikon Microphot SA microscope (Optical Apparatus, Ardmore, PA) with a Nikon Triple Wavelength Cube at 600 × magnification.
Control cells were blocked with bovine serum albumin as described above and then incubated with normal chicken IgY (from egg yolks) (Lampire Biological Laboratories, Pipersville, PA), diluted 1:1000, for serglycin, or with normal rabbit IgG (1:100) for vWF. The secondary and tertiary antibodies were the ones described above. Nonpermeabilized cells were also stained as above, except that the permeabilization step with 0.5% Triton X-100 was omitted.
To confirm the specificity of the antibody for serglycin, total HUVEC protein extracts were evaluated by Western blotting. No reactive bands were detected. The intact serglycin, which reacts with the antibody on slot blots, cannot be transferred electrophoretically to the nitrocellulose. Thus, the staining of the whole-cell preparations is expected to be specific for serglycin.
Double intracellular immunofluorescent labeling for confocal microscopy
HUVECs were grown on 1% gelatin-coated 2-well chamber slides until about 80% confluent. One well on each slide was used for the control and the other for the specific labeling. The slides were fixed, washed, permeabilized, and incubated with antibodies as described above. The slides were blocked with 4% normal donkey serum (Jackson ImmunoResearch Labs, West Grove, PA). The primary antibodies were chicken antihuman serglycin (diluted 1:20 in DPBS), rabbit antihuman vWF (Sigma) (diluted 1:200), and goat antihuman tissue plasminogen activator (Chemicon International, Temecula, CA) (diluted 1:100). The secondary antibodies were Rhodamine Red-X–conjugated donkey antichicken IgY, Cy5-conjugated donkey antigoat IgG, and fluorescein isothiocyanate–conjugated donkey antirabbit IgG (Jackson ImmunoResearch Laboratories). All secondary antibodies were diluted 1:100 in DPBS. Double labeling was performed with all 3 possible combinations of the primary antibodies. After the incubation with the first primary antibody, the slides were washed 3 times with DPBS and then incubated with the appropriate secondary antibody for 1 hour and washed 3 times. Slides were blocked again with donkey serum, and the second set of primary and secondary antibodies was applied as for the first set. Slides were washed 3 times in DPBS, and coverslips were applied to the slides.
Control wells were processed as described above. Controls were normal chicken IgY (1:1000) for serglycin, normal rabbit IgG (1:1000) for vWF, and normal goat IgG (Sigma) (1:1000) for tPA. To assure specificity of the labeling and absence of interaction among the antibodies used, single staining was also performed in the same experiment, and the double-labeled cells were equivalent in appearance to the single-labeled cells at the appropriate wavelengths. Backgrounds were negligible.
The images were obtained with a Bio-Rad MRC 600 Laser Scanning Confocal Imaging System equipped with a krypton-argon mixed gas laser that allows visualization at 488, 568, and 647 nm. Images were captured at a total magnification of 600 ×. The images were printed in the Adobe Photoshop program (Adobe Systems, San Jose, CA).
Results
Detection of serglycin mRNA in endothelial cells
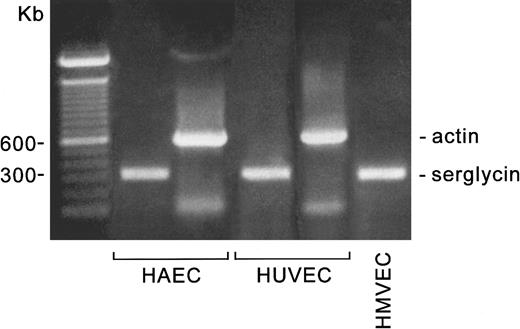
The RT-PCR products of HUVECs, an HAEC culture, and a human microvascular endothelial cell culture are shown in Figure1. The actin PCR reactions were performed from the same RT reactions as the serglycin PCR indicated for the HUVECs and HAECs, and the primers were at the same concentrations. The mRNA for serglycin appears to be similarly abundant in all 3 types of endothelial cells.
Detection of serglycin mRNA in 3 types of endothelial cells.
The cell cultures, mRNA extractions, and RT-PCR reactions were carried out as described in the text. The PCR products were analyzed on 1% agarose gels in Tris/acetate/EDTA buffer. The markers are the 100-bp ladder (Bio-Rad).
Detection of serglycin mRNA in 3 types of endothelial cells.
The cell cultures, mRNA extractions, and RT-PCR reactions were carried out as described in the text. The PCR products were analyzed on 1% agarose gels in Tris/acetate/EDTA buffer. The markers are the 100-bp ladder (Bio-Rad).
Characterization of 35S-labeled serglycin synthesized by HUVECs
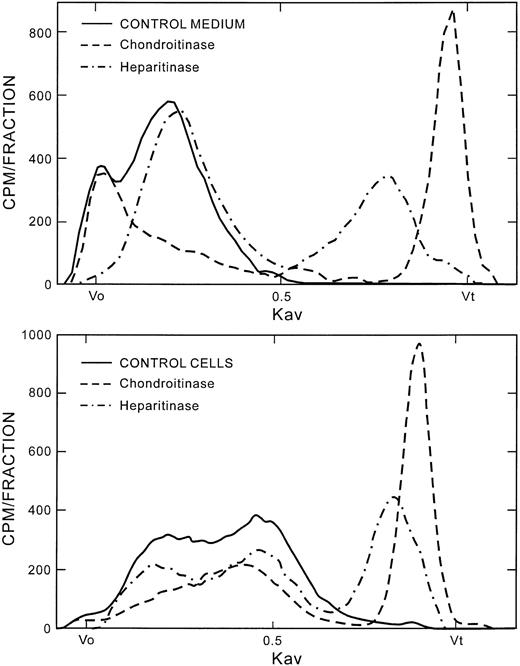
The incorporation of radioactivity into proteoglycans was measured in the cells and in the culture medium. About 85% to 90% of the radioactivity was found in the culture medium, demonstrating that most of the proteoglycans synthesized by HUVECs in culture are secreted. The cumulative amount of radioactivity at days 0 to 3 was about half that seen during the 3-to-6–day and 6-to-9–day labeling periods. The proteoglycans were analyzed on Sepharose CL-6B. Figure2 (top panel) shows the labeling patterns of the culture medium proteoglycans. The profile was the same whether labeling was started at day 0, 3, or 6. The proteoglycans from the culture medium migrated with peaks at the Vo and a broad peak centered at Kav 0.20, where the Kav is the relative distance of the peak between the Vo and the Vt of the column. Figure 2 (top panel) also shows the Sepharose CL-6B labeling patterns after digestion of the culture medium proteoglycans with either chondroitinase ABC or heparitinase. The peak near the Vo contained mainly heparan sulfate proteoglycans, and the Kav 0.20 peak contained primarily chondroitin sulfate proteoglycans. The secreted material consisted of approximately 55% chondroitin sulfate and 45% heparan sulfate proteoglycans.
Characterization of HUVEC-secreted and HUVEC-associated proteoglycans and their chondroitinase and heparitinase digestion profiles.
Proteoglycans were purified as described in the text. Intact proteoglycans and the chondroitinase and heparitinase digests from cells and culture medium were chromatographed on the Sepharose CL-6B column. Vo represents the void volume; Vt is the total volume of the column; and the Kav is the fractional distance of migration of the peak between the Voand Vt. The Vo and Vt were marked by blue dextran and phenol red, respectively. Approximately 50 fractions were collected between the Vo and Vt. CONTROL indicates undigested proteoglycans. Within each panel, the same amount of radioactivity was applied to the column for control and digested samples.
Characterization of HUVEC-secreted and HUVEC-associated proteoglycans and their chondroitinase and heparitinase digestion profiles.
Proteoglycans were purified as described in the text. Intact proteoglycans and the chondroitinase and heparitinase digests from cells and culture medium were chromatographed on the Sepharose CL-6B column. Vo represents the void volume; Vt is the total volume of the column; and the Kav is the fractional distance of migration of the peak between the Voand Vt. The Vo and Vt were marked by blue dextran and phenol red, respectively. Approximately 50 fractions were collected between the Vo and Vt. CONTROL indicates undigested proteoglycans. Within each panel, the same amount of radioactivity was applied to the column for control and digested samples.
The Sepharose CL-6B labeling profile from the cells (Figure 2, bottom panel) was different from that of the culture medium. There is only a trace of radiolabeled material at the Vo. There appear to be 2 overlapping peaks, at about Kav 0.22 and Kav 0.43. The first peak is consistent in part with the chondroitin sulfate–proteoglycan peak from the medium, since about half of the material under this peak was digested by chondroitinase ABC. The cell-associated proteoglycans were about 57% chondroitin sulfate.
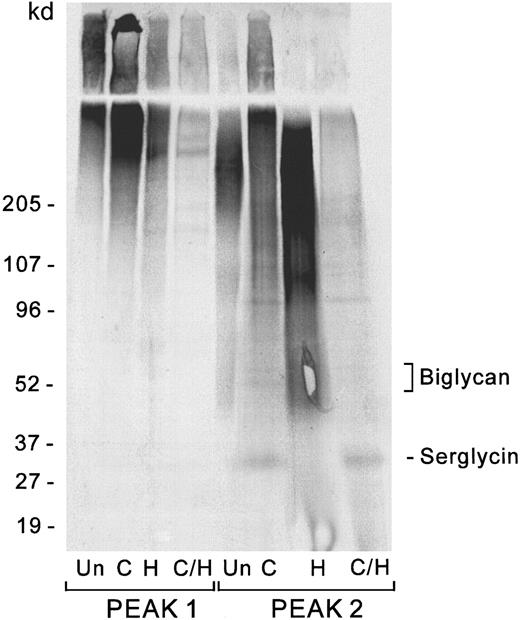
The 2 major peaks from the Sepharose CL-6B profile of the culture medium were subjected to chondroitinase and heparitinase digestion to determine whether a core protein that would be consistent with serglycin might be present. Figure 3shows the 35S-labeled core proteins obtained from the Vo and Kav 0.20 peaks after digestion with chondroitinase, heparitinase, or both enzymes together. Data are shown for the labeling period beginning on day 3 of culture, but identical results were obtained for the preceding and succeeding 3-day intervals. The Vo peak contains only the high–molecular weight core proteins at greater than 200 kd, probably versican and perlecan, which appear after heparitinase digestion. The second peak contains a predominant core protein of approximately 31 kd, consistent with the presence of the serglycin core protein, which appears only after chondroitinase digestion. Since serglycin core protein was detected by35S-sulfate labeling at all three 3-day time intervals tested, we presume that serglycin is synthesized continuously throughout the culture period. Although the cDNA for serglycin predicts a molecular weight (MW) of 14.4 kd for the mature protein, it should be noted that the core protein from platelet serglycin and putative serglycins from hematopoietic tumor cells migrate at about twice this size on SDS-PAGE even in the presence of β-mercaptoethanol.11,19,20 Part of the increase in apparent molecular weight is due to the approximately 4 to 6 residual hexasaccharide stubs, each 1.2 kd, expected to remain after the GAG depolymerizations, but the nonglycosylated core protein itself migrates at a higher apparent molecular weight than is expected from the known amino acid sequence of the protein (see section on recombinant serglycin below). The prominence of this band in the chondroitinase digest of the endothelial proteoglycans suggests that it represents the major secreted HUVEC chondroitin sulfate core protein. Since the secreted proteoglycans account for 85% of the total proteoglycan synthesis and 55% of the GAG chains of the secreted proteoglycans are chondroitin sulfate, and this band contains almost all the35S-sulfate visualized in the chondroitinase digest of the Kav 0.2 peak from the CL-6B column, secreted serglycin may account for nearly 40% of the chondroitin sulfate mass in proteoglycans synthesized by HUVECs under our culture conditions. A faint doublet is present at approximately 95 to 100 kd, which may arise from the chondroitin sulfate form of CD4435 or thrombomodulin,36 which could have been cleaved from the membrane. Another faint doublet appears at 50 to 55 kd, consistent with reports of biglycan in bovine aortic endothelial cells.37-41
Core proteins of endothelial cell–secreted proteoglycans.
Digestions were performed as described in the text. The digestion products were separated by SDS-PAGE and visualized by autoradiography. Proteoglycans from the Sepharose CL-6B column were pooled in 2 fractions: the Vo peak (peak 1) and the entire Kav 0.2 peak (peak 2). Un indicates undigested; C, chondroitinase; H, heparitinase; C/H, double digestion with chondroitinase and heparitinase. Undigested material represents 10% of the sample, and each digest represents 30%.
Core proteins of endothelial cell–secreted proteoglycans.
Digestions were performed as described in the text. The digestion products were separated by SDS-PAGE and visualized by autoradiography. Proteoglycans from the Sepharose CL-6B column were pooled in 2 fractions: the Vo peak (peak 1) and the entire Kav 0.2 peak (peak 2). Un indicates undigested; C, chondroitinase; H, heparitinase; C/H, double digestion with chondroitinase and heparitinase. Undigested material represents 10% of the sample, and each digest represents 30%.
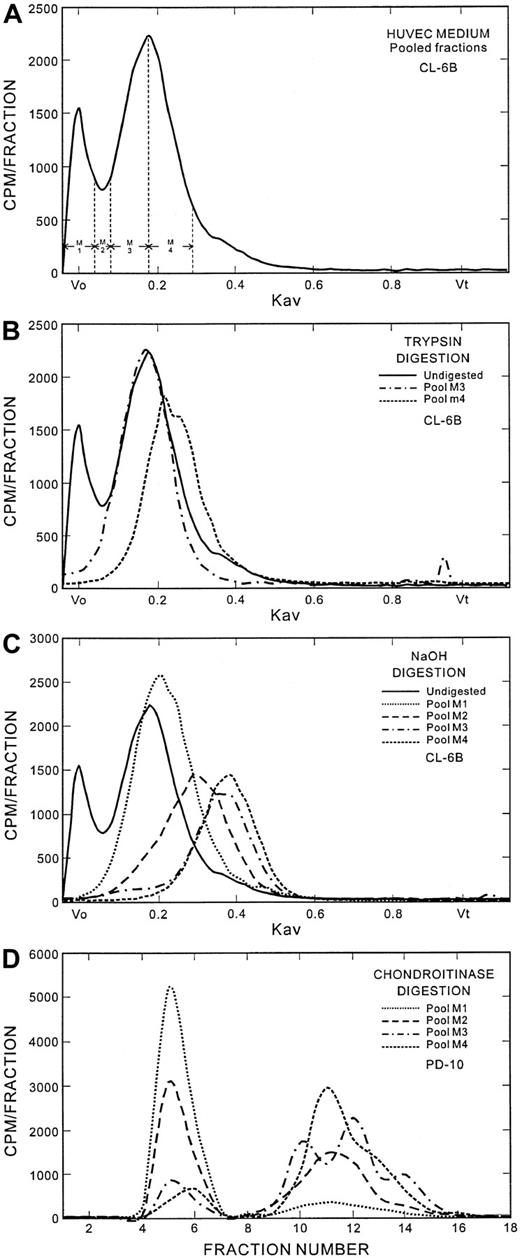
To provide further information on the structure of the putative serglycin proteoglycan, the analyses shown in Figure4 were performed. The Sepharose CL-6B column eluate from the culture medium proteoglycans was pooled into 4 fractions as shown in the top panel. The Sepharose CL-6B chromatography pattern of the trypsin digestion of the proteoglycans under the Kav 0.20 peak (pools M3 and M4) is shown in the second panel. A large proportion of the material in this peak was resistant to trypsin digestion. This is consistent with the behavior of the serglycin proteoglycan, which retains the GAG-attachment region intact after trypsin digestion, resulting in only a small decrease in overall size due to some proteolysis of the core protein. Pool M4 contained an additional component, seen as a shoulder at approximately Kav 0.26, which may be biglycan. The sizes of the GAG chains from the Sepharose CL-6B pools M1 through M4 are shown in the third panel, also shown as the Sepharose CL-6B profile. The GAGs from pool M1 are predominantly heparan sulfate chains, which elute at Kav 0.20 through 0.22, and are therefore much larger in size than the GAGs from pools M2 through M4. The GAGs from pool M2, which would have a mixture of proteoglycans from the Vo and Kav 0.20 peaks, migrated at a mean Kav of 0.31. The GAG peaks from both pools M3 and M4 were at Kav 0.38 through 0.40. The chains associated with serglycin were derived from peaks M3 and M4, since these fractions were also identified by antiserglycin immunostaining (see below) of a slot blot prepared from aliquots of the Sepharose CL-6B column fractions. The chondroitinase ABC digest of each pool was analyzed on PD-10 columns (Figure 4, bottom panel). The percentages of chondroitin sulfate in pools M1 through M4 were, respectively, 12.3%, 50%, 86.6%, and 88.3%. The chondroitinase ABC digestion products of pools M3 and M4 showed 3 low–molecular-weight peaks on PD-10 (Sephadex G-25) chromatography, most likely resulting from production of polysaccharides during the digestion, suggesting that the chains do not have a structurally homogeneous chondroitin sulfate disaccharide repeat pattern. The peak positions of the chondroitinase digestion products of pools M3 and M4 also differed from each other.
Analysis of the secreted endothelial proteoglycans.
(A) Fractionation of the secreted proteoglycans for analysis. The Sepharose CL-6B fractions were pooled as indicated for analyses shown in the lower panels of this Figure. (B) Trypsin digestion of secreted proteoglycans. Trypsin digests of fractions 3 and 4, which contain the putative serglycin proteoglycans, were analyzed on Sepharose CL-6B. (C) GAGs associated with the secreted proteoglycans. The GAG chains were released from the core protein by alkaline digestion as described in the text and analyzed on Sepharose CL-6B columns. (D) Chondroitinase ABC digestion of secreted proteoglycans. The digests were performed as described in the text and analyzed on PD-10 (Sephadex G-25) columns.
Analysis of the secreted endothelial proteoglycans.
(A) Fractionation of the secreted proteoglycans for analysis. The Sepharose CL-6B fractions were pooled as indicated for analyses shown in the lower panels of this Figure. (B) Trypsin digestion of secreted proteoglycans. Trypsin digests of fractions 3 and 4, which contain the putative serglycin proteoglycans, were analyzed on Sepharose CL-6B. (C) GAGs associated with the secreted proteoglycans. The GAG chains were released from the core protein by alkaline digestion as described in the text and analyzed on Sepharose CL-6B columns. (D) Chondroitinase ABC digestion of secreted proteoglycans. The digests were performed as described in the text and analyzed on PD-10 (Sephadex G-25) columns.
Production and characterization of recombinant serglycin protein and specificity of the antibody
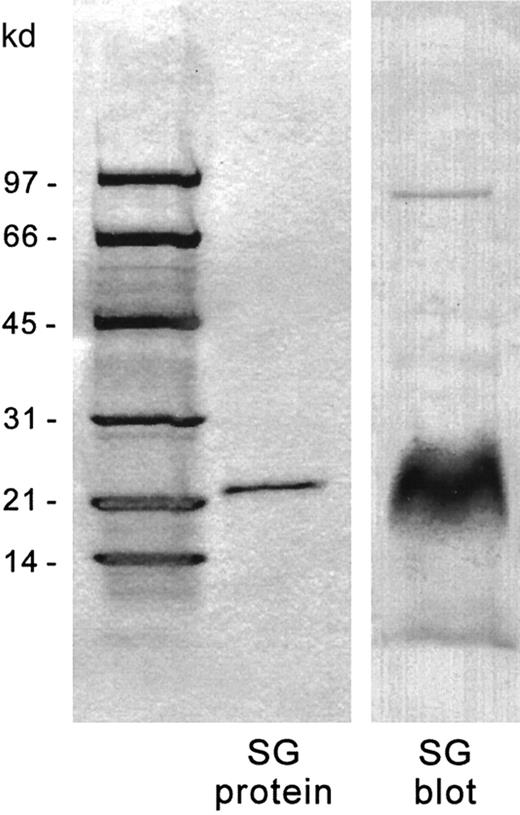
Recombinant serglycin exons 2 and 3 (predicted MW, 14.4 kd) migrated on SDS-PAGE at approximately 22 kd. The sequence of the first 10 amino acids of the purified protein band was identical to that expected from the nucleotide sequence. The recombinant protein was recognized by the affinity-purified antibody as expected on Western blots (Figure 5). To assess the ability of the antibody to interact with serglycin from human hematopoietic cells, the 35S-sulfate–radiolabeled chondroitinase ABC–digested proteoglycans from HL-60 cells, a human hematopoietic cell line that has very high expression of serglycin, were transferred to nitrocellulose, and autoradiography and immunolabeling were performed on the same blot (Figure 6). The antibody recognized only the major 35S-labeled core protein band at about 32 kd. The same results were found with the CHRF 288-11 cells (not shown). The antibody also recognized intact proteoglycan on nitrocellulose slot blots (data not shown) and so was useful in identifying the regions of the Sepharose CL-6B eluate of the35S-labeled proteoglycans which contained serglycin, as described above.
Recombinant serglycin and immunoblotting by chicken antiserglycin IgY.
The recombinant serglycin was electrophoresed on 4% to 20% SDS-PAGE gradients and immunoblotted onto nitrocellulose. In each lane, 2 μg recombinant protein were applied. Left panel: Coomassie stain. Right panel: Immunostain.
Recombinant serglycin and immunoblotting by chicken antiserglycin IgY.
The recombinant serglycin was electrophoresed on 4% to 20% SDS-PAGE gradients and immunoblotted onto nitrocellulose. In each lane, 2 μg recombinant protein were applied. Left panel: Coomassie stain. Right panel: Immunostain.
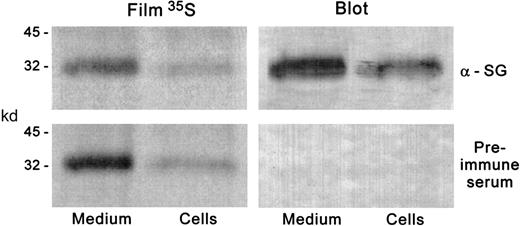
Immunoblotting and autoradiographic detection of serglycin in HL-60 cells.
HL-60 cells were incubated with 35S-sulfate, and proteoglycans were purified and subjected to chondroitinase digestion as described in the text. The digests were electrophoresed on SDS-PAGE gradient gels in duplicate and transferred to nitrocellulose. One blot was immunostained with the antiserglycin IgY and the other with preimmune serum. The blots were subjected to autoradiography. The radioactive band presumably representing the serglycin core protein was coincident with the immunostaining on the blot treated with the antiserglycin IgY. The same results were obtained with CHRF-288-11 cells.
Immunoblotting and autoradiographic detection of serglycin in HL-60 cells.
HL-60 cells were incubated with 35S-sulfate, and proteoglycans were purified and subjected to chondroitinase digestion as described in the text. The digests were electrophoresed on SDS-PAGE gradient gels in duplicate and transferred to nitrocellulose. One blot was immunostained with the antiserglycin IgY and the other with preimmune serum. The blots were subjected to autoradiography. The radioactive band presumably representing the serglycin core protein was coincident with the immunostaining on the blot treated with the antiserglycin IgY. The same results were obtained with CHRF-288-11 cells.
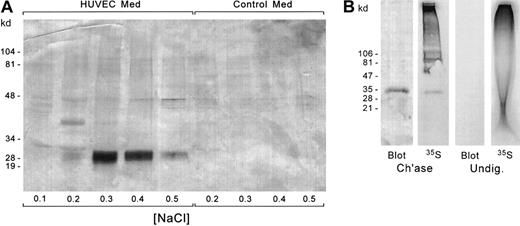
Immunoblotting of human serglycin core protein from HUVEC-conditioned culture medium and from the cell layer
The core proteins that were obtained by chondroitinase ABC digestion of the culture-medium proteoglycans were further purified by DEAE-Sephacel chromatography to remove proteins introduced during the enzymatic digestions and to confirm the anionic nature of the band that was recognized by the antibody. The fractions from the DEAE column were electrophoresed on SDS-PAGE, and the proteins were electroblotted onto nitrocellulose. The blots were labeled with the chicken antiserglycin antibody to identify the core protein (Figure7A). The core protein with its sulfated hexasaccharide stubs was seen in the 0.3 to 0.4 M NaCl eluate and migrated at about 31 kd on the SDS-PAGE gel. Residual intact heparan sulfate proteoglycans eluted at 0.5 M NaCl. The undigested chondroitin sulfate proteoglycans also would have eluted at 0.5 M NaCl or more, so the elution position of the immunolabeled band is consistent with the presence of the core protein with the sulfated stubs. An equivalent amount of culture medium that had not been exposed to cells was analyzed in parallel. The antibody did not detect any proteins of the size range of serglycin in the fresh medium that had not been exposed to the endothelial cells. Thus, the core protein that we detected in the HUVEC-conditioned medium had indeed been derived from the human endothelial cells. When the culture-medium proteoglycans were digested with chondroitinase ACII, the immunostained core protein band was identified at about 26 to 28 kd (data not shown), consistent with the removal of an additional sulfated disaccharide on each GAG chain relative to the chondroitinase ABC digestion, leaving an unsulfated tetrasaccharide rather than a sulfated hexasaccharide. Each chondroitin sulfate disaccharide unit is about 0.5 kd, so that if there are 4 to 6 GAG chains, this would account for an apparent loss of about 2 to 3 kd.
Immunoblotting of serglycin core protein from secreted and intracellular HUVEC proteoglycans.
(A) The core proteins from the chondroitinase-digested proteoglycans from HUVEC-conditioned medium and an equal amount of non–cell-conditioned medium were fractionated on DEAE-Sephacel to separate the glycosylated core proteins from chondroitinase enzyme and other contaminating proteins in the carrier albumin. The DEAE-Sephacel fractions were electrophoresed by SDS-PAGE and transferred to nitrocellulose. The blots were immunostained with antiserglycin IgY. (B) Identification of intracellular serglycin core protein by35S-sulfate labeling and immunoblotting. Lanes 1 and 2: Chondroitinase-digested cellular proteoglycans were subjected to SDS-PAGE and Western blotting. The blot was subjected to autoradiography. Lanes 3-4: Undigested proteoglycans were electrophoresed in duplicate. One aliquot was transferred to nitrocellulose for Western blotting, and the other was subjected to autoradiography in the gel.
Immunoblotting of serglycin core protein from secreted and intracellular HUVEC proteoglycans.
(A) The core proteins from the chondroitinase-digested proteoglycans from HUVEC-conditioned medium and an equal amount of non–cell-conditioned medium were fractionated on DEAE-Sephacel to separate the glycosylated core proteins from chondroitinase enzyme and other contaminating proteins in the carrier albumin. The DEAE-Sephacel fractions were electrophoresed by SDS-PAGE and transferred to nitrocellulose. The blots were immunostained with antiserglycin IgY. (B) Identification of intracellular serglycin core protein by35S-sulfate labeling and immunoblotting. Lanes 1 and 2: Chondroitinase-digested cellular proteoglycans were subjected to SDS-PAGE and Western blotting. The blot was subjected to autoradiography. Lanes 3-4: Undigested proteoglycans were electrophoresed in duplicate. One aliquot was transferred to nitrocellulose for Western blotting, and the other was subjected to autoradiography in the gel.
Figure 7B shows the analysis of the serglycin core protein in the endothelial cells. Chondroitinase-digested and undigested proteoglycans were subjected to SDS-PAGE and Western blotting, and the blot was exposed to autoradiographic film. Additionally, undigested proteoglycans were subjected to SDS-PAGE for autoradiography of the gel to discern whether any radioactive bands of the size of the serglycin core protein were present in the DEAE-purified proteoglycans. This was done because the intact proteoglycans cannot be transferred to nitrocellulose. The 31-kd band from the chondroitinase-treated sample was both radiolabeled and immunostained. No bands appeared at 31 kd in the undigested samples.
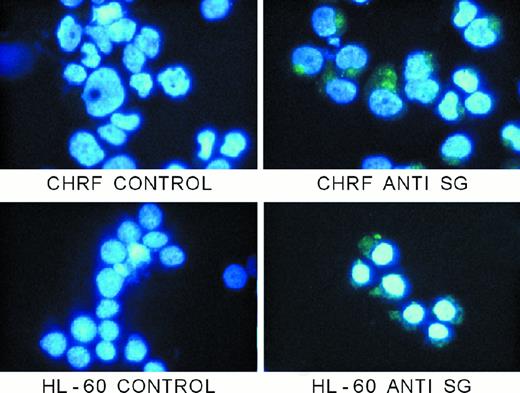
Immunostaining of hematopoietic cells
Figure 8 shows the immunostaining patterns of 2 human hematopoietic cell lines (CHRF 288-11 and HL-60) that are known to express serglycin mRNA and to synthesize a proteoglycan with the physical properties of serglycin. The pattern of staining is granular, as expected, with a strong Golgi stain as well. Although previous autoradiographic electron microscopic studies of 35S-sulfate–labeled bone marrow cells42 43 have shown that proteoglycans are present in the hematopoietic cell granules, ours is the first to show the presence of serglycin proteoglycan in granules of hematopoietic cells with a specific immunostain.
Immunostaining of hematopoietic cells with antiserglycin IgY.
Cells were prepared as described in the text. Controls were treated with nonimmune IgY from egg yolks, and serglycin staining was achieved with the chicken antiserglycin. Nuclei were stained with DAPI. The cells and treatments are indicated in the figure. SG indicates serglycin.
Immunostaining of hematopoietic cells with antiserglycin IgY.
Cells were prepared as described in the text. Controls were treated with nonimmune IgY from egg yolks, and serglycin staining was achieved with the chicken antiserglycin. Nuclei were stained with DAPI. The cells and treatments are indicated in the figure. SG indicates serglycin.
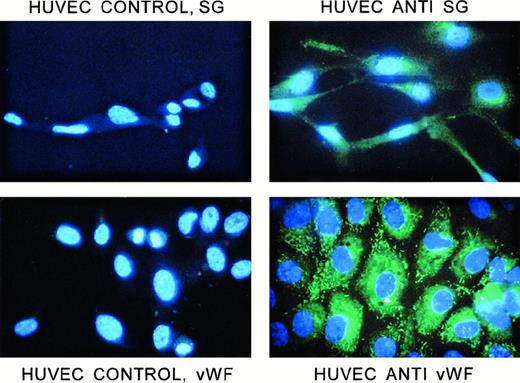
Immunostaining of HUVECs
Figure 9 shows the staining of HUVECs with the chicken anti–human serglycin antibody. The permeabilized cells were stained abundantly with this antibody. The pattern was granular; labeling was distributed throughout the cytoplasm; and in most cells, there was also an intense area of perinuclear labeling. Nonpermeabilized cells did not stain. Controls show only fluorescence from the nuclear DAPI stain. Thus, the staining pattern is comparable to that of hematopoietic cells that also make this proteoglycan and presumably store at least a portion of it in granules, as was shown in Figure 8. Figure 9 also shows the staining of the permeabilized HUVECs with anti-vWF (Sigma). However, the rodlike pattern, identical to that reported previously by others,44 45 was different from the rounder and smaller structures containing serglycin. The anti-vWF antibody did not immunostain nonpermeabilized cells.
Immunofluorescent staining of HUVECs with antiserglycin IgY and anti-vWF IgG.
Cells were prepared as described in the text. All cells were stained with DAPI to label the nuclei. The cells were labeled with either chicken antiserglycin or rabbit anti-vWF. Controls were, respectively, nonimmune IgY and normal rabbit serum. The cells and treatments are indicated in the figure. SG indicates serglycin.
Immunofluorescent staining of HUVECs with antiserglycin IgY and anti-vWF IgG.
Cells were prepared as described in the text. All cells were stained with DAPI to label the nuclei. The cells were labeled with either chicken antiserglycin or rabbit anti-vWF. Controls were, respectively, nonimmune IgY and normal rabbit serum. The cells and treatments are indicated in the figure. SG indicates serglycin.
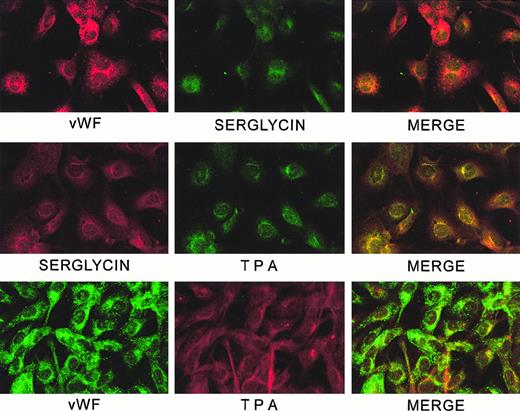
Confocal microscopic analysis of subcellular localization of serglycin, vWF, and tPA
Figure 10 shows confocal microscopy of combinations of serglycin/vWF, serglycin/tPA, and tPA/vWF. Serglycin and tPA are distributed similarly throughout the cytoplasm, and complete overlap appears evident in the perinuclear area. Neither serglycin nor tPA colocalizes with the very prominent tubular Weibel-Palade bodies, which contain the vWF. Staining of controls was negligible. The appearance of the cells when singly labeled was the same as the appearance when labeling with the same antibody was detected at the appropriate wavelength in the double-labeled samples.
Confocal analysis of localization of serglycin, vWF, and tPA in HUVECs.
Slides were prepared and examined by confocal microscopy as described in the text.
Confocal analysis of localization of serglycin, vWF, and tPA in HUVECs.
Slides were prepared and examined by confocal microscopy as described in the text.
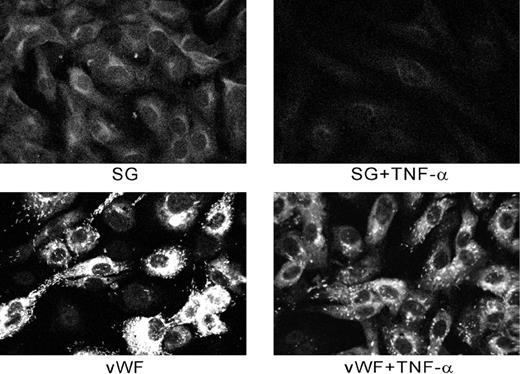
Figure 11 shows an experiment in which cells were examined by confocal microscopy with or without treatment with TNF-α. The cytokine decreased the amount of both serglycin and vWF associated with the cells and apparently induced a release that affected both storage compartments.
Effect of TNF-α on secretion of serglycin and vWF from HUVECs.
Cells were treated as described in the text. Staining of TNF-α–treated cells was greatly reduced compared with controls.
Effect of TNF-α on secretion of serglycin and vWF from HUVECs.
Cells were treated as described in the text. Staining of TNF-α–treated cells was greatly reduced compared with controls.
Discussion
We have shown for the first time that the serglycin proteoglycan is a major chondroitin sulfate proteoglycan of endothelial cells. While this article was in preparation, Kulseth et al31 published a report that the mRNA for serglycin was present in HUVECs, HAECs, and an HMVEC preparation, but they did not provide evidence for the synthesis of the protein, its subcellular localization, or its secretion. Our study, by analysis of the peaks in the Sepharose CL-6B profiles and the immunodetection and radiolabeling detection of the core proteins of the secreted and cellular chondroitin and heparan sulfate proteoglycans on SDS-PAGE, has shown that serglycin represents a substantial portion of endothelial cell proteoglycan synthesis and is the most abundant chondroitin sulfate proteoglycan secreted from HUVECs in culture. We have also shown by cyto-immunofluorescence and confocal microscopy that the proteoglycan appears to be stored in cytoplasmic vesicles in a distribution similar to that of tPA but distinct from vWF and Weibel-Palade bodies. However, treatment with TNF-α causes loss of serglycin and vWF from the HUVECs, suggesting that both storage pools are released by a common pathway and that the serglycin vesicles are secretory vesicles. Kulseth et al31 demonstrated that serglycin mRNA was elevated and that total 35S-labeling of secreted proteoglycans was increased after HUVECs were treated with TNF-α, but the amount of cell-associated proteoglycan was not quantitated. The Sepharose CL-6B profile in Kulseth et al31 would be consistent with increased release of serglycin to the culture medium in the presence of TNF-α.
Several proteoglycans are released to the extracellular environment by endothelial cells, either by secretion or by release from the plasma membrane. All these proteoglycans should be present in the material that we have isolated from the culture medium. Perlecan, the large heparan sulfate proteoglycan, is secreted by endothelial cells from a variety of sources.46 Several studies of bovine thoracic aorta endothelial cells have demonstrated secretion of the dermatan sulfate proteoglycan biglycan.37-41 The results of those studies, however, contained no data for the appearance of serglycin core protein. Several differences in methodology may account for our ability to detect serglycin in HUVECs. There is a species difference, ie, bovine vs human; different types of blood vessels were used (aortic endothelial vs umbilical vein); and the bovine endothelial proteoglycans were labeled beginning at 3 days post-confluence, whereas our cells were studied from the time of initiating the culture through about 2 days of confluence. In addition, the bovine studies analyzed the dermatan sulfate proteoglycan fractions from DEAE-Sephacel peaks or specific bands from gel separations, rather than the total proteoglycans or total dermatan sulfate/chondroitin sulfate proteoglycan.
In addition to proteoglycans that are secreted directly, endothelial cells are known to synthesize at least 3 transmembrane proteoglycans that may release their proteoglycan ectodomains to the surrounding medium by proteolytic cleavage at the cell surface. These proteoglycans are thought to be responsible for the presentation of an anticoagulant surface to the circulation. They include thrombomodulin, which may contain a chondroitin sulfate chain,36 syndecan-4 (ryudocan), which is a heparan sulfate proteoglycan that contains a small percentage of its GAG sequences as the specific antithrombin III binding sequence of heparin,47,48 and CD44, which may be present as a chondroitin sulfate proteoglycan,35 although there is disagreement about this question. On the basis of the percentage of chondroitin sulfate–associated radioactivity in the Vo and Kav 0.20 peaks on the Sepharose CL-6B columns, the mass of chondroitin sulfate GAG chain associated with serglycin is several times the amount associated with all the other secreted proteoglycans that remain soluble in the culture medium. Some35S-labeled core protein bands appear in trace amounts relative to serglycin after chondroitinase ABC digestion. The band at approximately 100 kd could be secreted thrombomodulin, since the cell-associated molecule migrates at MW 105 000 on SDS-PAGE under reducing conditions,36 or could be derived from CD4435 or an as-yet-unidentified proteoglycan. The faint labeling of bands of approximately 50 to 55 kd is consistent with biglycan.37-41 The relative intensity of each band would be proportional to the number of hexasaccharide stubs, and thus, on a molar basis, the human serglycin core protein, which is generally thought to have 4 GAG chains, would be labeled somewhat more heavily than the other core proteins with 1 to 3 GAG chains. However, since the chondroitin sulfate GAGs of the total secreted Kav 0.2 proteoglycans migrated as a single symmetrical peak on Sepharose CL-6B, the GAG chain lengths on these molecules were probably similar, and thus, the intensity of labeling of the serglycin band would be reasonably representative of the proportion of the total chondroitin sulfate associated with the proteoglycans.
Our immunostaining studies suggest that serglycin is contained within vesicles in the endothelial cells as well as the hematopoietic tumor cells, since the pattern of staining in the permeabilized cells in all these cases showed a definite granular appearance. What is the granule structure in which serglycin is contained in endothelial cells? Our immunofluorescence microscopy analyses suggest that the proteoglycan is not stored within the Weibel-Palade bodies, but within smaller vesicles that may also contain tPA. A recent study49 has demonstrated that tPA in lung endothelial cells is contained in granules that can be separated from the Weibel-Palade bodies on sucrose gradients, although another study by confocal microscopy has suggested that tPA is located both within the Weibel-Palade bodies and in different structures.50 It has been reported that several endothelial cell proteoglycans can bind to tPA,51 and the characteristics that we have described for serglycin are consistent with one of these proteoglycans. We speculate that serglycin may have a function related to packaging of tPA in secretory vesicles and thereby in facilitating secretion of tPA from endothelial cells. Thus, serglycin may play a role in modulating thrombolysis via tPA, similarly to the known importance of serglycin for stabilizing mast cell chymases.52 Another secreted endothelial protein, multimerin, appears to be in a secretory pool that is functionally separate from Weibel-Palade bodies.53Thus, our study provides additional evidence that secretory vesicles other than Weibel-Palade bodies are present in endothelial cells.
Although immunostaining had suggested that serglycin was present on the rat L2 yolk sac tumor cell membrane1 as well as within the cytoplasm, it seems unlikely that serglycin is a transmembrane proteoglycan, since there are no hydrophobic sequences in the mature protein that are obvious candidates for transmembrane structures. Alternatively, serglycin could be an external membrane proteoglycan; however, there was no staining of the nonpermeabilized HUVECs, so in this case serglycin does not appear to be on the cell surface.
We have found that about 85% of the serglycin made by endothelial cells during 4 days of culture is secreted under the culture conditions that we have used. It is interesting to compare this pattern of secretion with that of other serglycin-producing cells. Platelets and megakaryocytes from guinea pigs in vivo appear to retain their newly synthesized serglycin in storage granules, since the amount and characteristics of radiolabeled proteoglycan in platelets are consistent with the amount and characteristics in the normal megakaryocytes as platelet production from megakaryocytes is monitored during 5 days in animals injected with35S-sulfate.10 The entire platelet granule pool of proteoglycans, presumably serglycin, is secreted in response to thrombin while the putative membrane proteoglycan is retained by the platelets.10 In contrast, 3 hematopoietic tumor cell lines that we have studied—erythrocytic/megakaryocytic HEL,19megakaryoblastic CHRF 288-11,20 and promyelocytic HL-60 cells (B.S., unpublished data, 1996), secrete 55% to 70% of their serglycin under control conditions and secrete virtually 100% of their intact proteoglycans when stimulated by phorbol myristate acetate. Only free GAGs remain within the cells when HL-60 and CHRF cells are treated with phorbol myristate acetate.20 Our previous study has provided data that suggest that there are 2 pools of serglycin in CHRF-288-11 cells, a storage pool in which serglycin is retained with its core protein intact and a constitutive secretory pool that normally releases serglycin, also with its core protein intact. The constitutive secretory pool, however, undergoes severe proteolysis of the core protein surrounding the GAG-attachment site, but keeps the core GAG-attachment region intact when the cells are incubated with non–sequence-specific phosphorothioate oligodeoxynucleotides.54 The mechanism by which this occurs has not been established. Lymphocytes constitutively secrete serglycin, and serglycin synthesis is upregulated by stimuli,7 but lymphocytes have not been reported to have cytoplasmic storage granules. We have shown that serglycin is distributed throughout the cytoplasm of HUVECs in a vesicular pattern, is secreted constitutively during culture, and is secreted in response to TNF-α. Future studies will further elucidate the structure and function of the serglycin granules.
The size and structure of the HUVEC serglycin proteoglycan and its GAG chains are of interest. The serglycin proteoglycan from HUVECs is smaller than that of platelets of humans and other species.10,26 The HUVEC serglycin migrates at Kav of about 0.20 on Sepharose CL-6B columns, as compared with migration at Kav 0.12 for guinea pig or human platelets. The difference in size appears to be due to the size of the GAG chains, which in HUVECs are also considerably smaller—ie, they migrate at a higher Kav on Sepharose CL-6B—than those of many hematopoietic cells.10,19-21 The importance of chondroitin sulfate GAG chain length in regard to the ability of the proteoglycan to bind to ligands is not known. We have reported previously that Wistar-Furth rats, which have a severe platelet alpha granule defect, synthesize serglycin with nearly 3-fold smaller GAG chains than those of normal rats.26 It is not known whether this difference in size is responsible for changes in packaging of other granule substances, because of either loss of ability to interact with specific proteins or to changes of osmotic properties of the granules due to the much lower concentration of GAG chains. The chondroitin sulfate chains of the endothelial cell serglycin also appear to deviate from the normal repeating disaccharide chondroitin-4-sulfate structure, since the chondroitinase digestion products are varied in size, and these specific structures may determine substances with which this proteoglycan will interact in endothelial cells. Future studies will address the questions of the functions of the endothelial serglycin proteoglycan.
Acknowledgments
The authors thank Andrew Likens for expert preparation of the figures for this article, and Kristin (DiMezzes) Brodbeck for expert technical assistance in the early phases of this study.
Supported by National Institutes of Health grants HL-29282 (B.P.S.) and HL-53590 (J.D.S.A.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Barbara P. Schick, Cardeza Foundation for Hematologic Research, Thomas Jefferson University, 1015 Walnut St, Philadelphia, PA 19107; e-mail: barbara.schick@mail.tju.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal