Abstract
Lipid peroxidation plays an important role in atherogenesis. Previous studies suggested that autoantibodies against epitopes of oxidized low-density lipoprotein may indicate the extent or rate of progression of atherosclerosis. The aim of this study was to investigate whether autoantibodies to oxidized phospholipids, such as oxidized cardiolipin (OxCL), correlate with levels of isoprostane F2α-VI, a sensitive marker of in vivo lipid peroxidation, as well as with the extent of atherosclerosis. Two groups of apolipoprotein E-deficient mice were fed chow with or without vitamin E (2000 IU/kg diet) for 16 weeks. In untreated animals, autoantibodies against OxCL and urinary, plasma, and aortic isoprostane F2α-VI levels increased significantly. Vitamin E treatment significantly reduced antibody titers, isoprostane levels, and atherosclerosis at the end of the study, compared with untreated mice. Autoantibodies to OxCL correlated with aortic isoprostane F2α-VI levels (r2 = 0.42,P = .001 for IgG andr2 = 0.63, P < .001 for IgM). Both aortic isoprostane F2α-VI levels (r2 = 0.59, P < .001) and titers of OxCL antibodies (r2 = 0.70, P < .001 for IgG and r2 = 0.68,P < .001 for IgM) correlated with the extent of aortic atherosclerosis. The fact that the levels of autoantibodies to OxCL correlated with a sensitive direct measure of lipid peroxidation in vivo and that both autoantibodies and aortic isoprostane F2α-VI levels correlated with the extent of atherosclerosis suggests that antibodies to OxCL are a sensitive indicator of in vivo lipid peroxidation and atherosclerosis.
Introduction
Lipid peroxidation, in particular the formation of oxidized low-density lipoprotein (OxLDL) is believed to play an important role in atherogenesis.1-3 In vitro, OxLDL exhibits a broad spectrum of biologic effects that may promote atherogenesis, such as endothelial dysfunction, activation of endothelial adhesiveness, and monocyte and smooth muscle cell differentiation and proliferation. OxLDL is also rapidly internalized by macrophages via scavenger receptors, resulting in intracellular lipid accumulation and foam cell formation. The presence of OxLDL in atherosclerotic lesions has been established4 and substantial evidence now exists for the immunogenicity of OxLDL in vivo and the involvement of the immune system in atherosclerosis.5-7 During oxidation, a large number of oxidative neoepitopes are formed as a result of oxidative changes to the lipids of LDL and adduct formation between lipid peroxidation products and reactive amine groups of apolipoproteins. These trigger humoral immune responses, and circulating autoantibodies to several oxidation-specific epitopes have been demonstrated in humans and animal models of atherosclerosis.4-6,8-10 Atherosclerotic mice have particularly high autoantibody titers8,9 and natural monoclonal autoantibodies cloned from apolipoprotein E-deficient (apoE−/−) mice recognize oxidized phospholipid epitopes on OxLDL.10,11 The titer of autoantibodies binding to malondialdehyde-modified LDL (MDA-LDL) or copper-oxidized LDL (Ox-LDL) has been linked with the extent of atherosclerosis in LDL receptor-deficient (LDLR−/−) mice9 and in human subjects (reviewed in Witztum and Palinski5,6). Patients with the antiphospholipid antibody syndrome (APS), have traditionally been said to have autoantibodies to anionic phospholipids, such as cardiolipin, but current thinking also suggests that many of these autoantibodies are directed to the phospholipid-binding protein, β2GP1.12,13However, we have provided evidence that the antigens of some of these autoantibodies are in fact oxidized phospholipids, such as oxidized cardiolipin (OxCL), or adducts of OxCL with β2GP1.11,14 In a recent clinical study in patients with systemic lupus erythematosus (SLE), we reported increased antibody titers against OxCL in subjects with increased levels of F2-isoprostanes,15 specific and sensitive quantitative markers of in vivo lipid peroxidation.16These data suggest a relationship between a measure of lipid peroxidation and antibody titers to OxCL. However, a direct correlation of autoantibodies to OxCL and F2-isoprostanes with the extent of atherosclerosis has not yet been established. The purpose of this study was to investigate this relationship in apoE−/− mice with progressive spontaneous atherosclerosis in the absence or presence of an antioxidant (vitamin E) that reduces atherogenesis in this strain.17
Materials and methods
Animals
The animals used for this investigation were part of a study previously described in detail.17ApoE−/− mice (tenth generation backcrossed from 129/B6F1 heterozygous into C57Bl/6) were fed normal mouse chow. At 10 weeks of age (baseline), animals were randomized into 2 groups (n = 10, each). One was continued on chow (control group); the other received chow supplemented with vitamin E (2000 IU/kg chow) for 16 weeks. Levels of autoantibodies, isoprostane (iP) F2α-VI, and plasma lipids were determined at baseline and at the end of the study. Atherosclerosis and aortic iPF2α-VI levels were determined at the end of the study, ie, at age 26 weeks. All procedures and care of animals were approved by the Institutional Animal Care and Usage Committee of the University of Pennsylvania.
Determination of circulating autoantibodies
The plasma levels of autoantibodies binding to OxCL, MDA-LDL, and OxLDL were determined in individual plasma samples (n = 10 for each group) as previously described in detail, using a highly sensitive enzyme-linked immunoassay with a chemiluminescent detection system.11,18 Cardiolipin was allowed to undergo oxidation by exposure to air after plating on microtiter wells.11,18MDA-LDL, and OxLDL were generated as previously described11 19 and plated as antigen on microtiter wells. Wells were then incubated with a 1:100 dilution of mouse plasma, and IgG or IgM bound to the plated antigen was detected with alkaline phosphatase-labeled antimouse IgG or IgM antibodies. Results were expressed as relative light units per 100 milliseconds (RLU/100 ms).
To characterize the IgG from these mice, we isolated IgG from pooled plasma of the untreated and vitamin E-treated mice. Approximately 50 to 100 μL of plasma from each of the animals remaining at the end of the study was pooled to create one pool of approximately 1 mL from the untreated and vitamin E-treated mice, respectively. IgG was isolated from each pool by protein A chromatography, and the purity of the isolated IgG tested by Western blot analysis. Specifically, both purified IgG fractions were shown to be free of β2GP1 (apo H). To further characterize these antibodies, we initially prepared the wells with a fixed concentration of OxCL as described previously, and then added increasing amounts of IgG to the wells. The amount of IgG bound was then determined as described previously. In a second set of experiments, we plated increasing amounts of either OxCL or a “reduced” CL, analog in which all the unsaturated bonds had been hydrogenated,11 yielding a CL analog unable to undergo lipid peroxidation. To exclude any possibility of contamination with β2GP1, in this experiment we used gelatin (in place of bovine serum albumin) to dilute the antibody and to “post-coat” the wells after the antigen was plated. A fixed concentration of IgG was then added and the amount bound determined as described previously.
Biochemical analysis
Urinary, plasma, and total aortic iPF2α-VI levels were measured by a stable dilution isotope gas chromatography/mass spectrometry assay (GC/MS), as previously described.17Briefly, a known amount of internal standard [2H4]-iPF2α-VI was added to the samples. After solid phase extraction, the samples were derivatized and purified by 2 thin-layer chromatography steps. Finally, each sample was analyzed on a Fisons MD-800 GC/MS (Fisons, Beverly, MA). Plasma cholesterol and triglyceride levels were determined as previously described.17 Plasma vitamin E levels were measured by high-performance liquid chromatography (HPLC).20
Preparation of mouse aortas and measurement of vascular iPF2α-VI levels
After collection of the terminal blood, the mice were killed, and the aortic tree was perfused for 10 minutes with ice-cold phosphate-buffered saline (PBS) containing 20 μM BHT and 2 mM EDTA, pH 7.4, by inserting a cannula into the left ventricle and allowing free efflux from an incision in the vena cava.
After the removal of surrounding adventitial tissue, the aorta was opened longitudinally from the aortic root to the iliac bifurcation.8 The aorta was divided into 2 segments: the proximal part, including the arch, the thoracic, and supra-abdominal aorta, was used for quantitation of atherosclerosis (see below); and the distal abdominal aorta was used for total iPF2α-VI measurement as described previously.
Quantitation of aortic atherosclerosis
Statistics
Results were expressed as mean ± standard error of means (SEM). Total cholesterol, triglyceride, urinary, plasma, and tissue iPF2α-VI, autoantibody levels, and the extent of aortic atherosclerosis were analyzed by analysis of variance (ANOVA) and subsequently by Student unpaired 2-tailed t test. Correlations between parameters were tested by linear regression analysis.
Results
Untreated mice
Compared with baseline, urinary iPF2α-VI levels were significantly increased at the end of the study (1156 ± 60 vs 550 ± 51 pg/mg creatinine, respectively; P < .0001) (n = 10). The changes in plasma iPF2α-VI qualitatively resembled those observed in urine. Thus, 26-week-old apoE−/− mice had higher plasma levels of iPF2α-VI than 8-week-old animals (2.3 ± 0.15 vs 0.8 ± 0.009 ng/mL, respectively; P < .0001) (n = 10). Total plasma cholesterol levels increased significantly over the course of the study (from 560 ± 45 to 790 ± 50 mg/dL,P < .001), whereas no significant changes in triglyceride levels were observed (data not shown).
In the untreated mice, both IgG and IgM autoantibodies binding to OxCL increased significantly from baseline to the final time point of the study (Table 1). A similar increase was observed for autoantibodies binding to MDA-LDL and Ox-LDL (Table 1). Aortic iPF2α-VI levels at time of death at 26 weeks of age were 0.86 ± 0.09 pmol/μmol phospholipids (n = 10). Lesions were present throughout the aortic tree in all the mice studied. Atherosclerotic lesions covered 17.9% ± 5% of the total surface area considered. Total aortic iPF2α-VI levels showed a significant correlation with the extent of atherosclerosis (r2 = 0.87; P < .001). In analogy, titers of autoantibodies binding to OxCL also correlated with the extent of atherosclerosis (r2 = 0.83, P < .001 for IgG and r2 = 0.74,P = .002 for IgM). Finally, levels of both IgG and IgM anti-OxCL autoantibodies correlated with aortic (r2 = 0.83, P = .002,r2 = 0.84, P < .001, respectively), plasma (r2 = 0.70,P < .001, r2 = 0.68,P < .001, respectively) and urinary iPF2α-VI levels (r2 = 0.75, P < .001,r2 = 0.70, P < .01, respectively). By contrast, no correlation was observed between MDA-LDL or OxLDL autoantibodies titers and extent of atherosclerosis, aortic, plasma, and urinary iPF2α-VI levels (data not shown).
Plasma levels of autoantibodies against OxCL, MDA-LDL, and Ox-LDL in untreated apoE−/− mice on chow at 8 weeks and 26 weeks of age
| Autoantibodies (RLU/100 ms) . | Age 8 wk . | Age 26 wk . | P value . |
|---|---|---|---|
| IgG (OxCL) | 987 ± 222 | 10 662 ± 3144 | < .01 |
| IgM (OxCL) | 2120 ± 327 | 36 894 ± 8018 | <. 001 |
| IgG (MDA-LDL) | 7210 ± 620 | 19 414 ± 2043 | < .001 |
| IgM (MDA-LDL) | 22 059 ± 1585 | 47 637 ± 3414 | < .001 |
| IgG (Ox-LDL) | 1850 ± 249 | 4181 ± 466 | < .05 |
| IgM (Ox-LDL) | 4552 ± 434 | 10 313 ± 1470 | < .01 |
| Autoantibodies (RLU/100 ms) . | Age 8 wk . | Age 26 wk . | P value . |
|---|---|---|---|
| IgG (OxCL) | 987 ± 222 | 10 662 ± 3144 | < .01 |
| IgM (OxCL) | 2120 ± 327 | 36 894 ± 8018 | <. 001 |
| IgG (MDA-LDL) | 7210 ± 620 | 19 414 ± 2043 | < .001 |
| IgM (MDA-LDL) | 22 059 ± 1585 | 47 637 ± 3414 | < .001 |
| IgG (Ox-LDL) | 1850 ± 249 | 4181 ± 466 | < .05 |
| IgM (Ox-LDL) | 4552 ± 434 | 10 313 ± 1470 | < .01 |
Results are expressed as mean ± standard error of the mean, n = 10 for each age group.
OxCL indicates oxidized cardiolipin; MDA-LDL, malondialdehyde-modified low-density lipoprotein; Ox-LDL, oxidized low-density lipoprotein.
Effect of vitamin E
At the beginning of the study (baseline), there were no significant differences between mice in the control (n = 10) and vitamin E (n = 10) groups in urinary, plasma iPF2α-VI, cholesterol, vitamin E, and autoantibodies against OxCL, MDA-LDL or OxLDL levels (not shown). Compliance with the vitamin E supplementation was evident, as its plasma levels showed a marked increase in the treatment group at the end of the study (136 ± 20 vs 36 ± 7 μM;P < .001). Compared with the untreated animals, 16 weeks of treatment with vitamin E reduced levels of iPF2α-VI in urine (1156 ± 68 vs 530 ± 30 pg/mg creatinine;P < .001), plasma (2.3 ± 0.15 vs 1.25 ± 0.15 pg/mL;P < .001) and aortas (0.86 ± 0.09 vs 0.40 ± 0.07 pmol/μmol phospholids; P < .01) (n = 10 for each group). Similarly, total aortic lesion areas were significantly reduced by vitamin E treatment (17.9 ± 5 vs 6.1 ± 0.9%, respectively;P = .008). By contrast, the treatment did not influence total plasma cholesterol or triglyceride levels (data not shown).
After 16 weeks of vitamin E supplementation, levels of IgG and IgM autoantibodies binding to OxCL were lower in the vitamin E group (n = 10) compared with untreated controls (n = 10) for IgG, 3758 ± 1048 versus 10 662 ± 3144 RLU/100 ms (P = .05); for IgM, 8719 ± 2371 versus 36 894 ± 8018 RLU/100 ms (P < .005). No change, however, was observed for IgG and IgM autoantibody levels binding to MDA-LDL and Ox-LDL (not shown).
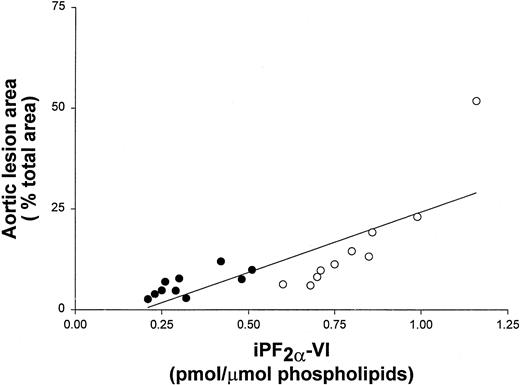
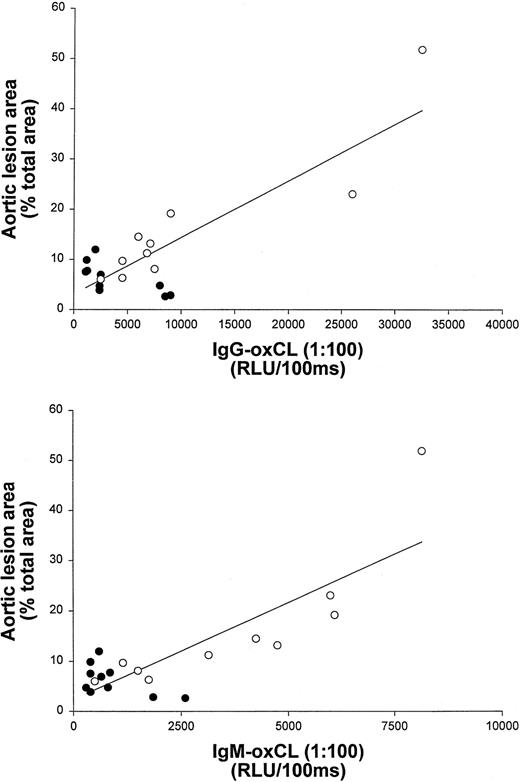
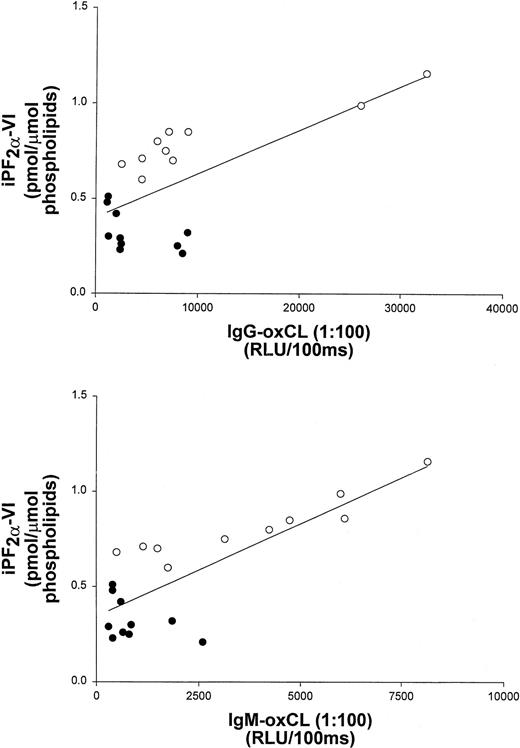
To further examine the correlation of iPF2α-VI and OxCL autoantibody levels with the extent of atherosclerosis, data from vitamin E-treated and untreated control mice at the end of the study were pooled. These data still showed a significant correlation between aortic atherosclerosis and aortic iPF2α-VI levels (r2 = 0.59; P < .001) (Figure 1), and between atherosclerosis and IgG or IgM autoantibodies to OxCL (r2 = 0.70; P < .001 andr2 = 0.68, P < .001, respectively) (Figure 2). Similarly, aortic iPF2α-VI levels and levels of IgG or IgM autoantibodies to OxCL were also still significantly correlated, albeit with lower regression coefficients (r2 = 0.42, P = .001 andr2 = 0.63, P < .001, for IgG and IgM, respectively) (Figure3).
Aortic lesion area and aortic iPF2α-VI levels correlate in apoE−/− mice.
Correlation between aortic lesion area and aortic iPF2α-VI levels in apoE−/− mice from both the control (open circles) and vitamin E-treated (closed circles) groups.
Aortic lesion area and aortic iPF2α-VI levels correlate in apoE−/− mice.
Correlation between aortic lesion area and aortic iPF2α-VI levels in apoE−/− mice from both the control (open circles) and vitamin E-treated (closed circles) groups.
Aortic lesion area and terminal plasma levels of IgG and IgM antibodies binding to OxCL correlate in apoE−/− mice.
Correlation between aortic lesion area and terminal plasma levels of IgG and IgM antibodies binding to OxCL in apoE−/− mice from both the control (open circles) and vitamin E-treated (closed circles) groups.
Aortic lesion area and terminal plasma levels of IgG and IgM antibodies binding to OxCL correlate in apoE−/− mice.
Correlation between aortic lesion area and terminal plasma levels of IgG and IgM antibodies binding to OxCL in apoE−/− mice from both the control (open circles) and vitamin E-treated (closed circles) groups.
Aortic iPF2α-VI levels and terminal plasma levels of IgG and IgM antibodies binding to OxCL correlate in apoE−/− mice.
Correlation between aortic iPF2α-VI levels and terminal plasma levels of IgG and IgM antibodies binding to OxCL in apoE−/− mice from both the control (open circles) and vitamin E-treated (closed circles) groups.
Aortic iPF2α-VI levels and terminal plasma levels of IgG and IgM antibodies binding to OxCL correlate in apoE−/− mice.
Correlation between aortic iPF2α-VI levels and terminal plasma levels of IgG and IgM antibodies binding to OxCL in apoE−/− mice from both the control (open circles) and vitamin E-treated (closed circles) groups.
Characterization of the OxCL autoantibodies
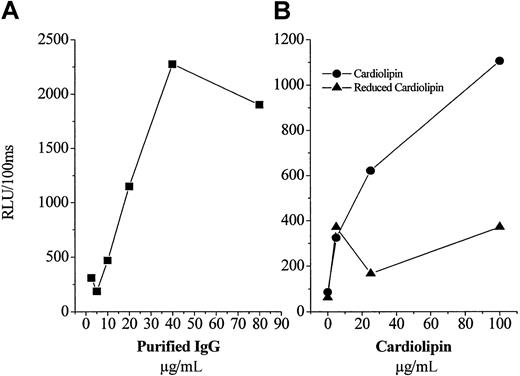
As noted in the “Introduction,” “antiphospholipid” autoantibodies in humans with the APS have been described to bind to a variety of antigens, including the ubiquitous phospholipid binding protein, β2GP1. To characterize the antigen to which the IgG from these mice was binding, we purified IgG from a pooled sample prepared from the untreated and vitamin E-treated mice. First, we demonstrated by Western blot analysis that each of the pools did not contain any β2GP1 (not shown). In an initial experiment, we added a fixed amount of OxCL to microtiter wells, and then added increasing concentrations of purified IgG (from vitamin E-treated mice) to the plate. As shown in Figure 4A, there was a dose-dependent binding of the IgG. Similar results were found for the IgG isolated from the untreated mice (not shown). To further characterize the IgG, next we added to microtiter wells increasing amounts of either OxCL or a “reduced” CL analog, in which the unsaturated bonds have been hydrogenated, rendering the CL unable to undergo lipid peroxidation.11 Furthermore, this experiment was conducted in the presence of gelatin, as the nonspecific protein used to postcoat wells, to eliminate the possibility that any β2GP1 was present. As shown in Figure 4B, there was a dose-dependent increase in the binding of IgG (from untreated mice) to the OxCL, but no significant binding to the reduced CL analog. These data clearly demonstrate that the isolated IgG from these animals bind to the OxCL, independent of β2GP1.
Characterization of protein A-purified IgG from pooled plasma of untreated and vitamin E-treated apoE−/− mice.
The 2 principal IgG preparations contained no β2GP1. (A) Increasing concentrations of IgG (from vitamin E-treated plasma), which contained no β2GP1, were added to microtiter wells containing a fixed amount of OxCL and the amount of IgG bound measured as described in “Materials and methods.” (B) A fixed content of purified IgG (from untreated plasma) was added to microtiter wells containing an increasing concentration of either OxCL or a “reduced” CL analog unable to undergo oxidation. Each point is the mean of triplicate determinations.
Characterization of protein A-purified IgG from pooled plasma of untreated and vitamin E-treated apoE−/− mice.
The 2 principal IgG preparations contained no β2GP1. (A) Increasing concentrations of IgG (from vitamin E-treated plasma), which contained no β2GP1, were added to microtiter wells containing a fixed amount of OxCL and the amount of IgG bound measured as described in “Materials and methods.” (B) A fixed content of purified IgG (from untreated plasma) was added to microtiter wells containing an increasing concentration of either OxCL or a “reduced” CL analog unable to undergo oxidation. Each point is the mean of triplicate determinations.
Discussion
Antiphospholipid autoantibodies (aPL) are found in patients with APS and in connection with certain states such as infections and certain drugs.13,22,23 Patients with aPL have recurrent venous or arterial thrombosis, history of increased fetal death, and autoimmune thrombocytopenia. Recently, it has been reported that these patients are at increased risk for myocardial infarction.24-26 It is known that there is considerable heterogeneity in the autoantibody populations found in patients with APS and in their pathogenic importance.13,23-25,27Moreover, there has been considerable controversy regarding the antigens to which so-called aPL bind. Although much of the original work in this area focused on the binding of autoantibodies to anionic phospholipids,22 it is currently widely thought that many, if not most of such antibodies bind to β2GP1, an apolipoprotein that has high phospholipid avidity.12,13,23Among the “antilipid” type autoantibodies, CL has traditionally, and most often, been used as the test antigen for aPL assays. Cardiolipin is a hydrophobic phospholipid that contains 4 polyunsaturated fatty acid chains. In standard clinical solid-phase enzyme-linked immunosorbent assays (ELISAs) for anticardiolipin antibodies (aCL), the CL, in organic solvent, is pipetted into a microtiter well and the organic solvent evaporated to “plate” the CL as antigen. We have previously shown that this procedure leads to almost immediate oxidation of the CL and that the binding of immunoglobulin from patients' sera is directed at epitopes of OxCL, due to the OxCL itself, or adducts formed between the OxCL and protein present in the microtiter well, such as β2GP1.11,14 We have previously shown that, whereas C57Bl/6 mice have no aCL, apoE−/− mice have elevated titers of aCL and that these selectively bind to oxidized epitopes of CL (OxCL) as well as to OxLDL.11 In this paper, we demonstrate that there are clearly populations of antibodies in the apoE−/− that bind exclusively to OxCL, independently of the presence of murine β2GP1. At present, the exact epitopes on OxCL to which these autoantibodies bind are not yet defined. In the whole sera, of course, there is an abundance of β2GP1, and it is possible that the titers of antibodies measured in this study also included, in part, autoantibodies directed against adducts of OxCL that would form with the β2GP1 present.
This paper explicitly demonstrates that atherogenesis per se is associated with an increase in circulating autoantibodies to OxCL and that plasma levels of these autoantibodies correlate with the overall extent of aortic atherosclerosis in apoE−/− mice. More importantly, it provides the first evidence that the titers of autoantibodies binding to OxCL correlate with tissue levels of isoprostane F2α-VI, a specific and direct measure of lipid peroxidation occurring in vivo.16
We have previously reported that the titer of autoantibodies binding to MDA-LDL increases in LDLR−/− mice during progressive atherogenesis,9 similar to the increase noted here in the apoE−/− mice (Table 1). In addition, a large number of clinical studies have established correlations between the titer of autoantibody binding to different oxidized lipids of LDL and various manifestations of cardiovascular disease or risk factors.5,6,28-36 The correlation of autoantibodies to OxCL with the extent of atherosclerosis observed in our study would be consistent with the majority of human studies. This is not surprising, because phospholipid-containing polyunsaturated fatty acids are prominent components of LDL and the site of initiation of oxidation when LDL is subjected to oxidative stress. Because OxLDL accumulates in lesions in the artery wall8 and because CL is a prominent phospholipid of LDL,27 it is likely that OxCL within the atherosclerotic plaque is an immunogen and could explain in part why the autoantibodies to OxCL correlate both with the measures of lipid peroxidation and with the extent of atherosclerosis. The fact that autoantibody titers to OxCL fell in parallel with decreased measures of lipid peroxidation and atherosclerosis in response to vitamin E suggests that these autoantibodies are a sensitive index of these parameters in this animal model, presumably because of a decreased extent of presentation of the OxCL antigen as a result of decreased content of OxLDL. The hypothesis that aCL antibodies could reflect the oxidative status in vivo was recently tested by us. Patients with SLE positive for aCL had higher F2-isoprostane levels than negative ones, and these levels were highly correlated with aCL titer.15
In this study, we were surprised by the observation that the titer of autoantibodies to MDA-LDL or Ox-LDL did not fall in response to the vitamin E feeding. Although we do not know the exact reason for this observation, it may relate to the extent and degree of change of atherosclerotic disease in this mouse model of the disease. Recently, we observed that the titers to MDA-LDL and Ox-LDL fell significantly in LDL receptor-deficient mice, first placed on an atherogenic diet for 6 months, and subsequently placed on a chow diet plus vitamins C and E for 6 months. In those experiments, there was actual lesion regression, which correlated to changes in antibody titers (Tsimikas et al, manuscript submitted). The design of the current experiments was different in that both treated and untreated apoE−/− mice had a progression of disease during the course of the study, though the rate was obviously much lower in the vitamin E group. The fact that, in the current study, titers to OxCL fell, but antibody titers to epitopes of OxLDL did not, may not only reflect the extent of antigen presentation in OxLDL as noted previously, but could also reflect the possibility that the immunogen was not simply OxLDL, but for example, could have been OxCL released from apoptotic and dying cells in the lesions as well.
The observation that the titer of autoantibodies to OxCL correlates with the extent of atherosclerosis in apoE−/− mice is of particular interest for several reasons. First, oxidized phospholipids or oxidized phospholipid-protein adducts seem to induce a particularly strong humoral immune response.38 This is also apparent in Table 1, which shows that the relative increase of IgG and IgM autoantibodies against OxCL during the evolution of the disease was much greater than that of autoantibodies to MDA-LDL and OxLDL in apoE−/− mice. This could also explain in part the absence of any correlation between the titer of autoantibodies to OxLDL and atherosclerosis or iPF2α-VI levels in this experiment. Second, monoclonal antibodies cloned from apoE−/− mice (“EO” antibodies) that bound well to oxidized phospholipids and to OxLDL—but not antibodies binding to MDA-LDL—block the uptake of OxLDL by macrophage scavenger receptors.38 It is therefore possible that antibodies to oxidized phospholipids are not only markers of the atherogenic process, but that they also possess important biologic properties that could influence atherogenesis. However, we do not know if the OxCL autoantibodies measured in this study have such properties. Third, other biologic effects of antibodies to oxidized phospholipids could include modulation of coagulation.13,20,21,23,35 39
A major limitation of clinical studies investigating the correlation between atherosclerosis and circulating autoantibody titers in humans is that neither the overall extent of atherosclerosis nor the amount of antigen in the artery wall can be accurately estimated by noninvasive measures. This study is not subject to these limitations, as both the extent of atherosclerosis and a sensitive reliable measure of in vivo lipid peroxidation were available. However, other theoretical caveats remain valid. For example, the antibody titer represents a balance between antibody formation and “consumption” and is therefore influenced by factors such as fluctuations in antigen formation over time, which may in turn be influenced by the cellular composition of lesions, in particular their content of macrophages and T cells.40 Formation of immune complexes, both in the circulation and in the artery wall may also affect antibody titers.
In summary, our results provide the first demonstration that the plasma levels of autoantibodies against OxCL correlate with iPF2α-VI levels in plasma and aortic wall, and that all of these parameters correlate with the extent of atherosclerosis in apoE−/− mice. Furthermore, vitamin E treatment reduces all of them in parallel with the extent of atherosclerosis. This suggests that autoantibodies to OxCL may be sensitive indicators of in vivo lipid peroxidation and atherogenesis. Further studies to test this hypothesis are warranted.
Supported in part by a grant-in-aid from the American Heart Association (9951223U) and grants from the NIH (M01RR00040) and NHLBI (HL57505).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Domenico Praticò, Department of Pharmacology, University of Pennsylvania, BRB II/III, Rm 812, 421 Curie Blvd, Philadelphia, PA 19104; e-mail: domenico@spirit.gcrc.upenn.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal