Abstract
Studies on nasal T/natural killer (NK)–cell lymphoma have been hampered by its tendency to cause necrosis. Thus, the establishment of cell lines of this neoplasm would seem to be valuable. This study attempted to establish cell lines from primary lesions of this tumor, and successfully obtained 2 novel Epstein-Barr virus (EBV)–positive cell lines, SNK-6 and SNT-8, by means of high-dose recombinant interleukin 2. Flow cytometry showed that SNK-6 had an NK-cell phenotype, CD3−CD4−CD8−CD19−CD56+T-cell receptor (TCR) α/β− TCR γ/δ−, whereas SNT-8 was CD3+CD4−CD8−CD19−CD56+TCR α/β− TCR γ/δ+. These were consistent with immunophenotypes of their original tumors, and the cell lines had monoclonal EBV clones identical to ones in their original tumors. Thus, the cell lines developed from cells forming the primary lesions. Genotypic analysis showed that SNK-6 had unrearranged TCR and immunoglobulin heavy-chain genes, supporting the conclusion that SNK-6 was of NK-cell lineage. On the other hand, SNT-8 had rearranged TCR β-, γ-, and δ-chain genes, and together with its phenotype, SNT-8 proved to be a γδ T-cell line. This is the first report of the establishment of cell lines from primary lesions of nasal T/NK cell lymphomas, and the results demonstrated that there are at least 2 lineages, NK- and γδ T-cell, in this neoplasm. Moreover, it has been suggested that nasal T/NK cell lymphomas of these lineages may belong to the same clinicopathologic entity because both types of cases shared common clinical and histopathologic features.
Introduction
Nasal T/natural killer (NK)–cell lymphoma is a distinct clinicopathologic entity characterized by progressive necrotic lesions in the nasal cavity, nasopharynx, and palate.1 It has been called angiocentric lymphoma, lethal midline granuloma, or polymorphic reticulosis.2 It is a relatively rare disease associated with a poor prognosis and more often seen in Asia than in the United States and Europe.3-14 Nasal T/NK-cell lymphomas can exhibit a broad histologic spectrum and usually show invasion of the vascular walls, which is associated with prominent ischemic necrosis of both tumor cells and normal tissue.
The most characteristic feature of the disease is a consistent and strong association with the Epstein-Barr virus (EBV); indeed, almost all cases are positive for EBV irrespective of ethnic difference,15-22 yet the precise role of the virus in the etiology of the disease is poorly understood. The cellular origin of the nasal T/NK-cell lymphoma has been controversial. It was initially thought to originate from the T-cell lineage based on immunophenotype.23-27 Later studies using combined immunophenotypic and genotypic analysis have suggested that most nasal T/NK-cell lymphomas are of NK-cell lineage, being CD56+, negative for surface CD3 (Leu4), and unassociated with rearrangements of the T-cell receptor (TCR) genes.11,28-30 On the other hand, some studies have reported the existence of T-cell lymphomas that express TCR proteins, that is α/β and γ/δ chains, associated with rearranged TCR genes; these were presumably αβ T-cell lymphomas10,30 and γδ T-cell lymphomas.18,31 In addition, there have been reports of atypical cases in which cell surface antigens were expressed discordantly; these were CD3(Leu4)+CD56+ cases without TCR gene rearrangements,32 and cases with TCR proteins expressed discordantly with their rearrangement status of the TCR genes.10,29,30 Moreover, it has recently been suggested that nasal T/NK-cell lymphomas of NK- and γδ T-cell lineages are derived from activated cytotoxic cells, have common clinical and histopathologic features, and belong to the same clinicopathologic entity.31 Therefore, precise phenotypes of this neoplasm including the T-cell subset in cases of T-cell lymphomas and their relationships to clinicopathologic features need to be studied. However, it is often difficult to obtain a sufficient amount of tissues from such a necrotic lesion. To solve this problem, the propagation of tumor cells in vitro seems to be useful. Recently, a novel NK-cell line, NK-YS, was established from peripheral blood of a patient with a leukemic-state nasal NK-cell lymphoma.33However, because nasal T/NK-cell lymphomas infrequently develop leukemia, a more applicable method for establishing a cell line is needed.
Therefore, in the present study, we aimed to establish cell lines from primary lesions of nasal T/NK-cell lymphomas. We established 2 novel EBV-positive cell lines of NK- and γδ T-cell lineages, SNK-6 and SNT-8. In the present report, we describe immunologic and molecular biologic features of these cell lines.
Patients, materials, and methods
Patients and nasal tumors
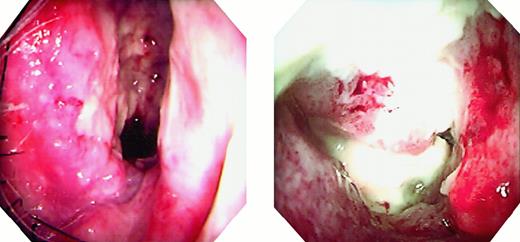
Tumor tissues obtained from 2 Japanese patients with nasal T/NK-cell lymphomas were used in this study. Patient 1 was a 62-year-old man and patient 2 was a 48-year-old woman. Both patients had tumors on their right inferior turbinates through the nasal vestibula. The tumors were composed of granulomatous parts and white necrotic parts (Figure 1). Biopsies were performed at the granulomatous parts, but small necrotic tissues were included. Freshly biopsied tissues were cut into several pieces, and portions of these tissues were used for establishing cell lines. The remaining tissues were fixed in 10% buffered formalin for paraffin sections or frozen in liquid nitrogen for immunophenotyping and molecular analysis.
Endoscopic findings.
The patients' right nasal cavities show granulomatous tumors and white necrosis. Left panel, patient 1; right panel, patient 2.
Endoscopic findings.
The patients' right nasal cavities show granulomatous tumors and white necrosis. Left panel, patient 1; right panel, patient 2.
Immunohistochemistry and in situ hybridization
Immunophenotyping of cells for B-, T-, and NK-cell differentiation antigens was performed on frozen sections as well as on paraffin sections. The antibodies used for the paraffin sections were anti-CD3ε polyclonal antiserum (Dako, Glostrup, Denmark), L26 (anti-CD20, Dako), UCHL-1 (anti-CD45RO, Dako), and anti-CD56 (Novocastra, Newcastle, UK) monoclonal antibodies. The antibodies used for the frozen sections were anti-CD2 (Leu5b) and anti-CD3 (Leu4) monoclonal antibodies (Becton Dickinson, San Jose, CA). A standard peroxidase antiperoxidase complex method followed by diaminobenzidine reaction was used to detect positive signals. Paraffin sections were also used for in situ hybridization with a digoxigenin-labeled oligonucleotide probe complementary to the EBV-encoded RNA (EBER-1): the sequence is 5′-AGACACCGTCCTCACCACCCGGGACTTG TA-3′. Hybridization was detected by the procedure described by Chang and colleagues.34
Cell culture and morphologic evaluation
The tumor tissues were minced, and strained through a 70-μm cell strainer (Becton Dickinson). The cells obtained through the strainer were then suspended in RPMI 1640 medium supplemented with antibiotics, 10% heat-inactivated human serum, and 700 U/mL of recombinant human interleukin 2 (IL-2). The cells were cultured in a humidified atmosphere at 37°C with 5% CO2 in air. One half of the medium was exchanged with fresh medium twice a week. The cultured cells from patients 1 and 2 have been maintained in the presence of IL-2 for over 20 months and 17 months, respectively, and designated as SNK-6 and SNT-8. Cytocentrifuge smears of the cells were prepared and stained with May-Giemsa for morphologic evaluation.
Flow cytometric analysis
The SNK-6 and SNT-8 cells were analyzed by 2-color immunofluorescence with a flow cytometer (EPICS XL, Beckman Coulter, Hialeah, FL) for the expression of surface markers. The following antibodies conjugated with fluorescein isothiocyanate (FITC) or phycoerythrin (PE) (Becton Dickinson) were used: anti-CD3, -CD4, -CD8, -CD16, -CD19, -CD21, -CD25, -CD56, -CD57, -HLA-DR, -TCR α/β, and -TCR γ/δ.
Southern blot analysis for TCR and immunoglobulin genes
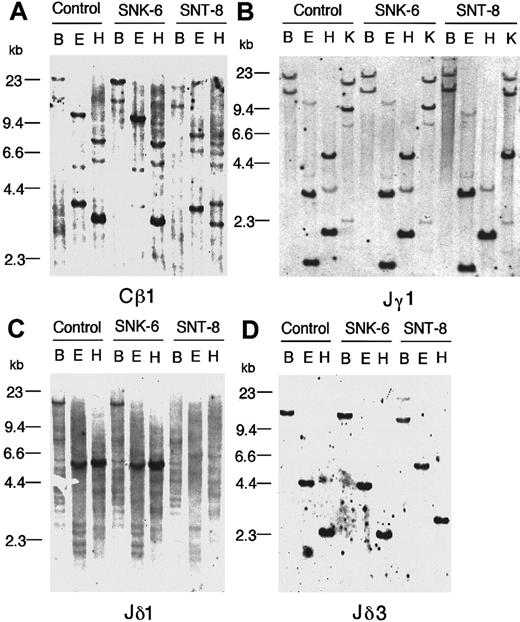
The SNK-6 and SNT-8 cells were analyzed for rearrangements of the TCR β, γ, and δ chains and the immunoglobulin heavy-chain genes by Southern blotting. DNA (3 μg) extracted from each of the cell lines was digested with a restriction endonucleaseBamHI, EcoRI, or HindIII (TAKARA, Kyoto, Japan), electrophoresed through a 0.7% agarose gel and transferred onto a nylon membrane. Human placenta DNA was used as a germline control. The membrane was subjected to a fluorescein-labeled Cβ1, Jγ1, Jδ1, or Jδ3 probe for the TCR genes, or a Jh probe for the immunoglobulin heavy-chain gene.35-39 The Jδ3 probe was a 1.5-kb XbaI genomic fragment of the Jδ3 region. In addition, DNA was digested with KpnI for the Jγ1 probe, and processed in the same manner. Hybridizations were visualized by a Fluorescein Gene Images System (Amersham, Buckinghamshire, UK) according to the manufacturer's instructions.
Assessment of clonality of EBV
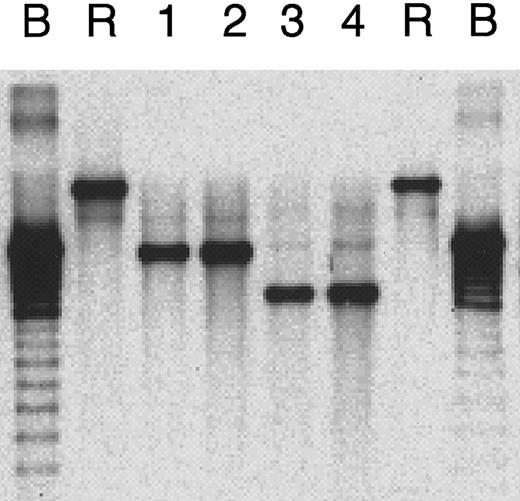
DNA extracted from the cell lines as well as the frozen tumor tissues were digested with BamHI, separated on a 0.7% agarose gel, and transferred to a nylon membrane. A 1.9-kbXhoI subfragment of BamHI-Dhet derived from EBV termini was used as a probe for terminal repeats of the EBV DNA40 according to the methods for detecting signals described above. Raji and B95-8 cells were used as controls for cell expansion with monoclonal EBV infection and polyclonal EBV replication, respectively.
Results
Primary lesions
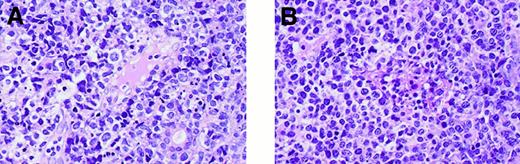
The tumors in both patients shared common histologic features. The normal structure of the nasal mucosa disappeared and was replaced by tumors, and nonsuppurative necrosis was readily noted. The tumors were composed of pleomorphic, medium- to large-sized atypical lymphoid cells. Infiltration of neoplastic cells to small vessels was observed (Figure 2). In situ hybridization showed that both tumors were positive for EBV. Immunophenotypes of both tumors were CD3ε+ CD20−CD56+, whereas the tumors showed a difference in positivity for the surface CD3 (Leu4) antigen. The tumor in patient 1 was CD3 (Leu4)−, and the tumor in patient 2 was CD3 (Leu4)+. These results indicated that the tumor in patient 1 had a phenotype of NK cells. The results are summarized in Table 1.
Histopathology.
Primary lesions show invasion of tumor cells to small blood vessels. (A) Patient 1. (B) Patient 2. (Hematoxylin-eosin, final magnification × 140).
Histopathology.
Primary lesions show invasion of tumor cells to small blood vessels. (A) Patient 1. (B) Patient 2. (Hematoxylin-eosin, final magnification × 140).
Summary of phenotypes of primary tumors
| Case . | CD2 (Leu5b) . | CD3 (Leu4) . | CD3ε . | CD20 . | CD45RO . | CD56 . | EBER-1 . | Developed cell line . |
|---|---|---|---|---|---|---|---|---|
| 1 | + | − | + | − | + | + | + | SNK-6 |
| 2 | + | + | + | − | + | + | + | SNT-8 |
| Case . | CD2 (Leu5b) . | CD3 (Leu4) . | CD3ε . | CD20 . | CD45RO . | CD56 . | EBER-1 . | Developed cell line . |
|---|---|---|---|---|---|---|---|---|
| 1 | + | − | + | − | + | + | + | SNK-6 |
| 2 | + | + | + | − | + | + | + | SNT-8 |
Morphology and phenotypes of the cell lines
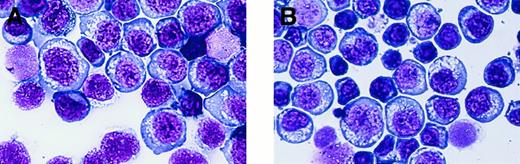
The morphologies of the SNK-6 and SNT-8 cells were similar to each other, except for their sizes. The SNK-6 cells were composed of medium to large cells, whereas the SNK-8 cells included small cells as well as medium to large ones (Figure 3). Large cells in both cell lines had large nuclei and abundant basophilic cytoplasm. Nuclei as well as cytoplasm became smaller and pycnotic as the cell sizes became smaller. Both cells had a number of cytoplasmic vacuoles, but scarcely any azurophilic granules.
Morphology.
Established cell lines show rich cytoplasmic vacuoles and no azurophilic granules. (A) SNK-6. (B) SNT-8. (Wright-Giemsa, final magnification × 440)
Morphology.
Established cell lines show rich cytoplasmic vacuoles and no azurophilic granules. (A) SNK-6. (B) SNT-8. (Wright-Giemsa, final magnification × 440)
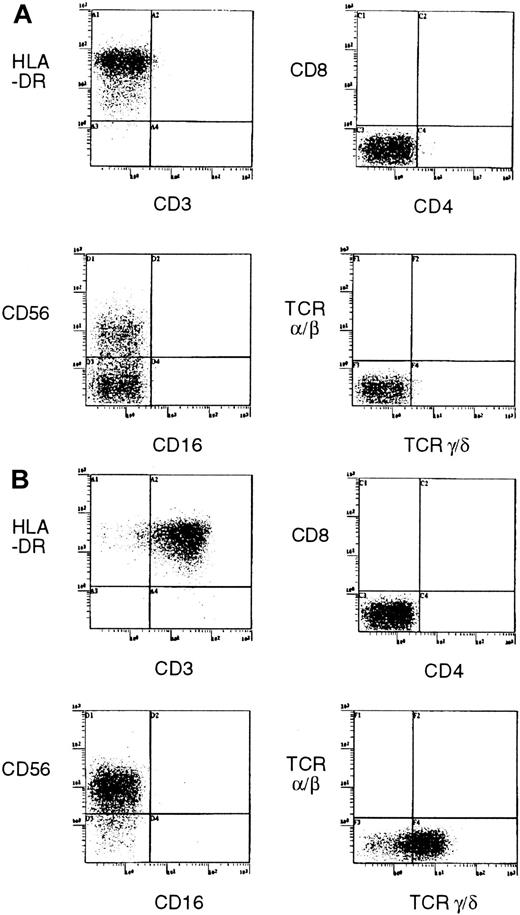
Flow cytometry showed that the SNK-6 cells were CD3− CD4−CD8−CD16−CD19−CD21−CD25+CD56+CD57−HLA-DR+, and TCR α/β− TCR γ/δ− (Figure4A). Thus, SNK-6 was shown to have an NK-cell phenotype. On the other hand, the SNK-8 cells were CD3+CD4−CD8−CD16−CD19−CD21−CD25+CD56+CD57−HLA-DR+, and TCR α/β− TCR γ/δ+ (Figure 4B).
Expression of cell surface markers for subset of T cells and NK cells by established cell lines.
(A) SNK-6. (B) SNT-8.
Expression of cell surface markers for subset of T cells and NK cells by established cell lines.
(A) SNK-6. (B) SNT-8.
Southern blot analysis for TCR and immunoglobulin genes
Neither of the cell lines showed a rearrangement of the immunoglobulin heavy-chain gene. In terms of the TCR genes, the SNK-6 cells showed no rearrangements of the TCR β-, γ-, and δ-chain genes, supporting that SNK-6 is of NK-cell lineage (Figure5). On the other hand, the SNT-8 cells showed rearrangements of the β-, γ-, and δ-chain genes. The rearrangements of the γ-chain gene were initially not detected by the Jγ1 probe hybridized with BamHI, EcoRI, orHindIII digest (Figure 5B). Then, we evaluated hybridization of the probe with the KpnI digest, because it has been reported that the Vγ9-JγP rearrangement frequently observed in rearranging γ loci can be detected with the KpnI digest, but the BamHI rearranged band has virtually the same size as the germline bands and the EcoRI and HindIII bands are in germline configuration in this rearrangement.41,42 As expected, a 9-kb germline band derived from a KpnI fragment including the JγP gene changed to a 12-kb band probably by assignment of the Vγ9 gene to the JγP gene (Figure 5B). With respect to the δ-chain gene, deletion was initially found in the Jδ1 region in the SNT-8 cells (Figure 5C), suggesting that rearrangements of the α-chain gene or deletion of the δ-chain gene occurred in the cells.43 Subsequently, we sought rearrangements of the δ-chain gene by hybridizing the blots with the Jδ3 probe, because the SNT-8 cells showed expression of the TCR γ/δ receptor by flow cytometry. As a result, the Jδ3 probe successfully detected rearranged bands, indicating that the deletion of the Jδ1 gene was a consequence of the usage of the Jδ3 gene in rearrangements of the δ locus. (Figure 5D).
Southern blot analysis.
Southern blot analysis shows no rearrangements of TCR genes in SNK-6 and rearrangements of the β-, γ-, and δ-chain genes in SNT-8. Panels show hybridization with the following probes: (A) the Cβ1 probe, (B) the Jγ1 probe, (C) the Jδ1 probe, (D) the Jδ3 probe. Human placenta DNA was used as a germline control. The letters above the pictures are BamHI (B), EcoRI (E),HindIII (H), and KpnI (K) digest. Note that the rearrangements of the γ-chain gene in SNT-8 cannot be detected by the BamHI, EcoRI, orHindIII digest. The lack of a 5-kb HindIII band hybridizing with the Jγ1 probe in SNT-8 is due to a polymorphism for a HindIII cleavage site.41
Southern blot analysis.
Southern blot analysis shows no rearrangements of TCR genes in SNK-6 and rearrangements of the β-, γ-, and δ-chain genes in SNT-8. Panels show hybridization with the following probes: (A) the Cβ1 probe, (B) the Jγ1 probe, (C) the Jδ1 probe, (D) the Jδ3 probe. Human placenta DNA was used as a germline control. The letters above the pictures are BamHI (B), EcoRI (E),HindIII (H), and KpnI (K) digest. Note that the rearrangements of the γ-chain gene in SNT-8 cannot be detected by the BamHI, EcoRI, orHindIII digest. The lack of a 5-kb HindIII band hybridizing with the Jγ1 probe in SNT-8 is due to a polymorphism for a HindIII cleavage site.41
Clonality of EBV
Southern blot analysis for the number of EBV terminal repeats showed a monoclonal band in each of the SNK-6 cells, the SNT-8 cells, and their original tumors. In addition, the bands in the cell lines corresponded to the bands in their respective original tumors (Figure6).
Clonality of EBV.
Southern blot analysis for clonality of EBV as evidence for monoclonal expansions of EBV-positive cells in primary tumor in patient 1 (lane 1), SNK-6 cells (lane 2), primary tumor in patient 2 (lane 3), and SNT-8 cells (lane 4). Controls are B95-8 cells (B) and Raji cells (R) for cells with monoclonal EBV expansion and polyclonal EBV replication, respectively.
Clonality of EBV.
Southern blot analysis for clonality of EBV as evidence for monoclonal expansions of EBV-positive cells in primary tumor in patient 1 (lane 1), SNK-6 cells (lane 2), primary tumor in patient 2 (lane 3), and SNT-8 cells (lane 4). Controls are B95-8 cells (B) and Raji cells (R) for cells with monoclonal EBV expansion and polyclonal EBV replication, respectively.
Discussion
The present study established 2 novel cell lines of T/NK-cell lineage from the primary lesions of nasal T/NK-cell lymphomas by means of a simple technique using high-dose IL-2. Established cell lines had monoclonal EBV clones identical to those in their respective original lesions. Moreover, the flow cytometric phenotypes of the cell lines corresponded with the immunohistochemical phenotypes of the primary lesions. Therefore, the cell lines were demonstrated to have developed from cells that formed the nasal T/NK-cell lymphomas in the patients. As far as we know, this is the first report describing the establishment of cell lines from primary lesions of the nasal T/NK-cell lymphoma.
The establishment of tumor cell lines seems to be valuable for the study of the nasal T/NK-cell lymphoma because the tendency of the disease to cause necrosis has hampered detailed analyses of the tumor. Recently, a novel NK-cell line NK-YS was established from the peripheral blood of a patient with a leukemic-state nasal NK-cell lymphoma.33 However, an association of leukemia with this disease is not common. Thus, we believe that a technique to establish a cell line from a primary lesion is more applicable to the disease. We have tried to date to establish cell lines from 5 primary lesions obtained from 5 independent patients and have succeeded in obtaining the 2 cell lines and in maintaining 2 other cells for over 1 month. Therefore, we believe that the technique described here will be generally useful for establishing cell lines from more cases of nasal T/NK-cell lymphomas.
The establishment of tumor cell lines appears to have advantages over biopsies for phenotypic and genotypic studies. In phenotypic studies, the expression of cell surface antigens can be analyzed more accurately by flow cytometry for suspended cells than by immunohistochemistry on tissue sections because most monoclonal antibodies for cell surface antigens were originally developed for flow cytometry, and the results of immunohistochemistry are occasionally difficult to interpret. Moreover, a cytoplasmic molecule such as CD3ε in NK cells, which is not expressed on the cell surface,30,44,45 may lead to false-positive results by immunohistochemistry, and can be assessed accurately by flow cytometry. With respect to genotypic studies, propagation of a clonal population of tumor cells appears to generate a clear result, whereas analysis of a biopsied tissue is associated with potentially equivocal findings because of the presence of nontumorous cells.46 Furthermore, the availability of a large amount of DNA allows us to repeat analysis and to try various DNA probes to confirm the reproducibility of our results.
In the present study, the results of phenotypic and genotypic analysis completely agreed with each other in the SNK-6 cells. The SNK-6 cells had an NK-cell phenotype CD3(Leu4)−CD4− CD8−CD19−CD56+TCR α/β− TCR γ/δ− and unrearranged TCR genes. On the other hand, the SNT-8 cells had a γδ T-cell phenotype CD3(Leu4)+CD4−CD8−CD19−TCR α/β− TCR γ/δ+ and showed rearrangements of the γ- and the δ-chain genes of the TCR, yet it was also CD56+ and associated with a rearranged β-chain gene. Because it was reported that peripheral γδ T-cell lymphomas frequently expressed CD5631,47-49 and showed rearrangements of the β-chain gene,47,49,50 SNT-8 was considered to be a tumor cell line of γδ T-cell lineage. Hence, the nasal T/NK-cell lymphoma in case 2 proved to be a γδ T-cell lymphoma. On the basis of previous reports, NK-cell is the predominant phenotype in nasal T/NK-cell lymphomas, and T cell is a minor phenotype.11,18,28,30 32 Therefore, SNT-8 should be useful for the study on the nasal T/NK-cell lymphoma of the γδ T-cell subset.
The genotypic studies of the SNT-8 cells gave us 2 lessons in evaluating rearrangements of the TCR genes in T-cell lymphomas. First, for analysis of the TCR γ-chain gene by using the Jγ1 probe,KpnI digest should be analyzed, when BamHI,EcoRI, or HindIII digest does not show a rearrangement.41,42 Second, when no signal is detected by the Jδ1 probe, another probe for the δ-chain gene should be applied to determine whether it is deleted or rearranged.39,43 However, to determine the subset of T-cell lymphomas, expression of the α/β or γ/δ TCR should be confirmed, because rearrangements of the γ-chain gene are commonly found in T-cell lymphomas irrespective of the type of TCR that is expressed51 and rearrangements of the β-chain gene are also found in γδ T-cell lymphomas as described above. In addition, consistency between the genotype and the phenotype seems necessary. Reviewing previously reported cases with nasal T-cell lymphomas with these considerations, we found only a small number of cases that can be clearly categorized as αβ or γδ T-cell lymphoma. Two of the 4 cases with nasal T-cell lymphomas reported by Chiang and coworkers29,30 (case 2 in reference 29 and case 9 in reference 30), a case in a series of 10 cases of T-cell lymphomas by Harabuchi and colleagues10 (case 17), and a CD3+ TCR α/β+ sinonasal case by Kanavaros and associates12 appeared to be αβ T-cell lymphomas. Interestingly, in contrast to most γδ T-cell lymphomas, 3 of these 4 cases were negative for CD56 and the other one was only weakly positive for CD56. On the other hand, one γδ T-cell case was reported by Kanavaros and coworkers,18 and 2 more cases were recently reported by the same group.31 Independently, 7 cases of possible γδ T-cell lymphomas were reported by Harabuchi and colleages10; these cases were positive for the δ-chain protein and negative for the β-chain protein, whereas 2 of them were CD3−, and none of them showed simultaneous rearrangements of the γ- and δ-chain genes. In addition, our case 2 was a γδ T-cell lymphoma. Therefore, it may be that the γδ T-cell subset is relatively common in the nasal T-cell lymphoma. The establishment of more cell lines appears to contribute to clarifying the features of the subset of the nasal T-cell lymphoma.
In the present study, the NK- and the γδ T-cell lymphomas formed similar tumors characterized by granulomatous appearance and necrosis in the nasal mucosa. Moreover, both tumors consisted of pleomorphic medium- to large-sized cells, which showed angioinvasion and were positive for EBV and CD56+. Recently, Arnulf and coworkers studied 11 cases of nonhepatosplenic peripheral γδ T-cell lymphomas31; 3 of these cases presented in the nasal cavity and the other cases were in other extranodal sites, including the larynx, lung, gastrointestinal tract, or skin. Interestingly, all of the 3 nasal cases were EBV positive, and 2 of them were CD56+, whereas only a few cases in the other sites were EBV positive, and none of them was CD56+. They concluded that nasal T/NK-cell lymphomas, whatever their NK- or γδ T-cell origin, have common clinical and histopathologic features, are associated with EBV, and are derived from activated cytotoxic cells, and thus most likely belong to the same clinicopathologic entity. The present results appear to support their conclusions.
Although the etiology of the nasal T/NK-cell lymphoma is largely unknown, it has been recognized that the disease occurs in a consistent and strong association with EBV.15-22 Likewise, SNK 6 and SNT-8 harbored monoclonal EBV clones. However, the mechanisms by which T/NK cell lineages are affected by the virus are poorly understood. In terms of the infecting route, CD21 is the only known receptor for the virus to date.52,53 Kaneko and colleagues showed that CD21 was expressed on normal NK cells activated with IL-2 as well as on isolated tumor cells from a nasal NK-cell lymphoma.54However, 2 EBV-positive NK-cell lines, SNK-6 in the present study and NK-YS,33 did not express the CD21 antigen. Likewise, 5 EBV-positive T-cell lines, SNT-8 in the present study and 4 cell lines reported previously,55 were negative for the CD21 antigen. With respect to the tumorigenicity of the virus, the latent membrane protein LMP-1 is known to possess transforming activity for rodent as well as human cells,56,57 and may also play a role in the nasal T/NK-cell lymphoma. Cytogenetic studies have shown frequent abnormalities in chromosome 6 in nasal T/NK-cell lymphomas,58,59 and there has also been a report that abnormalities of chromosome 6 were additionally recognized in cells isolated from patients with chronic active EBV infection and that the abnormalities might be caused by the integration of the virus to chromosome 6.60 Therefore, to clarify a role of the EBV in the etiology of the nasal T/NK-cell lymphoma, virologic, molecular biologic, and cytogenetic studies seem to be necessary, warranting the use of the established cell lines for future studies and necessitating further efforts to develop more cell lines from nasal T/NK-cell lymphomas.
Acknowledgment
The authors thank Mrs Aya Kasuya for her technical assistance.
Supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan (no. 11670207 and 09253103) and Grants-in-Aid for Education and Research Promotion Program 1999 from Tokyo Medical and Dental University.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Norio Shimizu, Department of Virology, Division of Virology and Immunology, Medical Research Institute, Tokyo Medical and Dental University, 1-5-45 Yushima, Bunkyo-ku, Tokyo 113-8510, Japan; e-mail: nshivir@mri.tmd.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal