Abstract
LL2, an anti-CD22 monoclonal antibody against B-cell lymphoma, was covalently linked to the amphibian ribonuclease, onconase, a member of the pancreatic RNase A superfamily. LL2 increased in vitro potency (10 000-fold) and specificity against human Daudi Burkitt lymphoma cells while decreasing systemic toxicity of onconase. Monensin further increased potency of LL2-onconase on Daudi cells (IC50, 20 and 1.5 pM, absence and presence of monensin, respectively). A 1-hour exposure to LL2-onconase was sufficient to kill Daudi cells in culture. These favorable in vitro properties translated to significant antitumor activity against disseminated Daudi lymphoma in mice with severe combined immunodeficiency disease. In mice inoculated with tumor cells intraperitoneally (ip), LL2-onconase (100 μg 5 times ip every day) increased the life span of animals with minimal disease 200%. The life span of mice with advanced disseminated Daudi lymphoma (tumor cells inoculated intravenously) was increased 135%. Mice injected with LL2-onconase tolerated a dose as high as 300 mg/kg. Because both onconase and LL2 are in clinical trials as cancer therapeutics, the covalently linked agents should be considered for treatment of non-Hodgkin lymphoma.
Introduction
The outlook for management of non-Hodgkin B-cell lymphomas (NHL) with monoclonal antibody-mediated therapies has improved. Encouraging response rates in patients treated with antibody constructs have been reported to range from 25% to 95% (reviewed in Press1). One of the best characterized monoclonal antibody targets on B cells is the CD22 antigen. CD22, a member of the sialoadhesin family of adhesion molecules, mediates cellular interactions by recognizing specific cell surface sialylated glycoconjugates. CD22 is attractive because it is not exposed on embryonic stem or pre-B cells nor is it normally shed from the surface of antigen-bearing cells.2 A murine anti-CD22 monoclonal antibody (mAb) (LL2, originally designated EPB-23) was developed for imaging and treatment of NHL. LL2 has a highly restricted specificity; it does not cross-react with peripheral blood cells, including the blood's normal B cells, yet is reactive with virtually all cases of NHL.4 Recently, preliminary results of a clinical trial in which the humanized version of LL2, Epratuzumab, was used have been reported.5 No dose-limiting toxicity nor human anti-Epratuzumab antibody response was observed at the highest dose administered (1000 mg/m2 per week), whereas objective responses were noted. Thus, the naked antibody is well tolerated and has clinical efficacy. Although these and other results1are hopeful, the durability of the responses is not yet known. In this regard, continued treatment with an Epratuzumab-onconase conjugate to remove residual tumor cells could improve treatment outcomes.
Antibodies linked to toxic plant or bacterial enzymes, immunotoxins, have been shown to have potential for the treatment of residual disease in blood-borne cancers (reviewed in Kreitman and Pastan,6Thrush et al,7 and Uckun and Reaman8). Hematologic malignancies are particularly appropriate for these relatively large (IgG, approximately 180 kd to sFv approximately 60 kd) hybrid proteins because the tumor cells are present in the blood and more easily accessible than in solid tumors. Anti-CD22 ricin A-chain immunotoxins have been well studied both preclinically7,9-11 and clinically.7,12-15The promise this approach holds is illustrated by the attainment of a complete sustained response of a large B-cell lymphoma with an anti-CD22 ricin A-chain immunotoxin. In this regard, LL2 possesses unique features compatible with its use in the construction of immunotoxins. It is rapidly internalized, and then the CD22 antigen is re-expressed on the cell surface.4 In fact, LL2 was previously linked to a derivative of Pseudomonas exotoxin by Kreitman et al16 and shown to very effectively induce regression of subcutaneous human B-cell lymphomas in mice.
Although immunotoxins made with plant or bacterial toxins certainly appear to have potential as therapeutics for blood-borne cancers, their clinical development has revealed problems related to nonspecific toxic side effects and immunogenicity (reviewed in Vitetta et al17). Members of the pancreatic ribonuclease family have been proposed as possible alternatives to plant and bacterial toxins because these small extracellular proteins normally reside in the plasma and tissues of humans (Rybak and Newton18 and references therein). The internalization of these proteins by microinjection,19 antibodies,20-23 or growth factors24 has shown them to be potent cytotoxins in vitro, although significantly less so than antibody toxin constructs. For the first time, this paper describes a conjugate that is built with an RNA-damaging enzyme and differs significantly from immunotoxins with respect to mechanism of action and delivery. Iordinov et al25 present evidence that onconase cytotoxic mechanism of action differs from that of plant and bacterial toxins because it is at least partly independent of protein synthesis inhibition. Onconase-treated cells displayed apoptosis-associated cell blebbing, nuclear pyknosis, DNA fragmentation, PARP cleavage, and activation of caspase-3–like activity. Additionally, onconase is being evaluated as a single therapeutic agent in phase III clinical trials for mesothelioma (S.M.M., unpublished data). This also distinguishes onconase from plant and bacterial toxins that cannot be administered to human patients without targeting agents, ie, onconase is a hybrid drug/enzyme analog with unique properties.26 27
Its small size, basicity, and homology to extracellular pancreatic RNases may explain why onconase can be given to humans repeatedly for years without problems associated with immunogenicity.28Because LL2 has demonstrated activity in patients with lymphoma29,30 and can increase onconase-specific tumor killing thousands of times, the covalent partnership of these 2 agents may usher in a new phase of antibody-targeted protein therapeutics that addresses the problems of toxicity and immunogenicity that has hampered the clinical development of immunotoxins.31
Materials and methods
Materials
LL2, a product of Immunomedics Inc, Morris Plains, NJ, UPC-10 (Cappel Organon Teknika, Durham, NC) murine monoclonal IgG2a antibody, and onconase (generic name, ranpirnase), a product of Alfacell Corp, Bloomfield, NJ, were applied to PD-10 columns (Pharmacia LKB Biotechnology, Piscataway, NJ), equilibrated, and eluted with 0.1 M NaPO4, pH 7.5, containing 0.1 M NaCl before use in the conjugation reaction. N-succinimidyl 3-(2-pyridyldithio) propionate (SPDP) and 2-iminothiolane (2-IT) were from Pierce Chemical Co, Rockford, IL; 5,5′ dithiobis(2-nitrobenzoic acid) (DTNB), dithiothreitol (DTT), and monensin were from Sigma (St Louis, MO). Daudi, Ramos, RL, Raji, CA-46 human lymphoma cell lines, and the HUT-102 T-cell lymphoma cell line were obtained from the American Type Culture Collection (Rockville, MD).
Mice
Female pathogen-free, CB17 SCID/NCr or BALB/c mice (6 to 8 weeks of age) were obtained from the Animal Production Area, National Cancer Institute (NCI)-Frederick Cancer Research and Development Center. The mice were kept in cages with filter bonnets under sterile conditions in a laminar air-flow unit. They were maintained on a diet of sterilized mouse chow (Ziegler Bros, Gardner, PA) and given water ad libitum. In all animal experiments, animal care procedures were in accordance with standards described in the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Preparation of conjugates
Conjugates were prepared at room temperature. Briefly, 0.27 μmol onconase was treated with a 2.5-fold excess of SPDP for 30 minutes to obtain a substitution of 0.9 to 1.2 mol SPDP/mol onconase. After excess SPDP was removed by PD-10 chromatography, the onconase-2-pyridyl disulfide derivative was treated with 2 mM DTT for 1 hour to reduce the 2-pyridyl disulfide bond. While onconase was being reduced, the antibodies (12.5 nmol) were incubated with a 20-fold excess of 2-IT (250 nmol) and 2.5 mM DTNB (final concentration) in 100 mM sodium borate, pH 8.5 for 1 hour. Immediately after the removal of excess 2-IT and DTNB from the antibody solution and DTT from the onconase solution by PD-10 chromatography, the 2 modified proteins (10:1 molar ratio, onconase:antibody) were combined and incubated overnight at room temperature. The reaction between onconase and antibody was monitored by the appearance of thionitrobenzoate ion (TNB), a by-product released as the disulfide bonds between the RNase and antibody are formed. The number of moles of onconase conjugated per mole of antibody was determined in 2 ways. (1) Spectrophotometrically at 412 nm by comparing the number of moles of TNB released [TNB, E412 nm = 13 60032] with the number of moles of antibody contained in the reaction mixture; (2) the number of moles of RNase conjugated per mole of antibody was also determined by SDS-polyacrylamide gel electrophoresis (PAGE) performed under reducing conditions with known standards of onconase and antibody. Identical results were obtained with the 2 methods. The conjugates were separated from unconjugated RNase by chromatography on a Toyo Soda TSK 3000 SW column (Toso Haas, Montgomeryville, PA) as described.33
Protein determination
Protein concentration was determined using the following extinction coefficients: onconase, E1%, 277 nm = 8.8; LL2 antibody, E1%, 280 nm = 14.0; LL2-onconase, E1%, 280 nm = 10.0.
Cytotoxicity assays
All human lymphoma cells were maintained in RPMI 1640 (Gibco BRL, Gaithersburg, MD) medium containing 10% heat-inactivated fetal bovine serum and 50 μg/mL gentamicin. Cells (10 000 in 0.1 mL) were placed in each well of a 96-well plate 24 hours before treatment. On the day of treatment, test samples (10 μL) were added to the appropriate well, and the cells were incubated for 3 days at 37°C in a humidified CO2 incubator. Protein synthesis was determined as described.33 Each point was in triplicate, and each experiment performed at least twice. The IC50, the concentration of test sample that inhibits protein synthesis by 50%, was determined from semilogarithmic plots in which protein synthesis as a percentage of control (buffer-treated cells) was plotted versus test protein concentration.
RNase assay
Ribonuclease activity using yeast transfer RNA (tRNA) (Sigma) was determined at 37°C by monitoring the formation of perchloric acid–soluble nucleotides as described.34 The following buffer was used (final concentration); 10 mM HEPES, pH 6.0, containing 160 μg/mL human serum albumin (HSA, Sigma). Final tRNA concentrations ranged from 0.1 to 1 mg/mL. Incubation times were 2 hours. Each assay was repeated at least twice, and the data pooled.
Binding studies
Daudi cells were washed twice with Dulbecco phosphate-buffered saline (DPBS) (Gibco BRL) before being placed in PBS containing 1% bovine serum albumin (BSA). Cells (1.5 to 2.0 × 105cells in 0.25 mL) were placed in 1.5-mL tubes, 10 μL of varying concentrations of LL2 and LL2-onconase was added, and the cells were incubated on ice for 15 minutes. [125I]LL2 (20 ng per assay, 1.5 × 108 cpm/nmol) was added to the cells, and the incubation continued for 2 to 3 hours on ice. The cells were washed 3 times with ice-cold 1% BSA/PBS, and the bound [125I]LL2 quantitated with a gamma counter. Duplicates from at least 2 experiments were pooled, and the amount of onconase conjugate that displaced [125I]LL2 was determined.
Stability of conjugates in plasma
LL2-onconase was diluted 1:10 in pooled human plasma (Sigma) and incubated for 3 days at 37°C. Cytotoxicity assays were performed to determine the activity of the treated conjugate in parallel with nontreated conjugate.
In vivo toxicity
Mice were injected intravenously (iv) or intraperitoneally (ip) on different schedules as described in “Results” with 0.2 mL PBS or PBS containing varying doses of onconase or LL2-onconase. The animals were monitored daily for weight loss and other signs of toxicity such as coat ruffling and changes in gait for at least 1 week after the final dosing. Toxicity severe enough to prevent feeding necessitated euthanasia. Both SCID and BALB/c mice were used for determining toxicity. To evaluate the dose-limiting toxicity of LL2-onconase, 3 SCID mice were treated with 2.0 mg conjugate per day (0.2 mL ip injections of 500 μg, 4 times per day). When it was believed that the mice would not survive another treatment, a complete necropsy was performed. The preparation and analysis of the slides was performed by the Pathology/Histology Laboratory, SAIC, Frederick, MD, (contract no. N01-CO-56000).
In vivo antitumor activity
Two in vivo animal models35 using female SCID mice 8 to 10 weeks of age obtained from the National Cancer Institute, Frederick, MD, were used to test the antitumor activity of LL2-onconase. In each model, the mice (5 to 10 mice in each group) were injected 24 hours in advance of the cell injection with 0.2 mL rabbit antiasialo GM1 (ASGM-1, 1:20 dilution in H2O, Wako Pure Chemical Industries, Richmond, VA). In the first model, 5 × 106 Daudi lymphoma cells were injected ip, followed 1 day later for 5 consecutive days with 0.2 mL ip injections of test substances. The total dose of LL2-onconase by weight (100 μg each day for 5 days, 25 mg/kg) was compared with equivalent amounts of each agent in the conjugate, ie, onconase alone (20 μg each day for 5 days, 5 mg/kg), LL2 alone (80 μg each day for 5 days, 20 mg/kg), or a mixture of these agents. The animals were monitored for the development of ascites.
In the second model, 5 × 106 Daudi lymphoma cells were injected iv, followed 1, 3, or 7 days later for 5 consecutive days with 0.2 mL iv injections of test substances. The animals were monitored for the development of hind limb paralysis, which occurred approximately 3 to 4 days before death,35 and were sacrificed as soon as paralysis developed. Histologic analysis of systemic cancer in the kidneys, a major affected organ, was determined in one experiment. The kidneys were removed 32 days after inoculation of tumor cells (5 days before the MPT), immersed in Bouins fixative (Ricca Chemical, Arlington, TX), paraffin-embedded, sectioned, and stained with hematoxylin and eosin. The preparation and analysis of the slides was performed by the Pathology/Histology Laboratory, SAIC (contract no. N01-CO-56000).
Results
Characterization of onconase conjugated to LL2
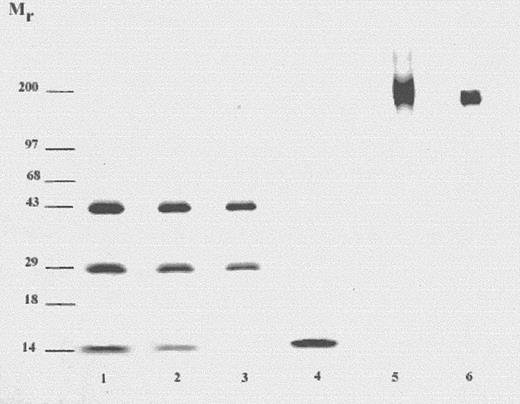
Amphibian onconase36 was coupled to the mAb, LL2. LL2 is a murine IgG2a antibody directed against the CD22 antigen on human B-cell lymphomas and leukemias.3,30 Intermolecular disulfide bond(s) between onconase and LL2 were formed by reacting onconase derivatized with the heterobifunctional cross-linking reagent, SPDP, with 2-iminothiolane–treated antibody. A 10-fold molar excess of SPDP-onconase over the derivatized antibody was used to drive the reaction toward producing the conjugate and limit-free antibody, following a procedure used previously in the preparation of RNase conjugates.33,37,38 The resulting conjugate was separated from unreacted onconase by gel filtration on a TSK-3000 high-performance liquid chromatography (HPLC) column. The conjugate pool was analyzed by SDS-PAGE (Figure 1). The Mr of LL2-onconase under nonreducing conditions was approximately 190 kd as expected for the monomeric antibody coupled to 3 mol of onconase (Mr 12 kd) (Table1). No free onconase was detected (compare lanes 4 and 5). High-molecular weight material (Mr > 200 kd) consistent with antibody-antibody conjugates, which also contain RNase38 appears to be present less than 5% by densitometric analysis (Figure 1, lane 5). Because the Mr of the conjugate (approximately 190 kd) is similar to that of the antibody (Mr 150 kd), it is difficult to show unequivocal separation of conjugate from free antibody on an SDS-PAGE under nonreducing conditions (compare lanes 5 and 6, Figure 1). Analysis of individual fractions across an unpooled HPLC peak (conjugate retention time, 28 to 34 minutes) revealed a very similar molar ratio of onconase to antibody (fractions eluting at 30, 32, and 34 minutes contain 2.8, 2.6, and 3.0 mol onconase per mole antibody, respectively). Determination of the cytotoxic activity of the individual fractions indicates that free antibody is not present in amounts significant enough to affect activity (IC50, 100 pM for each of the fractions eluting at 30, 32, and 34 minutes). As shown in Figure 1, lanes 1 and 2, onconase was only released from the conjugate under reducing conditions confirming the presence of a disulfide bond.
SDS-PAGE of LL2-onconase conjugate under reducing and nonreducing conditions.
LL2-onconase was prepared as described in “Materials and methods” and analyzed on a 4% to 20% polyacrylamide gel. Lanes 1-4, samples were reduced by boiling for 5 minutes in SDS sample buffer containing 2-mercaptoethanol. Lanes 1 and 2, 6 and 3 μg LL2-onconase, respectively; lane 3, 1 μg LL2; lane 4, 1 μg onconase. Lanes 5 and 6 were under nonreducing conditions. Lane 5, 3 μg LL2-onconase; lane 6, 1 μg LL2 antibody. The gel was stained with Coomassie Blue.
SDS-PAGE of LL2-onconase conjugate under reducing and nonreducing conditions.
LL2-onconase was prepared as described in “Materials and methods” and analyzed on a 4% to 20% polyacrylamide gel. Lanes 1-4, samples were reduced by boiling for 5 minutes in SDS sample buffer containing 2-mercaptoethanol. Lanes 1 and 2, 6 and 3 μg LL2-onconase, respectively; lane 3, 1 μg LL2; lane 4, 1 μg onconase. Lanes 5 and 6 were under nonreducing conditions. Lane 5, 3 μg LL2-onconase; lane 6, 1 μg LL2 antibody. The gel was stained with Coomassie Blue.
RNase activity of onconase or LL2-onconase conjugate
| RNase . | pH* . | Moles RNase per mole antibody† . | Km μM . | Kcat sec−1 . | Kcat/Km M−1 sec−1 . |
|---|---|---|---|---|---|
| Onc | 6.0 | N/A‡ | 7.7 | 0.02 | 2700 |
| LL2-Onc | 6.0 | 3.0 | 9.2 | 0.009 | 956 (0.4)1-153 |
| RNase . | pH* . | Moles RNase per mole antibody† . | Km μM . | Kcat sec−1 . | Kcat/Km M−1 sec−1 . |
|---|---|---|---|---|---|
| Onc | 6.0 | N/A‡ | 7.7 | 0.02 | 2700 |
| LL2-Onc | 6.0 | 3.0 | 9.2 | 0.009 | 956 (0.4)1-153 |
The RNase activity was measured at the pH most optimal for onconase. The data were derived from the initial rates of reactions containing 3 nM onconase and varying (0.1 to 1 mg/mL) concentrations of a yeast tRNA substrate.
The number of moles RNase conjugated per mole of antibody was determined spectrally at 412 nm by following the appearance of thionitrobenzoate ion (TNB). TNB is released from the 2-IT- and DTNB-treated antibody as disulfide bonds between the RNase and antibody are formed (see “Materials and methods”).
N/A, not applicable.
The number in parentheses indicates the percentage activity compared with onconase.
The conjugate was tested for RNase activity at the optimal pH for onconase and compared with the enzymatic activity of unconjugated onconase (Table 1). Although the Km (affinity of conjugated onconase for the substrate) remained similar to that of native onconase, the catalytic efficiency (Kcat/Km) was found to decrease to 40% that of the native amphibian RNase.
To determine the effect of conjugation on binding of LL2 to the CD22 antigen on human Daudi cells, the displacement of [125I]-labeled LL2 by the antibody or antibody conjugate was compared. The concentrations necessary to displace the labeled antibody by 50% were indistinguishable for each molecule (Figure 2).
Binding of LL2-onconase conjugate to Daudi cells.
Competitive binding analysis of LL2 or LL2-onconase inhibition of binding of [125I]-labeled LL2 IgG2a antibody to human Daudi lymphoma cells expressing the CD22 antigen. Binding analyses were conducted as described in “Materials and methods.” Duplicates from at least 2 experiments were pooled and plotted ± SEM. LL2, solid circles; LL2-onconase (LL2-Onc), open circles.
Binding of LL2-onconase conjugate to Daudi cells.
Competitive binding analysis of LL2 or LL2-onconase inhibition of binding of [125I]-labeled LL2 IgG2a antibody to human Daudi lymphoma cells expressing the CD22 antigen. Binding analyses were conducted as described in “Materials and methods.” Duplicates from at least 2 experiments were pooled and plotted ± SEM. LL2, solid circles; LL2-onconase (LL2-Onc), open circles.
In vitro cytotoxicity of LL2-onconase: potency and specificity
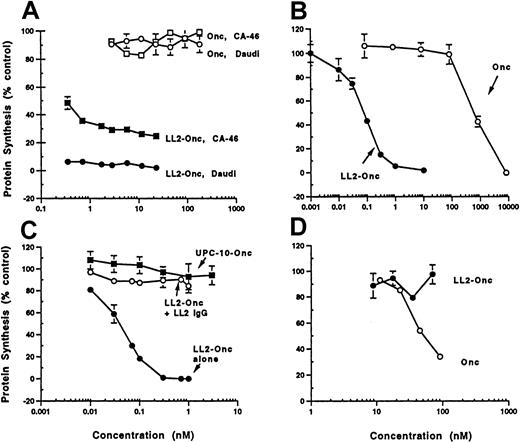
LL2-onconase was assayed for cytotoxicity by examining inhibition of protein synthesis on the human B-cell tumor cell lines Daudi and CA-46 (Figure 3). Both human tumor cell lines were sensitive to LL2-onconase (IC50s ranged from 10 to 100 pM in different experiments), resulting in an enhancement of onconase cytotoxicity by several orders of magnitude. Onconase cytotoxicity (IC50 700 nM) was increased 10 000-fold when coupled to LL2 (IC50 0.07 nM) (Figure 3B). The increase in cytotoxicity was due to specifically binding the CD22 antigen. Competition with excess LL2 antibody (300 nM) completely abrogated the cytotoxicity of the conjugate (Figure 3C). The possibility that enhanced cytotoxicity was due to nonspecific IgG-mediated internalization of onconase was eliminated because a nonrelevant antibody (UPC-10) of the same IgG2a isotype conjugated to onconase was not cytotoxic in Daudi cells (Figure 3C). Moreover, LL2-onconase did not inhibit protein synthesis in HUT-102 T cells that do not express the CD22 antigen, even though this cell line was more sensitive to unconjugated onconase than either Daudi or CA-46 cell lines (Figure3D). Whereas onconase (100 nM) decreased protein synthesis in HUT-102 T cells to 35% of mock-treated cells, it did not affect protein synthesis in either CA-46 or Daudi cells (Figures 3A,D).
Cytotoxicity and specificity of LL2-onconase.
(A) Cytotoxicity of conjugated (LL2-Onc) and unconjugated onconase (Onc) to Daudi (circle) or CA-46 (square) cells. Cells were treated with increasing concentrations of LL2-onconase (solid symbols) or onconase (open symbols). (B) LL2 increases the cytotoxicity of onconase. Daudi cells were treated with increasing concentrations of onconase (open circles) or LL2-onconase (solid circles). (C) LL2-onconase cytotoxicity on Daudi lymphoma cells is specific to the CD22 antigen. LL2-onconase cytotoxicy (solid circles) against Daudi cells is inhibited by excess (300 nM) LL2 (open circles); and a nonrelevant IgG2a (UPC-10) antibody-onconase conjugate is not cytotoxic to Daudi cells (solid squares). (D) Cytotoxicity of onconase (open circles) and LL2-onconase (solid circles) toward CD22-negative HUT102 cells. All cells were treated with the test substances for 3 days. Protein synthesis was measured as described in “Materials and methods.” Data are from at least 2 replicate experiments. The SEM of triplicate determinations are shown when they are greater than the symbol.
Cytotoxicity and specificity of LL2-onconase.
(A) Cytotoxicity of conjugated (LL2-Onc) and unconjugated onconase (Onc) to Daudi (circle) or CA-46 (square) cells. Cells were treated with increasing concentrations of LL2-onconase (solid symbols) or onconase (open symbols). (B) LL2 increases the cytotoxicity of onconase. Daudi cells were treated with increasing concentrations of onconase (open circles) or LL2-onconase (solid circles). (C) LL2-onconase cytotoxicity on Daudi lymphoma cells is specific to the CD22 antigen. LL2-onconase cytotoxicy (solid circles) against Daudi cells is inhibited by excess (300 nM) LL2 (open circles); and a nonrelevant IgG2a (UPC-10) antibody-onconase conjugate is not cytotoxic to Daudi cells (solid squares). (D) Cytotoxicity of onconase (open circles) and LL2-onconase (solid circles) toward CD22-negative HUT102 cells. All cells were treated with the test substances for 3 days. Protein synthesis was measured as described in “Materials and methods.” Data are from at least 2 replicate experiments. The SEM of triplicate determinations are shown when they are greater than the symbol.
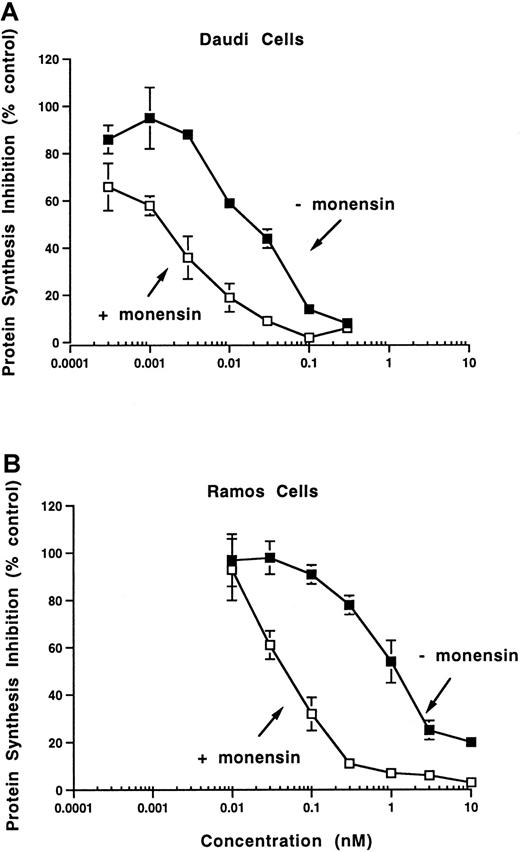
The in vitro potency as reflected by inhibition of protein synthesis was enhanced by monensin, a carboxylic ionophore previously shown to enhance the activity of anti-CD22 immunotoxins (reviewed in Casellas and Jansen39). Monensin enhanced cytotoxicity 13-fold on Daudi cells (IC50s 20 and 1.5 pM in the absence and presence of monensin) and 20-fold on Ramos cells (IC50s 1000 and 50 pM in the absence and presence of monensin, Figure 4). Unconjugated onconase cytotoxicity on Daudi cells is enhanced 2-fold in the presence of monensin, indicating that LL2-onconase and onconase internalize through similar compartments.
Monensin potentiates the cytotoxic activity of LL2-onconase.
Daudi (A) or Ramos (B) cells were treated with increasing concentrations of LL2-onconase in the absence (solid squares) or presence (open squares) of monensin for 3 days before protein synthesis was measured as described in “Materials and methods.” Monensin concentrations were 1 × 10−7 M and 1 × 10−8 M on Daudi and Ramos cells, respectively. Figure is representative of 2 experiments. The SEM of triplicate determinations are shown when they are greater than the symbol.
Monensin potentiates the cytotoxic activity of LL2-onconase.
Daudi (A) or Ramos (B) cells were treated with increasing concentrations of LL2-onconase in the absence (solid squares) or presence (open squares) of monensin for 3 days before protein synthesis was measured as described in “Materials and methods.” Monensin concentrations were 1 × 10−7 M and 1 × 10−8 M on Daudi and Ramos cells, respectively. Figure is representative of 2 experiments. The SEM of triplicate determinations are shown when they are greater than the symbol.
Although human Daudi cells were the most sensitive cell line to LL2-onconase, the conjugate was cytotoxic to other B-cell lines that expressed the CD22 antigen (Table 2). The IC50s of LL2-onconase required for protein synthesis inhibition in human Raji, Ramos, and RL lymphoma cells were 200, 1000, and 1200 pM, respectively. Unconjugated LL2 was not cytotoxic to any of the cell lines (IC50 > 20 000 pM).
Cytotoxicity of onconase or LL2-onconase on human cell lines
| Cell line . | LL2-onconase . | Onconase (−)monensin . | |
|---|---|---|---|
| (−)monensin . | (+)monensin . | ||
| IC50(pM) . | IC50 (pM) . | ||
| Daudi | 20 | 1.5 | 400 000 |
| Ramos | 1000 | 50 | >200 000 |
| Raji | 200 | ND* | >200 000 |
| RL | 1200 | ND | ND |
| HUT-102 | >100 000 | ND | 50 000 |
| Cell line . | LL2-onconase . | Onconase (−)monensin . | |
|---|---|---|---|
| (−)monensin . | (+)monensin . | ||
| IC50(pM) . | IC50 (pM) . | ||
| Daudi | 20 | 1.5 | 400 000 |
| Ramos | 1000 | 50 | >200 000 |
| Raji | 200 | ND* | >200 000 |
| RL | 1200 | ND | ND |
| HUT-102 | >100 000 | ND | 50 000 |
The concentration necessary to inhibit protein synthesis by 50% (IC50) is shown. LL2 antibody did not inhibit protein synthesis at the highest concentration tested (20 000 pM).
ND, not determined.
In vitro cytotoxicity of LL2-onconase: duration of exposure
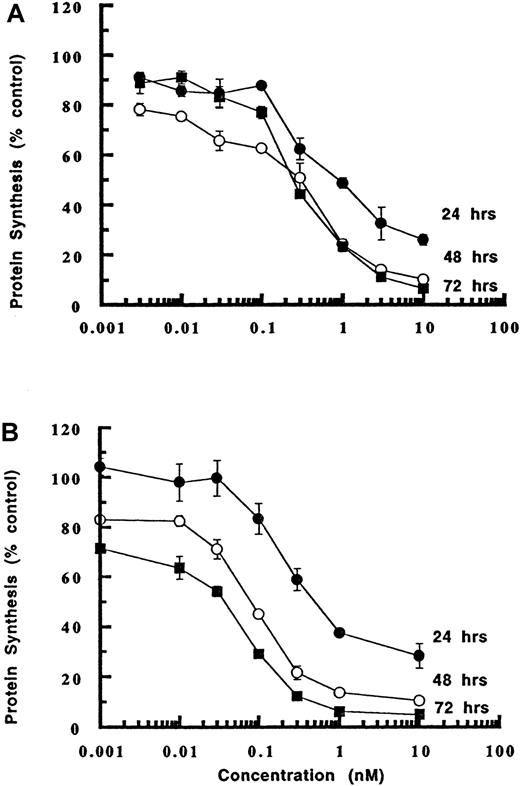
LL2 is known to be rapidly internalized4 and our experiments have shown that more than 95% of the antibody is specifically bound to the cell surface by 2 hours (data not shown). To determine whether a short-term exposure to the conjugate would be sufficient to cause cytotoxicity, inhibition of protein synthesis was examined in an experiment that compared the effects of LL2-onconase on Daudi cells after a 1-hour treatment compared with continuous exposure of Daudi cells to the conjugate (Figure5). Even a short 1-hour exposure to LL2-onconase, followed by washing and incubation in medium lacking the conjugate was sufficient to cause potent cytotoxicity, albeit less than that elicited by constant exposure. The IC50s were 1.8, 3.3, and 6.3 times less cytotoxic after a 1-hour exposure (IC50s 900, 300, 250 pM) compared with continual exposure (IC50s 500, 90, 40 pM) when measured at the same end points of 1, 2, and 3 days, respectively. Importantly, after a 1-hour exposure to cells, 10 nM of LL2-onconase was sufficient to inhibit protein synthesis by about 90% after 2 days (Figure 5). This implies that even a short exposure to LL2-onconase could result in tumor cell death in vivo.
Cytotoxicity of LL2-onconase to cells increases with time.
Daudi cells were treated for 1 hour (A) or continuously (B) with varying concentrations of LL2-onconase. Protein synthesis was measured 24, 48, or 72 hours later as described in “Materials and methods.” One of 2 representative experiments performed in triplicate is shown ± SEM.
Cytotoxicity of LL2-onconase to cells increases with time.
Daudi cells were treated for 1 hour (A) or continuously (B) with varying concentrations of LL2-onconase. Protein synthesis was measured 24, 48, or 72 hours later as described in “Materials and methods.” One of 2 representative experiments performed in triplicate is shown ± SEM.
Stability of LL2-onconase in plasma
For the disulfide-linked LL2-onconase to be effective as an antitumor agent in vivo, the disulfide bond must be stable to reduction in the bloodstream. To examine the stability of LL2-onconase, the disulfide-linked conjugate was incubated in human plasma at 37°C for 3 days. Cytotoxic activity of the treated conjugate was then compared with conjugate that was diluted in plasma and added to cells immediately with no incubation at 37°C. No change in activity compared with the unincubated controls was observed indicating that LL2-onconase is stable in human plasma at 37°C and that breakdown of the disulfide-linked conjugate is not rapid (unincubated LL2-onconase, IC50, 60 ± 2 pM; LL2-onconase after 3 days incubation in human plasma, IC50, 70 ± 3 pM).
In vivo effects of LL2-onconase: nonspecific toxicity in mice
Administered as a bolus, 1 mg of onconase injected ip caused severe weight loss and death to BALB/c (2 of 2) or SCID mice (2 of 2) (Table 3). Multiple injections of onconase were tolerated less well, especially when administered iv. The LD50 for iv injection of onconase was between 50 and 100 μg/d for 5 days or a total cumulative dose (TCD) of 19 mg/kg compared with ip administration of 100 μg/d (administered as 2 doses of 50 μg each, TCD of 25 mg/kg) (Table 3). Yet doses of 300 μg/d (TCD, 75 mg/kg) or 600 μg/d (TCD, 150 mg/kg) LL2-onconase caused no toxic symptoms when administered daily for 5 days. A dose of 1.2 mg LL2-onconase per day for 5 days (TCD, 300 mg/kg) caused mild (less than 10%) body weight loss that rapidly reversed on cessation of treatment. Because there are approximately 3 mol onconase per mole LL2, it represents 20% or 240 μg onconase per day. Thus, when conjugated to LL2, onconase is about 2-fold less toxic to mice. Increasing the dose of LL2-onconase to 1.6 mg/d was fatal to 3 of 3 mice. One mouse died after 4 injections, the other 2 after 8 injections. Thus, the LD50 lies between 1.2 and 1.6 mg of LL2-onconase administered intraperitoneally per day.
Toxicity in mice of onconase and LL2-onconase
| Drug . | Schedule . | Survivors/total . |
|---|---|---|
| Onconase | 1 mg ip × 1 | 0/4 |
| 25 μg iv QD × 5 | 2/2 | |
| 50 μg iv QD × 5 | 2/2 | |
| 100 μg iv QD × 5 | 0/2 | |
| 200 μg iv QD × 5 | 0/2 | |
| 2 × 50 μg ip QD × 5 | 1/2 | |
| 2 × 75 μg ip QD × 5 | 0/4 | |
| LL2-onconase | 300 μg iv QD × 5 | 2/2 |
| 2 × 300 μg ip QD × 5 | 2/2 | |
| 4 × 300 μg ip QD × 5 | 2/2 | |
| 4 × 400 μg ip QD × 5 | 0/3 |
| Drug . | Schedule . | Survivors/total . |
|---|---|---|
| Onconase | 1 mg ip × 1 | 0/4 |
| 25 μg iv QD × 5 | 2/2 | |
| 50 μg iv QD × 5 | 2/2 | |
| 100 μg iv QD × 5 | 0/2 | |
| 200 μg iv QD × 5 | 0/2 | |
| 2 × 50 μg ip QD × 5 | 1/2 | |
| 2 × 75 μg ip QD × 5 | 0/4 | |
| LL2-onconase | 300 μg iv QD × 5 | 2/2 |
| 2 × 300 μg ip QD × 5 | 2/2 | |
| 4 × 300 μg ip QD × 5 | 2/2 | |
| 4 × 400 μg ip QD × 5 | 0/3 |
Proteins diluted in phosphate-buffered saline (PBS) were administered once, twice, or 4 times daily (QD) for 5 consecutive days. Mice receiving more than one injection daily were compared with mice that received injections of PBS only. Mice were observed for signs of toxicity and weighed daily for several weeks after the last administration of drug.
ip indicates intraperitoneally; iv, intravenously.
To assess the dose-limiting toxicity of LL2-onconase, 3 mice were treated with 2 mg conjugate per day, and a complete necropsy was performed. Histopathologic examination revealed moderate-to-marked liver necrosis and moderate-to-marked depression of red blood cell precursors in the bone marrow and splenic red pulp. Kidney damage, which is dose-limiting for onconase in mice40 and clinical trails,28 was minimal and confined primarily to the proximal tubules.
In vivo effects of LL2-onconase: treatment of accessible minimal disseminated Daudi lymphoma in SCID mice
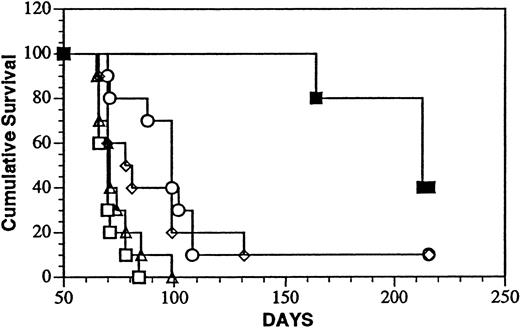
The in vivo antitumor activity of LL2-onconase was first studied in a system in which the tumor cell burden was minimal as well as accessible to the conjugate. After ip inoculation, Daudi cells localize and progressively grow in the abdominal cavity and viscera of SCID mice eventually leading to death of the animal.35 Survival after treatment with the onconase conjugate ip 1 day after tumor cell injection is shown in Figure 6. The dose and schedule selected for treatment experiments (5 mg/kg, administered daily for 5 days) corresponded to approximately 8% of the highest tolerated dose of LL2-onconase determined in the toxicity studies. The median survival time (MST, 216 days) of the conjugate-treated mice surpassed that of every other group that included mice injected with PBS (MST, 71 days; P < .001); unconjugated LL2 (MST, 99 days; P < .02); unconjugated onconase (MST, 70 days;P < .001) or a mixture of unconjugated LL2 and onconase (MST, 80 days; P < .02). Thus, the covalently linked conjugate significantly increased the life span of Daudi-bearing mice over that of vehicle-injected mice by 200%. Forty percent (4 of 10) mice were free of gross tumor on autopsy in the conjugate-treated group compared with 10% (1 of 10) in the LL2- and LL2-plus-onconase–treated groups and no long-term survivors in the PBS and onconase-treated groups. LL2 also significantly increased the life span of tumor-bearing mice (39%) over that of the PBS-treated mice (P < .002).
Treatment of minimal Daudi lymphoma disease with LL2-onconase or component proteins.
Daudi lymphoma cells (5 × 106 cells) were injected ip, followed 1 day later for an additional 4 consecutive days with ip injections of PBS (open triangles), 80 μg LL2 (open circles), 20 μg onconase (open squares), 80 μg LL2 + 20 μg onconase (open diamonds), or 100 μg LL2-onconase (solid squares) as described in “Materials and methods.” The mice treated with LL2-onconase survived significantly longer than PBS (P < .001); LL2 (P < .02); onconase (P < .001); LL2 plus onconase (P < .02).
Treatment of minimal Daudi lymphoma disease with LL2-onconase or component proteins.
Daudi lymphoma cells (5 × 106 cells) were injected ip, followed 1 day later for an additional 4 consecutive days with ip injections of PBS (open triangles), 80 μg LL2 (open circles), 20 μg onconase (open squares), 80 μg LL2 + 20 μg onconase (open diamonds), or 100 μg LL2-onconase (solid squares) as described in “Materials and methods.” The mice treated with LL2-onconase survived significantly longer than PBS (P < .001); LL2 (P < .02); onconase (P < .001); LL2 plus onconase (P < .02).
In vivo effects of LL2-onconase: treatment of minimal and advanced systemic Daudi lymphoma in SCID mice
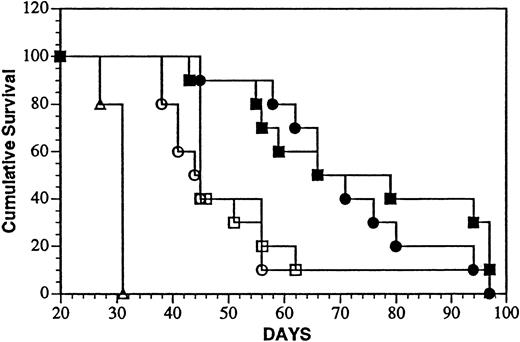
Intravenous injection of Daudi cells results in widely disseminated neoplasia with a more rapid onset of death compared with mice inoculated in the peritoneal cavity with the same number of tumor cells.35 Compression of the spinal cord causing hind limb paresis was previously shown to be predictive of survival time and was used as an end point (MPT). Survival of mice after treatment with the onconase conjugate iv 1 or 7 days, after tumor cell injection is shown in Figure 7. The MPT (69 and 73 days) of mice treated 1 or 7 days after tumor cell injection surpassed that of every other group that included mice injected with PBS (MPT, 31 days;P < .0001) or a mixture of unconjugated LL2 and onconase after 1 (MPT, 44 days; P < .0002) or 7 (MPT, 45 days;P < .0002) days. Again, LL2-onconase significantly increased the life span of Daudi-bearing mice over that of vehicle-injected mice by 123% and 135% when injected 1 or 7 days after tumor cell injection, respectively. Under these conditions, only 10% (1 of 10) of the mice were free of gross tumor on autopsy in 3 of 4 groups that contained LL2, 2 in the mixture and one in the conjugate-treated group in which treatment was delayed for 1 week after the administration of Daudi cells.
Treatment of aggressive minimal and advanced Daudi lymphoma disease with LL2-onconase or component proteins.
Daudi lymphoma cells (5 × 106 cells) were injected iv, followed 1 or 7 days later with daily iv injections for 5 consecutive days of PBS (open triangles), 80 μg LL2 + 20 μg onconase (open circles and squares, treatment days 1 to 5 and 7 to 11, respectively), or 100 μg LL2-onconase (solid circles and squares, treatment days 1 to 5 and 7 to 11, respectively) as described in “Materials and methods.” Both groups of mice treated with LL2-onconase survived significantly longer than PBS (P < .0001) or a mixture of LL2 plus onconase (P < .0002) treated mice.
Treatment of aggressive minimal and advanced Daudi lymphoma disease with LL2-onconase or component proteins.
Daudi lymphoma cells (5 × 106 cells) were injected iv, followed 1 or 7 days later with daily iv injections for 5 consecutive days of PBS (open triangles), 80 μg LL2 + 20 μg onconase (open circles and squares, treatment days 1 to 5 and 7 to 11, respectively), or 100 μg LL2-onconase (solid circles and squares, treatment days 1 to 5 and 7 to 11, respectively) as described in “Materials and methods.” Both groups of mice treated with LL2-onconase survived significantly longer than PBS (P < .0001) or a mixture of LL2 plus onconase (P < .0002) treated mice.
In another experiment mice were inoculated intravenously with Daudi cells and treatment was begun 3 days after tumor cell injection. A significant increase in life span (154% P < .005) was observed in the conjugate-treated group as well as the group containing an equimolar noncovalent mixture of the antibody and RNase (46%P < .05; data not shown). At the termination of the experiment, 40% of the animals in the conjugate-treated group were free of gross tumor on autopsy. Five days before the achievement of the MPT in the PBS-treated group (32 days after tumor cell injection), kidneys were removed from 2 mice in each group for histologic examination. No significant tumor lesions were observed in the conjugate-treated group compared with moderate (PBS-treated) and mild (LL2 plus onconase-treated) tumor foci found in the other groups.
Discussion
Covalently, linking onconase to LL2 increases its cytotoxicity thousands of times (onconase vs LL2-onconase, IC50s 0.7 and 0.00007 μM, respectively) even though the enzymatic activity of onconase in the conjugate was decreased to 40% that of the native protein. Multiple studies have shown that ribonuclease activity of onconase is essential for the manifestation of cytotoxicity when added to the extracellular media of cultured cells41,42 or directly microinjected into cells.19 Therefore, methods of linking onconase to LL2 that do not affect enzymatic activity might further enhance the potency of this conjugate. Certainly, results presented here demonstrate that increased cytotoxicity of LL2-onconase is feasible. In the presence of monensin, the potency of LL2-onconase on Daudi and Ramos cells was increased 13- and 20-fold, respectively. Monensin facilitates transport of RNases into the cytosol through a disrupted Golgi apparatus.43 Apparently, onconase can also enter the cytosol through a disrupted Golgi when internalized by LL2 suggesting that the pathways of onconase and LL2-onconase converge at some point after internalization. All together, the in vitro potency and specificity of LL2-onconase on Daudi cells (IC50s 10 to 100 pM) is comparable to anti-CD22 immunotoxin conjugates constructed with plant, eg, anti-CD22 ricin A-chain (IC50s 1 to 30 pM)11,44 and bacterial toxins, eg, anti-CD22Pseudomonas exotoxin derivatives (IC50s 1 to 40 pM).16 45
Conjugation to LL2 decreased systemic toxicity of onconase to mice in addition to enhancing the potency and specificity. Under current conjugation conditions aliquots of the highest achievable concentration of the conjugate (1.5 to 2.0 mg/mL) caused no signs of toxicity when injected once or twice a day for 5 days. Increasing the concentration by injecting drugs at 2-hour intervals, 4 times a day for 5 days (1.2 mg/d; total dose 300 mg/kg) did demonstrate toxicity as assessed by significant weight loss but the animals recovered when treatment was stopped. A further increase in dose to 1.6 mg/d was lethal. Toxicity studies in which other iterations of anti–CD22-cytotoxic RNase conjugates were evaluated concur with these results and demonstrate that mice can tolerate from 300 to 500 mg/kg without serious toxicities (D.L.N., unpublished observations). These doses compare favorably to the toxic doses of other anti-CD22 immunotoxins administered on a daily basis. Multiple dose LD50s of anti-CD22 Pseudomonas exotoxin conjugates ranged from 1 to 5 mg/kg.45 The 50% lethal dose of anti-CD22 ricin A-chain administered in a bolus was about 14 mg/kg.11 Moreover, the conjugate was less toxic than an equimolar dose of onconase. Mice that received just one half the molar equivalent dose of onconase in the conjugate survived only 12 injections and were found dead on the fourth day. These results are encouraging because the systemic toxicity of some toxic plant proteins is actually increased by conjugation to antibodies because of the increased time in the circulation.46,47 In mice, onconase is taken up and retained by kidneys more extensively than other monomeric pancreatic-type RNases because of binding sites in the molecule.28,48 Histopathologic examination of the kidneys show that onconase damages the proximal tubules of the kidney.40 Furthermore, renal toxicity is dose limiting in clinical trials.28 Slower clearance with less accumulation in the kidneys may explain the decreased toxicity of the conjugate in mice to the kidneys over that of the protein itself. In that regard, toxicity of the LL2-onconase conjugate was minimal to the kidneys but moderately to markedly severe to the liver. More valid toxicity assessments of LL2-onconase remain to be determined in a species in which the antibody recognizes a CD22 determinant.
Treatment schedules in immunodeficient mice were designed to assess tumor prevention as well as treatment of minimal and more advanced Daudi lymphoma. The results of the 3 experiments presented in this paper are representative of a total of 6 therapy experiments with LL2-onconase. In all the experiments, the conjugate was always effective in significantly increasing the survival of Daudi tumor–bearing mice. Additionally, the conjugate was always more effective than the individual agents that comprised it; LL2, onconase, or a mixture of the 2 agents. Onconase alone was not more effective than vehicle-treated mice, but LL2 alone or mixed with onconase did significantly prolong survival. Results showing an effect of the antibody are consistent with responses observed in patients to whom LL2 has been administered.29
LL2-onconase compares very favorably with reports using conventional immunotoxins. Treatment using the same model of minimal disseminated Daudi lymphoma with RFB4-dgA at 40% the LD50 achieved 40%11 and 0%49 cures compared with the 40% cures reported here. Other models in which disseminated Ramos lymphoma was treated report cure rates of 60% and 100% for anti-CD22 saporin50 and anti-CD22–RA,51 respectively. Studies are now underway varying the dosage, the route of administration, and the treatment schedules to further improve the efficacy of the anti-CD22–RNase conjugate.
In conclusion, an anti-CD22–cytotoxic RNase conjugate is potent and specific in cell culture and in SCID mice against disseminated Daudi B-cell lymphoma. Although the potency and specificity is comparable to anti-CD22 immunotoxins made with plant and bacterial toxins, it appears to cause less nonspecific side effects in mice because a dose of at least 300 mg/kg can be achieved. Significant increases in survival were obtained with a dose of 25 mg/kg (8% of highest dose tolerated). LL2-onconase is the most effective targeted RNase-based agent described to date and should be considered for further preclinical development as a potential therapeutic for non-Hodgkin lymphoma.
Acknowledgments
The technical support of Dale Ruby is gratefully acknowledged. We thank Dr William Murphy for helpful advice on SCID mouse tumor models. We very much appreciate our excellent administrative help and thank Ms Beverly A. Bales, Robin L. Reese, and Jamie M. Tammariello. We are most grateful for the interest and support of Dr Edward A. Sausville.
Supported in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health (NIH), under contract no. NO1-CO-56000.
D.M.G. is supported by an Outstanding Investigator Grant (CA39841) from the National Cancer Institute, NIH.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Susanna M. Rybak, NCI-FCRDC, Bldg 567, Rm 162, Frederick, MD 21702-1201; e-mail: rybak@ncifcrf.gov.

![Fig. 2. Binding of LL2-onconase conjugate to Daudi cells. / Competitive binding analysis of LL2 or LL2-onconase inhibition of binding of [125I]-labeled LL2 IgG2a antibody to human Daudi lymphoma cells expressing the CD22 antigen. Binding analyses were conducted as described in “Materials and methods.” Duplicates from at least 2 experiments were pooled and plotted ± SEM. LL2, solid circles; LL2-onconase (LL2-Onc), open circles.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/2/10.1182_blood.v97.2.528/5/m_h80210610002.jpeg?Expires=1767710972&Signature=gRN5u5bJjtLELLbI96O0h43nmO8p7tWlFz09BtYE8o-lgaP9TPaHkH3t4cGr~IwEH3k1hbzNKpPyyMXGWOT2SHqzQZHrvXG7HzJNZ6Dtel~VGJd4yvKv5NWp5R~1L6KdblMJ5OAC-52j2fRFkjhrUafJ~s~W81XPfYPmS48UNxszTWLubCGpGoKU5ibm6Xc2XXSqYZ-biTq6F2MhW2CDIwSfmW8qr8B~Fvu7vcJc3Oi1-mwJks-TDFR85cllJ2rp-wIsALLNQclG6PaQ25F7Zas3i5GmSFNfQiH0fmvnTohzHQwDL6x8hNnuXEu7QOEfCJgi~qVndSj0JWUmxnYIDA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal