Abstract
Activation of the collagen receptor glycoprotein VI (GPVI) by a collagen-related peptide (CRP) induces stimulation of platelets and megakaryocytes through the phosphatidylinositol (PI) 3-kinase–dependent pathway leading to activation of Bruton tyrosine kinase (Btk) and phospholipase Cγ2 (PLCγ2). Here, we present evidence that both proteins undergo PI 3-kinase–dependent translocation to the plasma membrane on CRP stimulation that is markedly inhibited by wortmannin and LY294002. Translocation of PLCγ2 but not Btk is also seen in megakaryocytes from X-linked immunodeficiency mice, which have a mutation that reduces the affinity of the pleckstrin homology (PH) domain of Btk for PI 3,4,5-trisphosphate (PI 3,4,5-P3). Activation of PC12 cells by epidermal growth factor (EGF) results in increased PI 3-kinase activity and high PI 3,4,5-P3 levels that trigger translocation of the green fluorescent protein (GFP)–labeled PH of Btk, but not the GFP-labeled PH and tandem Src homology 2 (SH2) domains of PLCγ2. In contrast to the results with CRP, the G protein–coupled receptor agonist thrombin stimulates PI 3-kinase–independent translocation of Btk but not PLCγ2. In conclusion, these results demonstrate that in mouse megakaryocytes, CRP leads to PI 3-kinase–dependent translocation of PLCγ2 and Btk that are independent of one another, whereas thrombin only induces translocation of Btk through a pathway that is independent of PI 3-kinase activity.
Introduction
Adhesion of platelets to the subendothelium at sites of vascular damage leads to their activation and subsequent recruitment of other platelets to form a hemostatic plug. Subendothelial collagen fibers play a critical role in this process, supporting adhesion and activation. Although platelets express several receptors for collagen, the integrin α2β1 is thought to have a major role in supporting adhesion, whereas the recently cloned glycoprotein VI (GPVI) has been shown to support activation.1 2
GPVI is associated with the Fc receptor γ-chain in the membrane and induces activation via a tyrosine kinase–dependent pathway that is similar to that used by immune receptors.3Cross-linking of GPVI by collagen-related protein (CRP) leads to tyrosine phosphorylation of the immunoreceptor tyrosine-based activation motif (ITAM) on the Fc receptor γ-chain via a Src family kinase,4-6 which binds the tyrosine kinase Syk via its tandem Src homology 2 (SH2) domains, causing activation.7 Syk triggers a series of downstream events that culminate in the activation of phospholipase Cγ2 (PLCγ2)–producing second messengers inositol 1,4,5-trisphosphate and 1,2-diacylglycerol. A number of proteins play critical but undefined roles in this sequence of events, including the adapters LAT and SLP-76,8,9 Bruton tyrosine kinase (Btk) and the p85/110 kd heterodimeric form of phosphatidylinositol (PI) 3-kinase.8 10-12
The role of PI 3-kinase in the regulation of Btk and PLCγ2 by GPVI has received close attention. The PI 3-kinase inhibitors, wortmannin and LY294002, partially block CRP or collagen activation of Btk and PLCγ2 in human platelets.13,14 Additionally, the Btk pleckstrin homology (PH) domain, which has a high affinity for PI 3,4,5-trisphosphate (PI 3,4,5-P3), inhibits the Ca++response to CRP in mouse megakaryocytes, whereas a low PI 3,4,5-P3–affinity mutant form of this domain (R28C) has no significant effect.13 This suggests that PI 3,4,5-P3 is the active lipid that mediates the effect of the PI 3-kinase pathway. A similar conclusion has been made for the regulation of PLCγ2 by FCγRIIA in platelets.10
The PI 3-kinase pathway also has a role at the level of Btk, which has a PH domain that binds selectively to PI 3,4,5-P3. In B cells, Btk activation is downstream of Syk and PI 3-kinase,15 and there is evidence for a role for Btk in PLCγ2 activation via a B-cell linker protein (BLNK).16,17 In human platelets, GPVI activation also leads to PLCγ2 phosphorylation through a pathway that is partially dependent on Btk.11 However, the presence of BLNK or a homologue has not been described in platelets and the relative position of Btk in the regulation of PLCγ2 remains unclear.
In this study, we provide evidence for a major role of PI 3-kinase in supporting translocation of PLCγ2 and Btk in human platelets as well as in murine megakaryocytes in response to CRP and demonstrate that the movement of PLCγ2 is independent of Btk. In addition, we show that thrombin receptor activation does not induce PLCγ2 translocation but provokes the translocation of Btk through a pathway that is independent of PI 3-kinase.
Materials and methods
Materials
CRP (GCP*[GPP*]10GCP*G, P* = hydroxyproline; the peptide is cross-linked through the C-terminal cysteines) was a kind gift of Drs Barnes, Knight, and Farndale (Department of Biochemistry, Cambridge, United Kingdom).18 Bovine thrombin, apyrase, and poly-L-lysine (70 000 g/mol) and prostacyclin were from Sigma (Poole, Dorset, United Kingdom). Rabbit polyclonal anti-PLCγ2, Q20 was from Santa Cruz Biotechnology (Santa Cruz, CA) and rabbit anti-Btk was a generous gift from Dr M. G. Tomlinson (DNAX Research Institute, Palo Alto, CA). Oregon green-488 conjugate goat antirabbit IgG (H+L), Fura-2, and calcium calibration standards were from Molecular Probes (Eugene, OR). Other reagents were from previously described sources.13 Wortmannin and LY294002 were dissolved in DMSO. All other reagents were made up in saline. The concentration of DMSO in the incubation never exceeded 1:1000 final dilution, and an equivalent volume of DMSO was always present in control samples.
Preparation of mouse megakaryocytes
Mouse megakaryocytes were obtained as described.6The 50 μL of megakaryocyte suspension was plated onto poly-L-lysine–coated coverslips and allowed to sediment for 3 minutes. Excess of suspension was removed and replaced by Hank's buffer, containing 143 mM NaCl, 5.6 mM KCl, 0.2 mM CaCl2, 2 mM MgCl2, 10 mM HEPES, 10 mM glucose, and 0.4% bovine serum albumin (BSA), pH 7.2. Experiments were carried out at room temperature, with an external concentration of Ca++ of 200 μM.
Preparation of human platelets
Human blood was taken from drug-free volunteers on the day of the experiment by using acidic citrate dextrose (ACD; 120 mM sodium citrate, 110 mM glucose, and 80 mM citric acid) as anticoagulant. Platelet-rich plasma was obtained as previously described.13 Washed platelets were resuspended at a concentration of 5 × 108 cells/mL in Tyrode-HEPES buffer containing EGTA (1 mM) and indomethacin (10 μM).
Immunostaining of megakaryocytes and platelets
Cells were activated by CRP (4 μg/mL) or thrombin (1 U/mL) for the indicated time before fixation by paraformaldehyde 3.2% (wt/vol) in phosphate-buffered saline (PBS) for 10 minutes. Cells were permeabilized by Triton X-100 (0.02%) in PBS for 10 minutes and nonspecific sites were saturated with BSA (2%; wt/vol) for 30 minutes. Megakaryocytes were immunostained for PLCγ2 or Btk. Primary antibodies were detected by Oregon green-488 conjugate goat antirabbit IgG. Cells were viewed in an inverted microscope (Axiovert S 100, Carl Zeiss, Herts, United Kingdom) under 40 × or 100 × oil immersion lens and analyzed by deconvolution using Openlab software (Improvision, Warwick, United Kingdom).
Measurement of Ca++ in single megakaryocytes
Megakaryocytes prepared as described previously were viewed on an inverted microscope and stage III/IV megakaryocytes, identified on the basis of size and morphology, as previously described6were microinjected with Fura-2 and recombinant protein with an Eppendorf micromanipulator 5170 and microinjector 5242 (Eppendorf, Cambridge, United Kingdom). Fura-2 pentapotassium salt was dissolved in standard intracellular buffer19 and was present in the injection needle at a concentration of 2.5 mM, resulting in an estimated intracellular concentration of 200 μM or less. Single-cell digital imaging was carried out by using Openlab software. Fluorescence video images and calculation of intracellular Ca++ ([Ca++]i) were made as previously described.13
Cloning of pleckstrin homology and tandem Src homology 2 domains of PLCγ2
Two pairs of primers (sense 5′-ATGAAGAGAGCCCTGGACC-3′ and antisense 5′-CATCGCTTCCTGGTGTAAG-3′) and (sense 5′-GAATTCCTCGAGATGATTGCTGATGCCAAGCT-3′ and antisense 5′-GAATTCGGCGCGCTCCAGGAGCTCGGGGGTCT-3′ containing theEcoRI restriction site) were designed to amplify respectively the PH and tandem SH2 domains of PLCγ2 by polymerase chain reaction (PCR) (30 cycles with each cycle being 1 minute at 94°C, 1 minute at 50°C, and 2 minutes at 72°C). The PH domain of PLCγ2 was subcloned into the EcoRV site of the vector pMosBlue (Amersham Pharmacia Biotech, Little Chalfont Bucks, United Kingdom) and then digested with the restriction enzymeHindIII and KpnI and subcloned into the corresponding restriction sites of pEGFP-N3 (Clontech, Hampshire, United Kingdom). The PCR product of SH2 tandem of PLCγ2 was digested with the appropriated restriction enzyme and subcloned into the corresponding restriction sites of pEGFP-C1. The construct was fully sequenced before use. The green fluorescent protein (GFP)–fused PH domain of Btk construct was kindly given by Dr T. Balla (Endocrinology and Reproduction Research Branch, NICHD, Bethesda, MD).20
Cell cultures, transfections, and confocal microscopy
Translocation of GFP-labeled PH domains was monitored in PC-12 cells after stimulation with epidermal growth factor (EGF) as described.21 After transfection (18 to 24 hours), cells were serum starved for 2 hours before fluorescence analysis at 37°C in Krebs-Ringer phosphate buffer (136 mM NaCl, 4.7 mM KCl, 1.25 mM MgSO4, 1.25 mM CaCl2, 5 mM sodium phosphate) containing 2 mM NaHCO3 and 25 mM HEPES (pH 7.4). Fluorescence imaging was performed with a Leica DMIRBE inverted confocal microscope controlled with TCS-NT4 software (Leica, Bucks Milton Keynes, United Kingdom) as previously described.21
Analysis of data
Each experiment was performed on megakaryocytes from a minimum of 3 different mice. Data are presented as representative single cells or as mean ± SE. Results were analyzed by an analysis of variance (ANOVA) test. In each case, P < .05 was taken as the minimum value to indicate statistical significance.
Results
CRP induces PI 3-kinase–dependent translocation of PLCγ2 and Btk and PI 3-kinase–independent translocation Syk to the plasma membrane in human platelets
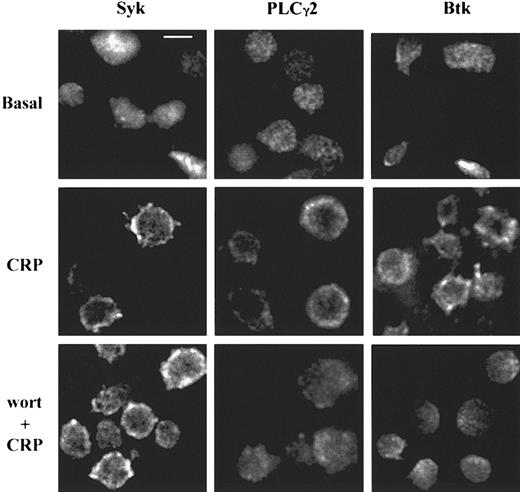
The location of Syk, Btk, and PLCγ2 was analyzed in human platelets before and after CRP stimulation with deconvolution microscopy. The proteins were found to be cytosolic in basal conditions and underwent translocation to the plasma membrane after 150 seconds of stimulation by CRP (Figure 1). To test the role of PI 3-kinase, platelets were pretreated by wortmannin for 5 minutes before stimulation by CRP. The inhibition of PI 3-kinase activity had no effect on the translocation of Syk, but both PLCγ2 and Btk translocation were found to be reduced, suggesting that the PI 3-kinase pathway is involved downstream of Syk but upstream of PLCγ2 and Btk.
CRP induces PI 3-kinase–dependent translocation of PLCγ2 and Btk or PI 3-kinase–independent translocation of Syk in human platelets.
Human platelets, stimulated by CRP (4 μg/mL) for 150 seconds, were fixed and stained for Syk, PLCγ2, or Btk as described in “Materials and methods” to study the protein localization. Platelets were pretreated with wortmannin (wort) 100 nM for 15 minutes before the stimulation by CRP to investigate the role of PI 3-kinase. Scale bar = 2 μm.
CRP induces PI 3-kinase–dependent translocation of PLCγ2 and Btk or PI 3-kinase–independent translocation of Syk in human platelets.
Human platelets, stimulated by CRP (4 μg/mL) for 150 seconds, were fixed and stained for Syk, PLCγ2, or Btk as described in “Materials and methods” to study the protein localization. Platelets were pretreated with wortmannin (wort) 100 nM for 15 minutes before the stimulation by CRP to investigate the role of PI 3-kinase. Scale bar = 2 μm.
CRP induces PI 3-kinase–dependent translocation of PLCγ2 and Btk to the plasma membrane in mouse megakaryocytes
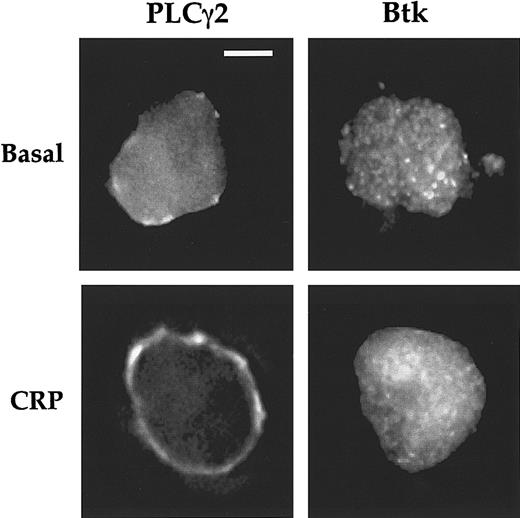
Translocation of PLCγ2 and Btk after activation of GPVI was analyzed in single murine megakaryocytes with deconvolution microscopy. As shown in Figure 2A, PLCγ2 and Btk were localized in the cytosol under basal conditions and underwent translocation to the plasma membrane after CRP stimulation (Figure 2A). The redistribution of the 2 proteins was measured by comparing the line intensity plots calculated for a cross section of the cell as shown in Figure 2B for PLCγ2. The distribution ratio of protein was calculated by dividing the fluorescence intensity at the plasma membrane (Ipm) by the intensity in the cytosol (Icyt). The distribution ratio of PLCγ2 was less than 1.0 in nonstimulated cells (Figure 2Bi), and this value increased to 2.3 after stimulation by CRP, corresponding to an increase in plasma membrane localization relative to the cytosol (Figure 2Bii). Translocation of PLCγ2 was detected at 60 seconds and peaked between 120 to 150 seconds before returning to the basal level by 600 seconds (Figure 2C). Translocation of Btk to the membrane occurred before that of PLCγ2, with significant translocation detected at 30 seconds (Figure 2C).
CRP induces PI 3-kinase–dependent translocation of PLCγ2 and Btk to plasma membrane.
(A) Micrographs show the localization of PLCγ2 and Btk in wild-type Balb-c mouse megakaryocytes. The megakaryocytes have been preincubated with wortmannin (wort) (100 nM) or LY294002 (50 μM) for 15 minutes and then activated with CRP (4 μg/mL) for 150 seconds. Cells were fixed and stained for PLCγ2 and Btk as described in “Materials and methods.” Distribution was analyzed by fluorescence microscopy and deconvolution for basal, CRP-activated megakaryocytes; wort-pretreated megakaryocytes; and LY294002 (LY)–pretreated megakaryocytes. Scale bar = 20 μm. (B) Distribution of fluorescence intensity in megakaryocytes along the lines shown in panel A was calculated by densitometry. The distributions in (i) and (ii) correspond to basal- and CRP-stimulated samples, respectively. (C) The time course of protein translocation was measured in megakaryocyte in response to CRP (4 μg/mL). The results are shown as distribution ratio (Ipm/Icyt) over time (described in “Results”). (D) Control and wort- or LY294002-pretreated megakaryocytes were stimulated by CRP for 150 seconds, and cells stained for PLCγ2 or Btk as described in “Materials and methods.” The amount of protein at the membrane was calculated as above. All results are expressed as a mean ± SE, and are from at least 8 megakaryocytes from 3 different experiments. * and ** correspond to results significantly different from basal or after stimulation with CRP for 2.5 minutes, respectively.
CRP induces PI 3-kinase–dependent translocation of PLCγ2 and Btk to plasma membrane.
(A) Micrographs show the localization of PLCγ2 and Btk in wild-type Balb-c mouse megakaryocytes. The megakaryocytes have been preincubated with wortmannin (wort) (100 nM) or LY294002 (50 μM) for 15 minutes and then activated with CRP (4 μg/mL) for 150 seconds. Cells were fixed and stained for PLCγ2 and Btk as described in “Materials and methods.” Distribution was analyzed by fluorescence microscopy and deconvolution for basal, CRP-activated megakaryocytes; wort-pretreated megakaryocytes; and LY294002 (LY)–pretreated megakaryocytes. Scale bar = 20 μm. (B) Distribution of fluorescence intensity in megakaryocytes along the lines shown in panel A was calculated by densitometry. The distributions in (i) and (ii) correspond to basal- and CRP-stimulated samples, respectively. (C) The time course of protein translocation was measured in megakaryocyte in response to CRP (4 μg/mL). The results are shown as distribution ratio (Ipm/Icyt) over time (described in “Results”). (D) Control and wort- or LY294002-pretreated megakaryocytes were stimulated by CRP for 150 seconds, and cells stained for PLCγ2 or Btk as described in “Materials and methods.” The amount of protein at the membrane was calculated as above. All results are expressed as a mean ± SE, and are from at least 8 megakaryocytes from 3 different experiments. * and ** correspond to results significantly different from basal or after stimulation with CRP for 2.5 minutes, respectively.
To investigate the role of the PI 3-kinase activity in the translocation of PLCγ2 and Btk, megakaryocytes were preincubated with the 2 structurally unrelated PI 3-kinase inhibitors, wortmannin or LY294002, for 15 minutes at concentrations that result in a complete inhibition of formation of PI 3,4,5-P3 in response to CRP in platelets.13 Both inhibitors completely blocked the translocation of Btk in response to CRP and reduced translocation of PLCγ2 by more than 90% (Figure 2A,D). This demonstrates that PI 3-kinase activity is involved in GPVI signaling by recruiting PLCγ2 and Btk to the plasma membrane.
The PH and tandem SH2 domains of PLCγ2 do not translocate on elevation of PI 3,4,5-P3
Membrane translocation of a number of PH domain-containing proteins such as Btk, Grp1, or centaurin α1, has been shown to be dependent on PI 3-kinase activity in NIH 3T3 and PC12 cells.19,21,22 To investigate the mechanism of translocation of PLCγ2, we transiently transfected PC12 cells with chimera proteins of GFP fused to the N-terminus PH domain and tandem SH2 domains of PLCγ2. The GFP-fused PH domain of Btk was used as a positive control in these studies. Using laser scanning confocal microscopy, we studied the resultant subcellular localization of GFP-PH and GFP–tandem SH2 domains of PLCγ2 before and during stimulation with EGF, an agonist that induces an increase in plasma membrane PI 3,4,5-P3 within these cells.23 In resting PC12 cells, GFP-PH domains of PLCγ2 and Btk were localized in the cytosol as well as in the nucleus, whereas the GFP–tandem SH2 domain was found primarily in the nucleus (Figure 3). Stimulation with EGF (100 ng/mL) resulted in an almost complete translocation of cytosolic GFP-PH Btk to the plasma membrane but had no effect on the localization of GFP-PH and GFP–tandem SH2 domains of PLCγ2 (Figure 3). These results demonstrate that, in contrast to Btk, neither the N-terminal PH or SH2 tandem domains of PLCγ2 are able to undergo translocation to the membrane on elevation of PI 3,4,5-P3. Similar results were also obtained with the PH domain of PLCγ1 (not shown).
The PH and tandem SH2 domains of PLCγ2 do not translocate on EGF stimulation of PC12 cells.
C12 cells were transfected with pEGFP-PH domain of Btk or pEGFP-PH or pEGFP–tandem SH2 domain of PLCγ2. After 24 hours, cells were starved of serum and imaged by laser-scanning confocal microscopy. Images are of PC12 cells in the absence or 1 minute after stimulation with EGF (100 ng/mL).
The PH and tandem SH2 domains of PLCγ2 do not translocate on EGF stimulation of PC12 cells.
C12 cells were transfected with pEGFP-PH domain of Btk or pEGFP-PH or pEGFP–tandem SH2 domain of PLCγ2. After 24 hours, cells were starved of serum and imaged by laser-scanning confocal microscopy. Images are of PC12 cells in the absence or 1 minute after stimulation with EGF (100 ng/mL).
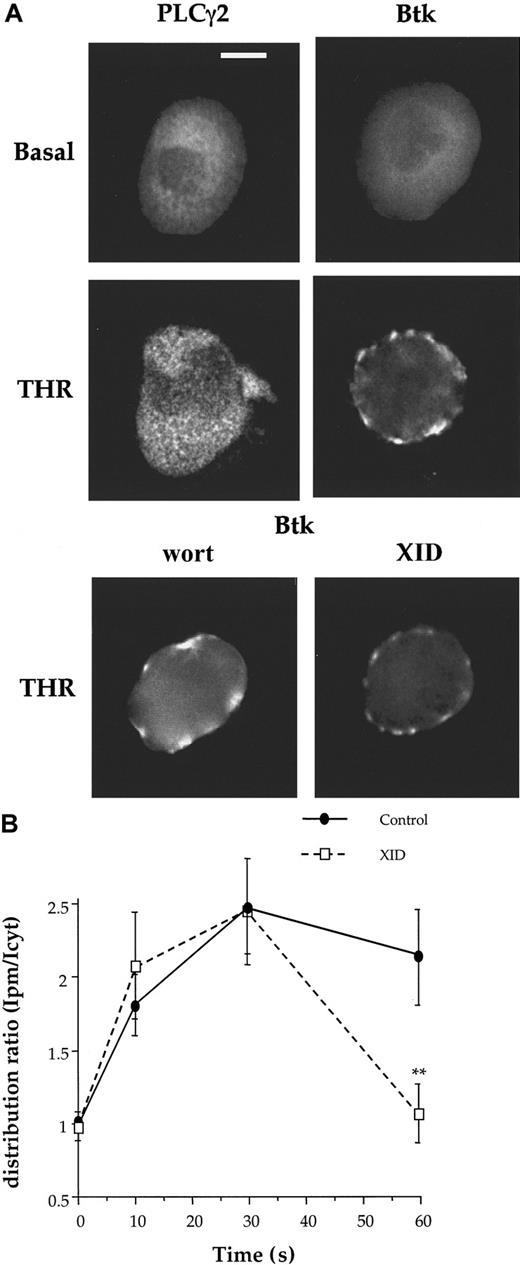
CRP induces translocation of PLCγ2 but not Btk to the plasma membrane in X-linked immunodeficiency mouse megakaryocytes
The fact that both Btk and PLCγ2 translocate through PI 3-kinase–dependent pathways, with a greater delay for PLCγ2 than for Btk, raises the question of their interdependence. To determine whether Btk is involved in the translocation of PLCγ2, we monitored translocation in megakaryocytes from X-linked immunodeficiency (XID) mice. XID mice express a mutant form of Btk in which arginine 28 within the PH domain is substituted by cysteine, resulting in a marked reduction in affinity for PI 3,4,5-P3.24 This mutation results in the loss of Btk translocation to the plasma membrane of the megakaryocyte after CRP activation (Figure4), demonstrating the importance of the interaction of PI 3,4,5-P3 with this domain. In contrast, translocation of PLCγ2 to the membrane in XID megakaryocytes is maintained, taking place over a similar time course and with a similar intensity to that seen in controls (Figure 4 and not shown). It was notable that a low level of PLCγ2 was present in the plasma membrane in the basal XID megakaryocytes, whereas it was absent in controls (Figure 4). This weak membrane localization is reduced when cells were preincubated with wortmannin (100 nM) over 30 minutes suggesting that it is dependent on PI 3-kinase activity (not shown). Wortmannin also inhibited the translocation of PLCγ2 to plasma membrane in XID megakaryocytes in response to CRP (not shown). These results demonstrate that PI 3-kinase activity is directly involved in the translocation of PLCγ2, and that this is not dependent on Btk translocation.
Localization and translocation of PLCγ2 and Btk in XID megakaryocytes.
XID mouse megakaryocytes were stimulated by CRP (4 μg/mL; 90 seconds) and stained for PLCγ2 and Btk. Cells were analyzed by fluorescence microscopy and deconvolution as described in Figure 1. Scale bar = 20 μm. Each image is representative of 7 megakaryocytes obtained from a minimum of 3 experiments on different animals.
Localization and translocation of PLCγ2 and Btk in XID megakaryocytes.
XID mouse megakaryocytes were stimulated by CRP (4 μg/mL; 90 seconds) and stained for PLCγ2 and Btk. Cells were analyzed by fluorescence microscopy and deconvolution as described in Figure 1. Scale bar = 20 μm. Each image is representative of 7 megakaryocytes obtained from a minimum of 3 experiments on different animals.
Wortmannin inhibits calcium elevation in response to CRP in control and XID mouse megakaryocytes
The elevation of [Ca++]i in response to CRP was investigated using single-cell video imaging. CRP stimulated a rapid increase in [Ca++]i before decaying to a plateau.13 Similar time course and magnitude of response was seen in XID megakaryocytes in response to CRP, with the basal level unchanged in both cell types (not shown). The latter observation suggests that the low level of PLCγ2 associated with the membrane in XID megakaryocytes is unlikely to be functional. The peak [Ca++]i response to CRP was 208 ± 23 nM (n = 13) and 220 ± 43 nM (n = 9) in control and XID megakaryocytes, respectively (Figure 5). These results demonstrate that translocation of Btk is not important for the initial peak of the Ca++ response to CRP. There was, however, a 20% reduction in the size of the plateau phase of the [Ca++]i response to CRP in XID megakaryocytes,25 which may reflect the involvement of Btk in the maintenance of the sustained Ca++ signal. This is similar to the pathway described in B cells after activation of the B-cell receptor.26
CRP elevates Ca++ in XID megakaryocytes.
Single megakaryocytes from control and XID mice were microinjected with Fura-2, pretreated or not for 15 minutes with wortmannin (wort) (100 nM), and stimulated by CRP (4 μg/mL). Intracellular Ca++was determined as described in “Materials and methods.” The increase in [Ca++]i in response to CRP is shown as histogram showing the mean ± SE. Results are from 8 to 10 cells from a minimum of 4 different mice. *corresponds to results significantly different from basal.
CRP elevates Ca++ in XID megakaryocytes.
Single megakaryocytes from control and XID mice were microinjected with Fura-2, pretreated or not for 15 minutes with wortmannin (wort) (100 nM), and stimulated by CRP (4 μg/mL). Intracellular Ca++was determined as described in “Materials and methods.” The increase in [Ca++]i in response to CRP is shown as histogram showing the mean ± SE. Results are from 8 to 10 cells from a minimum of 4 different mice. *corresponds to results significantly different from basal.
The inhibition of PI 3-kinase activity by wortmannin reduces the peak increase in Ca++ in response to CRP in control megakaryocytes to 60 ± 15 nM (n = 9), a lowering of approximately 70% as reported previously.13 XID megakaryocytes were slightly less sensitive to the PI 3-kinase inhibitor wortmannin, with the [Ca++]i response being reduced by 110 ± 23 nM (n = 5), a lowering of approximately 50% (Figure 5).
Thrombin induces Btk translocation in XID megakaryocytes
Thrombin was observed to stimulate translocation of Btk to the membrane of mouse megakaryocytes, but to have no significant effect on the distribution of PLCγ2 (Figure 6A). Btk was not evenly distributed on the membrane in thrombin-stimulated cells suggesting localization to specific regions in the membrane. Translocation of Btk in response to thrombin was maintained in the presence of wortmannin (100 nM) and in XID megakaryocytes when measured after 30 seconds (Figure 6A). This demonstrates that translocation is not dependent on the interaction of the PH domain of Btk with PI 3,4,5-P3. Btk was present at the plasma membrane for a shorter period in the XID megakaryocytes compared with controls, returning to the cytosol within 60 seconds of stimulation (Figure 6B). This suggests that thrombin targets Btk to the membrane through a novel pathway that is independent of PI 3,4,5-P3 but that the interaction between Btk and PI 3,4,5-P3 is required to keep the protein at the membrane. The peak elevation of [Ca++]i in response to thrombin was similar in control and XID megakaryocytes. It was also not altered in the presence of wortmannin (Table 1). These results are consistent with our previous report that the peak response to thrombin is not significantly altered after the microinjection of the PH domain of Btk, at a concentration that has been shown to block the response to CRP.13
Thrombin stimulates the translocation of Btk to the membrane.
(A) Mouse megakaryocytes were stimulated by thrombin (THR) and analyzed for expression of PLCγ2 and Btk as described in Figure 2. As labeled for each panel, Btk-stained megakaryocytes are unstimulated, THR-stimulated, wortmannin (wort)–treated, or XID megakaryocytes. Scale bar = 20 μm. Each image is representative of 8 megakaryocytes obtained from a minimum of 3 experiments on different animals. (B) The time course of Btk translocation was measured in control and XID megakaryocytes in response to THR (1 U/mL). The results are shown as distribution ratio (described in “Results” ).
Thrombin stimulates the translocation of Btk to the membrane.
(A) Mouse megakaryocytes were stimulated by thrombin (THR) and analyzed for expression of PLCγ2 and Btk as described in Figure 2. As labeled for each panel, Btk-stained megakaryocytes are unstimulated, THR-stimulated, wortmannin (wort)–treated, or XID megakaryocytes. Scale bar = 20 μm. Each image is representative of 8 megakaryocytes obtained from a minimum of 3 experiments on different animals. (B) The time course of Btk translocation was measured in control and XID megakaryocytes in response to THR (1 U/mL). The results are shown as distribution ratio (described in “Results” ).
Intracellular Ca++ increase after thrombin activation
| . | [Ca++]i response (in nM) . | ||
|---|---|---|---|
| Mean . | SEM . | n . | |
| Thrombin | 335 | 39 | 14 |
| Thrombin + wortmannin | 504 | 88 | 8 |
| Thrombin + Btk PH domain | 384 | 67 | 7 |
| XID + thrombin | 273 | 63 | 4 |
| . | [Ca++]i response (in nM) . | ||
|---|---|---|---|
| Mean . | SEM . | n . | |
| Thrombin | 335 | 39 | 14 |
| Thrombin + wortmannin | 504 | 88 | 8 |
| Thrombin + Btk PH domain | 384 | 67 | 7 |
| XID + thrombin | 273 | 63 | 4 |
Mouse megakaryocytes microinjected with Fura-2 were stimulated by thrombin under control conditions, after treatment for 15 minutes with wortmannin (100 nM), after microinjection of the wild-type PH domain of Btk, and in XID mice. [Ca++]i was measured using single-cell imaging. The increase of [Ca++]i is shown as mean ± SEM for the number of cells indicated. An ANOVA test of the calcium response under these conditions shows no significance.
[Ca++]I indicates intracellular Ca++; Btk, Bruton's tyrosine kinase; PH, pleckstrin homology.
Discussion
In this study, we show that PI 3-kinase activity is required for membrane targeting of PLCγ2 and Btk in platelets and megakaryocytes using the structurally distinct inhibitors, wortmannin and LY294002. In contrast, Syk translocation is not affected by wortmannin, indicating that PI 3-kinase activity is involved downstream of Syk. Translocation of PLCγ2 and Btk are distinct events because translocation of PLCγ2 in response to CRP is not altered in XID megakaryocytes that express a mutant form of Btk that does not move to the membrane. The inhibition of translocation of PLCγ2 by wortmannin and LY294002 is likely to account for the ability of these PI 3-kinase inhibitors to reduce the peak Ca++ response to CRP in megakaryocytes by approximately 70%, despite the absence of effect on the phosphorylation of PLCγ2.13 In contrast, the peak Ca++ response to CRP is unaltered in XID megakaryocytes, although there is a slight inhibition (20%) of the plateau phase suggesting a role of Btk in mediating sustained Ca++entry.25 A more significant role for Btk in the sustained phase of the [Ca++]i response has been proposed in human B cells and platelets on stimulation by the B-cell receptor and GPVI, respectively, based on studies on X-linked agammaglobulinemia (XLA) patients deficient in the kinase.11,27 It seems that Btk plays a greater role in the regulation of PLCγ2 in humans than in the mouse, in line with the increased severity of the immunodeficiency in humans. It is possible that other Tec family kinase proteins could be involved in this activation pathway14 and compensate for the lack of Btk to different degrees in mouse and human systems. Phosphorylation of Btk in response to CRP is reduced in the presence of wortmannin and LY294002. This is consistent with the proposal that phosphorylation of Btk is mediated by a Src family kinase after translocation to the plasma membrane.28
Studies were performed to identify the domain within PLCγ2, which supports PI 3-kinase–dependent translocation to the membrane. The GFP-labeled PH domain of PLCγ2 did not undergo translocation in PC12 cells after elevation of PI 3,4,5-P3. In contrast, the GFP-fused PH domain of Btk underwent translocation to the membrane in PC12 cells confirming previous reports.21 Similarly, the N-terminal PH domain of PLCγ1 is able to translocate to the membrane on PI 3-kinase activation.19 Bae et al29 have proposed that the tandem SH2 domain supports the interaction of PLCγ1 with PI 3,4,5-P3. However, there was no detectable translocation to the membrane of the GFP-labeled tandem SH2 domains of PLCγ2 on elevation of PI 3,4,5-P3 in PC12 cells. It is possible that the lack of translocation is due to the expressed domain being primarily compartmentalized in the nucleus. Translocation of PLCγ2 may therefore be mediated via another domain in the phospholipase such as the split PH domain, which flanks the tandem SH2 and SH3 domains or via an intermediate protein that has a PI 3,4,5-P3–binding domain. Alternatively, the interaction between the PH and/or tandem SH2 domains may not be strong enough to support translocation to the membrane, but may be sufficient to hold PLCγ2 at the membrane in the presence of other supporting interactions. For example, the adapter protein LAT, which is anchored in the plasma membrane, has been shown to associate with PLCγ2 probably via its C-terminal SH2 domain30 and to play a critical role in the regulation of the phospholipase.9
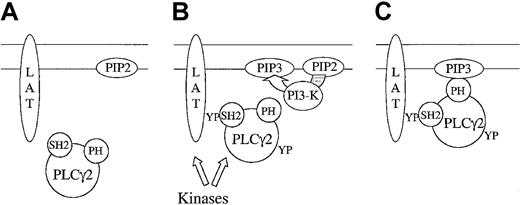
The observations that PI 3-kinase inhibitors block translocation of PLCγ2 to the membrane, but inhibit the elevation of Ca++by 70%, have minimal effect on tyrosine phosphorylation of the phospholipase,13 and have important implications for the regulation of the phospholipase. On the face of it, these results could indicate that phosphorylation of PLCγ2 takes place in the cytosol and that it is unlikely to play a role in the PI-3 kinase–independent increase in Ca++. This does not take into account, however, the presence of other domains within PLCγ2 that are able to target it to the plasma membrane such as the C-terminal SH2 domain of PLCγ2, which has been shown to bind to the membrane-localized adapter LAT.30 It is possible that the role of PI 3-kinase is to stabilize PLCγ2 at the membrane in combination with other interactions. In the presence of PI 3-kinase inhibitors, the association of PLCγ2 with the membrane would be transient, falling below the level of detection, although it may be long enough for phosphorylation to take place. A model illustrating this is shown in Figure 7.
Two-step translocation of PLCγ2.
Under basal conditions (A), PLCγ2 is cytosolic and not phosphorylated, the transmembrane adapter protein LAT is not phosphorylated, and the level of PI 3,4,5-P3 is minimal. After activation of GPVI (B), LAT becomes phosphorylated on tyrosine and associates with the C-terminal domain of PLCγ2. In addition, GPVI stimulates PI 3-kinase, leading to the formation of PI 3,4,5-P3 in the membrane. The localization of PLCγ2 in the membrane is stabilized by binding of PI 3,4,5-P3 to the PH domain of PLCγ2 (C).
Two-step translocation of PLCγ2.
Under basal conditions (A), PLCγ2 is cytosolic and not phosphorylated, the transmembrane adapter protein LAT is not phosphorylated, and the level of PI 3,4,5-P3 is minimal. After activation of GPVI (B), LAT becomes phosphorylated on tyrosine and associates with the C-terminal domain of PLCγ2. In addition, GPVI stimulates PI 3-kinase, leading to the formation of PI 3,4,5-P3 in the membrane. The localization of PLCγ2 in the membrane is stabilized by binding of PI 3,4,5-P3 to the PH domain of PLCγ2 (C).
Interestingly, in this context, there was a residual level of PLCγ2 on the plasma membrane in basal XID megakaryocytes that could be removed by wortmannin. This suggests that, in the absence of an intact Btk PH domain, a low basal level of PI 3,4,5-P3 may be sufficient to support limited translocation of PLCγ2 to the membrane. This residual membrane PLCγ2 is not correlated to an increase of basal [Ca++]i, suggesting that this alone is not sufficient to induce PLCγ2 activation. It is likely that PLCγ2 must be targeted to a specific region of the membrane and requires other proteins for activation.
The protease thrombin signals through G protein–coupled receptors leading to activation of PLCβ2. In contrast, thrombin stimulates minimal tyrosine phosphorylation of PLCγ2 suggesting that this does not play a functional role. Thrombin has been reported to stimulate tyrosine phosphorylation of Btk in human platelets through direct and indirect actions, the latter being mediated through αIIbβ3.11,31 In this study, we show that thrombin also stimulates translocation of Btk but not PLCγ2 to the membrane of mouse megakaryocytes through a pathway that is insensitive to wortmannin and LY294002 and that is maintained in XID mice. Translocation of Btk by thrombin could be mediated through a direct interaction with G protein α or βγ subunits,32-34 rather than via PI 3-kinase as seen in response to CRP. The translocation response to thrombin in megakaryocytes is independent of αIIbβ3, as there was no added fibrinogen or von Willebrand factor, and neither adhesion molecule is released from stimulated cells. The PH domain of Btk is required to anchor Btk at the membrane at increased stimulation times by thrombin as membrane association is lost after 1 minute in XID cells but not in control cells. Previously, we have reported that Btk has no significant role in human platelet aggregation or peak Ca++ elevation in response to thrombin, as neither response is altered in XLA patients or in the presence of wortmannin.11 13 However, the observation that translocation is not greatly altered in response to thrombin in XID megakaryocytes suggests a need for caution in regard to this conclusion.
To conclude, CRP stimulates, via GPVI, PI 3-kinase–dependent translocation of PLCγ2 and Btk through independent pathways. In contrast, thrombin induces translocation of Btk through a PI 3,4,5-P3–independent pathway. These different pathways of Btk translocation may give rise to synergistic interactions between G protein–coupled and tyrosine kinase–linked receptor agonists.
Supported by the British Heart Foundation (BHF) and the Wellcome Trust. S.P.W. is a BHF Senior Research Fellow.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Régis Bobe, Department of Pharmacology, University of Oxford, Mansfield Rd, OX1 3QT Oxford, United Kingdom; e-mail: regis.bobe@pharm.ox.ac.uk.



![Fig. 5. CRP elevates Ca++ in XID megakaryocytes. / Single megakaryocytes from control and XID mice were microinjected with Fura-2, pretreated or not for 15 minutes with wortmannin (wort) (100 nM), and stimulated by CRP (4 μg/mL). Intracellular Ca++was determined as described in “Materials and methods.” The increase in [Ca++]i in response to CRP is shown as histogram showing the mean ± SE. Results are from 8 to 10 cells from a minimum of 4 different mice. *corresponds to results significantly different from basal.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/3/10.1182_blood.v97.3.678/6/m_h80310652005.jpeg?Expires=1766018829&Signature=3AcVl~l~bIBbdPGUD99px2cDcJG3l0KszY8VNXKs9hLJURZS8LbhoCFc4W0Hx-GuVCTZxaf1aoeamUvs6pWAzLb-xnVliRoSd2aS~dS0CZR~zsUwYxzQcGvfDGPpy0Jkom3O1tNg45vy2I0wZuuygpp36Fzkv1l0cW694EOOiTllyREFN~rdq1vUDy9j1ts3Dkjbi6ax0B8UfXzwlPC77-P6593To2qACg3tJDqVSULgTDhXYoliHXQI2FaWODPIjBMwPYs94adX5jEqnRpSBjgAxq~5Nx-XNzQynNkOUvkpCHmasrg8LeVx9Qg5AiFvqnUpSulJO5wPS7L253F92g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal