Abstract
Interleukin 4 (IL-4) suppresses the growth of acute lymphoblastic leukemia (ALL) cells, but its clinical usefulness is limited by proinflammatory activity due mainly to the interaction of cytokine with endothelial cells and fibroblasts. Stroma-supported cultures of leukemic lymphoblasts were used to test the antileukemic activity of an IL-4 variant, BAY 36-1677, in which the mutations Arg 121 to Glu and Thr 13 to Asp ensure high affinity for IL-4Rα/IL-2Rγ receptors expressed by lymphoid cells, without activation of the IL-4Rα/IL-13Rα receptors mainly expressed by other cells. BAY 36-1677 (25 ng/mL) was cytotoxic in 14 of 16 cases of B-lineage ALL; the median reduction in cell recovery after 7 days of culture was 85% (range, 17%-95%) compared to results of parallel cultures not exposed to the cytokine. Twelve of the 14 sensitive cases had t(9;22) or 11q23 abnormalities; 3 were obtained at relapse. BAY 36-1677 induced apoptosis in leukemic lymphoblasts but did not substantially affect the growth of normal CD34+ cells, thus conferring a growth advantage to normal hematopoietic cells over leukemic lymphoblasts in vitro. BAY 36-1677 had antileukemic activity equal or superior to that produced by native IL-4, but it lacked any effects on the growth of endothelial cells and fibroblasts. The molecular manipulation of IL-4 to abrogate its proinflammatory activity has generated a novel and therapeutically promising cytokine for the treatment of high-risk ALL.
Introduction
Despite treatment with intensive chemotherapy, residual disease persists in approximately 20% of children and 65% of adults with acute lymphoblastic leukemia (ALL).1,2Recurrent disease is particularly resistant to chemotherapy, as are some subtypes of ALL, such as those with the t(9;22)(q34;q11) andMLL gene rearrangements.1,2 For most of these patients, the current arsenal of antileukemic drugs given at tolerable doses is clearly insufficient. Bone marrow ablation with high-dose chemotherapy or total body irradiation, followed by hematopoietic stem cell engraftment, may be curative in patients resistant to conventional therapy, but this option is restricted by the availability of suitable donors. Even when effective, chemotherapeutic regimens for ALL carry the risk for severe toxicity, including secondary malignancy, cardiomyopathy, and neuropsychological abnormalities.1 3-7Thus, there is an urgent need to develop new antileukemic drugs with better therapeutic indexes.
We and others have observed that interleukin-4 (IL-4), an immunomodulatory cytokine produced by T cells,8,9 induces growth arrest and apoptosis in leukemic lymphoblasts in vitro.10-12 These findings were confirmed in experiments with human leukemic cells engrafted in immunodeficient mice.13 The clinical usefulness of IL-4, however, is limited by the pleiotropic activities of cytokine, which can include renal, hepatic, neurologic, and gastrointestinal toxicities as well as capillary leak syndrome.14-19 Although the pathogenesis of these side effects is unclear, the available evidence suggests that it may relate in part to IL-4 signal transduction in fibroblasts and endothelial cells.18
The receptor for IL-4 (IL-4R) consists of a primary binding subunit, IL-4Rα, which, together with other components, forms the signaling complex. Lymphoid cells express mainly one IL-4R subtype, IL-4Rα/IL-2Rγ, whereas endothelial cells and fibroblasts express another, IL-4Rα/IL-13Rα.20,21 Shanafelt et al22 produced variants of IL-4 by mutating the region of the D-helix implicated in the interaction with IL-2Rγ and IL-13Rα. One variant, containing the substitution Arg 121 to Glu, has high affinity for IL-4Rα/IL-2Rγ receptors, but it does not activate IL-4Rα/IL-13Rα receptors.22 Variants containing this substitution stimulate T-cell proliferation, up-regulate CD23 expression in mature B cells, and suppress lipopolysaccharide-induced tumor necrosis factor-α secretion from monocytes, but they do not induce IL-4–mediated responses in endothelial cells.22Another recently developed variant, BAY 36-1677, contains the additional substitution of Thr 13 to Asp, which improves the affinity of the molecule for IL-4Rα. We used stroma-supported cultures of leukemic cells and normal hematopoietic cells to assess the cytotoxic activity of this IL-4 variant.
Patients, materials, and methods
Lymphoid-specific IL-4 variant BAY 36-1677
BAY 36-1677, containing the substitutions Thr 13 to Asp and Arg 121 to Glu (Figure 1), was generated by site-directed mutagenesis of human IL-4 using the method of Kunkel et al.23 BAY 36-1677 was produced in a mammalian expression system and purified with an anti–human IL-4 monoclonal antibody affinity matrix followed by reverse-phase liquid chromatography, lyophilized, and resuspended in sterile phosphate-buffered saline (PBS). This procedure yielded a more than 99% pure compound, as determined by SDS-PAGE and high-performance liquid chromatography. The primary structure was confirmed by N-terminal sequencing and mass spectrometry.
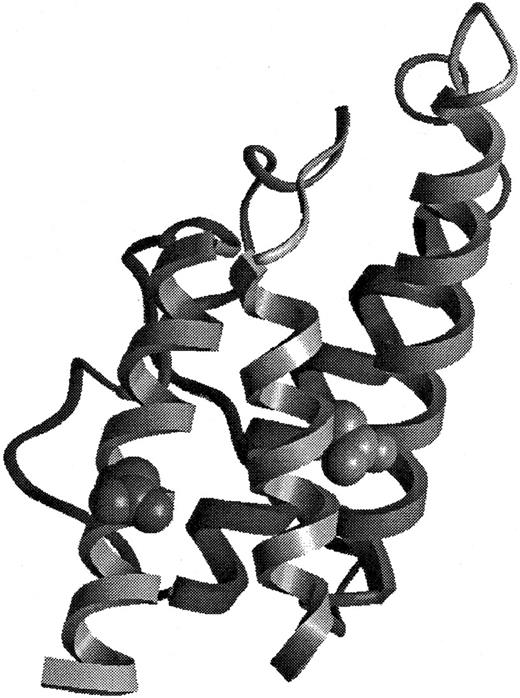
Structural representation of BAY 36-1677.
The model of BAY 36-1677 is derived from the structure of Müller et al.41 The mutations Thr 13 to Asp (right) and Arg 121 to Glu (left) have been introduced into the structural representation.
Structural representation of BAY 36-1677.
The model of BAY 36-1677 is derived from the structure of Müller et al.41 The mutations Thr 13 to Asp (right) and Arg 121 to Glu (left) have been introduced into the structural representation.
Receptor-binding assay
The extracellular domain of IL-4Rα was recovered using polymerase chain reaction from a Jurkat cell λgt11 library (Stratagene Cloning Systems, La Jolla, CA), and it was fused to DNA corresponding to the peptide sequence Ser-Ala-Trp-Arg-His-Pro-Gln-Phe-Gly-Gly at the C-terminus generating sIL-4Rα-STX. The peptide sequence Ser-Ala-Trp-Arg-His-Pro-Gln-Phe-Gly-Gly exhibits streptavidin-binding properties. Produced using the baculovirus system, sIL-4Rα-STX protein was purified by affinity chromatography using an IL-4–coupled matrix (CNBr Sepharose 4B) and stored in PBS at 4°C. Streptavidin-coated FlashPlates (DuPont NEN, Boston, MA) were coated with 100 μL of 2 μg/mL sIL-4Rα-STX in 100 mM Tris, 0.1% bovine serum albumin (BSA), pH 7.0, for 2 hours at 20°C, and they were washed with PBS, 0.1% BSA, pH 7.6. Coated plates were incubated with 200 pM 125I-IL-4 (DuPont NEN) and varying concentrations of native IL-4 or BAY 36-1677 (produced in the baculovirus system) in 100 μL PBS, 0.1% BSA, pH 7.6, for 1.5 hours at 20°C in quadruplicate. Assays were repeated at least twice, with native IL-4 or BAY 36-1677 assayed in parallel on the same plate. Bound radioactivity was measured in a TopCount scintillation counter (Packard Instrument, Meriden, CT), and KI values were calculated using the sigmoidal dose-response (variable slope) fit provided by the PRISM program (GraphPad Software, San Diego, CA).
Cells
Bone marrow cells were collected at diagnosis (n = 13) or relapse (n = 3) from patients with B-lineage ALL, ages 2 months to 18 years (median, 9.5 years; Table 1). In all 16 patients, the leukemic lymphoblasts were positive for CD19, CD22, HLA-DR, and terminal deoxynucleotidyl transferase. Cord blood samples were obtained after normal full-term deliveries. By trypan blue dye exclusion, the viability of cells was greater than 90% in all samples. These studies were approved by the Institutional Review Board of St Jude Hospital, and informed consent was given by the patients, their parents or guardians, or both.
Presenting features and sensitivity to BAY 36-1677 among cases of acute lymphoblastic leukemia
| Patient . | Age (y) . | Sex . | Leukocyte count (109/L) . | Karyotype . | Phenotype . | Cell survival on stroma (%)* . | Inhibition of cell recovery with BAY 36-1677 (%)† . |
|---|---|---|---|---|---|---|---|
| 11-153 | 17 | M | 1.5 | t(9;22) | Pre-B | 60 | 57 |
| 21-153 | 15 | F | 531.0 | 11q23 | Pre-B | 231 | 31 |
| 31-153 | 11 | M | 70.7 | t(9;22) | Early pre-B | 115 | 88 |
| 4 | 0.2 | F | 138.9 | 11q23 | Early pre-B | 93 | < 1 |
| 5 | 12 | F | 165.5 | t(9;22) | Early pre-B | 161 | 95 |
| 6 | 15 | M | 455.0 | 11q23 | Pre-B | 151 | 85 |
| 7 | 2 | F | 117.0 | t(9;22) | Pre-B | 102 | 69 |
| 8 | 6 | M | 7.3 | t(9;22) | Pre-B | 132 | 95 |
| 9 | 18 | M | 78.6 | t(9;22) | Early pre-B | 86 | < 1 |
| 10 | 5 | M | 4.1 | 53 chrom | Early pre-B | 71 | 94 |
| 11 | 13 | F | 20.7 | t(9;22) | Early pre-B | 67 | 87 |
| 12 | 2 | F | 28.0 | 48 chrom | Early pre-B | 62 | 85 |
| 13 | 6 | M | 72.7 | t(9;22) | Early pre-B | 76 | 78 |
| 14 | 18 | M | 367.0 | t(9;22) | Early pre-B | 57 | 35 |
| 15 | 4 | M | 83.5 | t(9;22) | Early pre-B | 147 | 90 |
| 16 | 8 | M | 497.0 | t(9;22) | Early pre-B | 80 | 17 |
| Patient . | Age (y) . | Sex . | Leukocyte count (109/L) . | Karyotype . | Phenotype . | Cell survival on stroma (%)* . | Inhibition of cell recovery with BAY 36-1677 (%)† . |
|---|---|---|---|---|---|---|---|
| 11-153 | 17 | M | 1.5 | t(9;22) | Pre-B | 60 | 57 |
| 21-153 | 15 | F | 531.0 | 11q23 | Pre-B | 231 | 31 |
| 31-153 | 11 | M | 70.7 | t(9;22) | Early pre-B | 115 | 88 |
| 4 | 0.2 | F | 138.9 | 11q23 | Early pre-B | 93 | < 1 |
| 5 | 12 | F | 165.5 | t(9;22) | Early pre-B | 161 | 95 |
| 6 | 15 | M | 455.0 | 11q23 | Pre-B | 151 | 85 |
| 7 | 2 | F | 117.0 | t(9;22) | Pre-B | 102 | 69 |
| 8 | 6 | M | 7.3 | t(9;22) | Pre-B | 132 | 95 |
| 9 | 18 | M | 78.6 | t(9;22) | Early pre-B | 86 | < 1 |
| 10 | 5 | M | 4.1 | 53 chrom | Early pre-B | 71 | 94 |
| 11 | 13 | F | 20.7 | t(9;22) | Early pre-B | 67 | 87 |
| 12 | 2 | F | 28.0 | 48 chrom | Early pre-B | 62 | 85 |
| 13 | 6 | M | 72.7 | t(9;22) | Early pre-B | 76 | 78 |
| 14 | 18 | M | 367.0 | t(9;22) | Early pre-B | 57 | 35 |
| 15 | 4 | M | 83.5 | t(9;22) | Early pre-B | 147 | 90 |
| 16 | 8 | M | 497.0 | t(9;22) | Early pre-B | 80 | 17 |
Chrom indicates chromosome.
Percentage leukemic cell recovery after 7 days of culture on bone marrow–derived stroma, compared to the number of cells originally seeded.
Percentage inhibition of cell recovery in cultures containing BAY 36-1677 (25 ng/mL), compared to control cultures without the cytokine.
Studies at first (patients 1 and 2) and second (patient 3) relapse.
Mononuclear cells were separated by density gradient centrifugation (Lymphoprep; Nycomed, Oslo, Norway) and washed 3 times in PBS and once in AIM-V (Gibco, Grand Island, NY), a serum-free, cytokine-free tissue culture medium. Cord blood CD34+ cells were separated with a MACS separation system (Miltenyi Biotec, Bergisch Gladbach, Germany), which, by flow cytometry, consistently afforded a purity of 90% or greater.24
Six B-lineage ALL cell lines—KOPN57bi, OP-1, RS4;11, 380, NALM6, and REH (all available in our laboratory)—were maintained in RPMI 1640 tissue culture medium (BioWhittaker, Walkersville, MD) with 10% fetal calf serum (FCS; BioWhittaker).
Cell cultures
Bone marrow stromal cells, depleted of T cells by CD6- and CD8-mediated rabbit complement lysis, were derived from healthy bone marrow donors. Stroma was prepared in 96-well flat-bottomed plates (Costar, Cambridge, MA) and fed with RPMI-1640, 10% FCS, and 10−6 M hydrocortisone (Sigma, St Louis, MO), as previously described.12 25-30 Human umbilical-cord endothelial cells (HUVEC), purchased from the American Type Culture Collection (Manassas, VA), were cultured in Endothelial-Growth Medium (Clonetics, San Diego, CA) with 10% FCS. Skin fibroblasts were derived from a healthy donor by punch skin biopsy and then fed with Dulbecco modified Eagle medium (BioWhittaker) and 10% FCS.
To prepare test cultures of leukemic cells from patients, we removed the media from the bone marrow stroma and washed the adherent cells 7 times with AIM-V tissue culture medium.12 25-30 The cells were then resuspended in AIM-V, and 3 to 4 × 105 cells were placed on the stromal layer in each well. We then added BAY 36-1677 (Bayer Biotechnology, Berkeley, CA), native IL-4 (Genzyme, Cambridge, MA), or IL-13 (R&D Systems, Minneapolis, MN) to the test wells at final concentrations ranging from 1 ng/mL to 100 ng/mL. A neutralizing antibody to IL-4, an antibody to IL-4Rα (CD124) that blocks IL-4 signaling, and an antibody to IL-2Rγ (CD132) were also used in some experiments (all antibodies were from R&D Systems). Cultures were incubated for 7 days at 37°C, 5% CO2, and 90% humidity. We used an identical procedure to culture normal CD34+ cells on stroma, except that the number of cells added to each well was lower (3 × 104). As in cultures of leukemic cells, we used serum-free AIM-V medium, with no addition of hematopoietic growth factors.
Cell counting
Cell numbers and phenotypes were determined by flow cytometry, when cultures were established, and again after 7 days, as described.12 25-30 Briefly, stromal cultures were transferred to Falcon tubes (Becton Dickinson, San Jose, CA). B-lineage ALL cells were incubated with CD19 conjugated to fluorescein isothiocyanate (FITC) and CD3 conjugated to phycoerythrin (PE); in some experiments, cells were stained with CD19 PE and CD10 FITC. Normal CD34+ bone marrow cells were incubated with a combination of CD34 conjugated to peridin chlorophyll protein (PerCP), CD19 PE, and CD13 and CD33, both conjugated to FITC. All monoclonal antibodies and isotype-matched unreactive controls were purchased from Becton Dickinson, with the exception of CD19 PE, CD10 FITC, CD13 FITC, and CD33 FITC, which were purchased from DAKO (Carpinteria, CA). After they were washed twice in PBS with 0.2% BSA and 0.2% sodium azide, the cells were resuspended in 0.5% paraformaldehyde and analyzed with a FACScan flow cytometer and Cell Quest software (Becton Dickinson).
At the beginning of each culture, we set “gates” around the area of the light-scatter dot plot that included virtually all leukemic cells. These gates were used to count cells with the predetermined light-scattering properties present after culture with or without IL-4 (variant and native). These cell numbers were corrected for the percentage of cells in each sample expressing a given immunophenotype. Relative cell recovery after drug treatment was calculated by the formula (number of cells recovered with drug/number of cells recovered in parallel culture without drug) × 100. All results are reported as the means of at least duplicate experiments.
Colony-forming assays
Cord blood mononuclear cells were first suspended in Iscoves modified Dulbecco medium (BioWhittaker) and 2% FCS, and then in complete methylcellulose medium (Methocult GF H4434; StemCell Technologies, Vancouver, BC, Canada) at a 1:10 (vol/vol) ratio, to a final cell concentration of 5 × 103/mL and 2 × 104/mL. The methylcellulose medium contains 30% FCS and the following recombinant human growth factors: erythropoietin (3 U/mL), stem cell factor (50 ng/mL), granulocyte–monocyte colony-stimulating factor (10 ng/mL), and IL-3 (10 ng/mL). Petri dishes (35-mm; Nalge Nunc, Naperville, IL) were filled with 1.1 mL cell suspension. Triplicate cultures with BAY 36-1677 (25 ng/mL) and without the variant IL-4 were placed in an incubator set at 37°C, 5% CO2, and 90% humidity. Colonies (more than 50 cells) were scored after 14 days.
Determination of apoptosis
DNA content was analyzed as previously described31using the ModFit software (Becton Dickinson). DNA fragmentation was evaluated after cell permeabilization and staining with fluorescein–12-dUTP.32 To detect apoptosis, we also labeled phosphatidylserine residues exposed on the cell surface with FITC-conjugated annexin-V (Trevigen, Gaithersburg, MD),33following the manufacturer's instructions. In these experiments, cell membrane permeabilization was demonstrated by labeling cells with 5 μg/mL propidium iodide (Trevigen) for 15 minutes at 20°C.
Results
Antileukemic activity of BAY 36-1677
Most of the ALL cases selected for testing had genetic features that confer a poor prognosis—either t(9;22)(q34;q11) (n = 11) or 11q23 abnormalities with MLL gene rearrangements (n = 3). Additionally, in 3 of these patients, the samples were collected at the first or second relapse (Table 1).
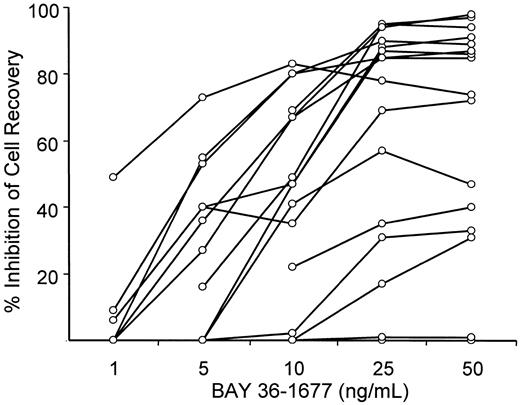
To maintain cell viability of leukemic lymphoblasts in vitro, we seeded them onto allogeneic bone marrow stromal layers, which suppress spontaneous apoptosis of ALL cells.27 34 After 7 days of culture, the number of leukemic lymphoblasts recovered from stromal layers ranged from 57% to 231% (median, 89.5%) of those originally seeded. Treatment with BAY 36-1677 (25 ng/mL) reduced the recovery of leukemic cells by 17% to 95% (median, 85%) compared with results of parallel cultures not exposed to the cytokine (Table 1). Only 2 of the cases (nos. 4 and 9) showed absolute resistance to the cytokine (less than 1% inhibition of cell recovery). The cytotoxic effect of BAY 36-1677 was dose dependent (Figure2).
BAY 36-1677 inhibits recovery of leukemic cells after culture in a dose-dependent manner.
Each point represents inhibition of cell recovery in 7-day stroma-supported cultures of leukemic lymphoblasts containing BAY 36-1677 at the indicated concentration relative to cell recovery in parallel control cultures without the cytokine. Values represent the means of the results of 2 cultures for each concentration.
BAY 36-1677 inhibits recovery of leukemic cells after culture in a dose-dependent manner.
Each point represents inhibition of cell recovery in 7-day stroma-supported cultures of leukemic lymphoblasts containing BAY 36-1677 at the indicated concentration relative to cell recovery in parallel control cultures without the cytokine. Values represent the means of the results of 2 cultures for each concentration.
Two of the 6 experimental cell lines studied (KOPN-57bi and OP-1), both carrying t(9;22)(q34;q11), were also susceptible to the IL-4 variant. After 7 days of culture, the respective reductions in cell recovery were 94.1% ± 2.3% (mean ± SD; n = 8) and 56.1% ± 9.8% (n = 6). The cytotoxic effects of BAY 36-1677 were less pronounced but readily apparent with the 380 cell line (29.0% ± 15.0%; n = 6), whereas among the remaining 3 cell lines (RS4;11, REH, and NALM6) cell killing was negligible. The growth-suppressive effects of BAY 36-1677 on KOPN-57bi, OP-1, and 380 were completely abrogated by an IL-4–neutralizing antibody (data not shown). All the leukemic cell lines tested (except REH) reacted with an antibody against the IL-4Rα chain (anti-CD124), which also abolished the cytotoxic effects of BAY 36-1677 (not shown). All cell lines, including REH, also reacted with an antibody against the IL-2Rγ chain (anti-CD132; not shown).
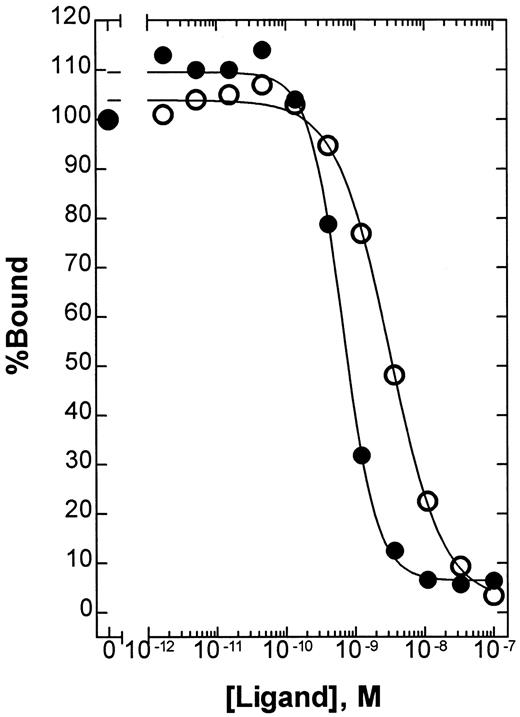
To determine the relative affinity of BAY 36-1677 and native IL-4 for IL-4Rα, we used a solid-phase competitive-binding assay. The absolute KI values were 2.8 × 10−9 M (95% confidence interval [CI], 2.3 to 3.5 × 10−9 M) for native IL-4 and 6.1 × 10−10 M (95% CI, 4.9 to 7.4 × 10−10 M) for BAY 36-1677 (Figure3). In this assay, the mutation Arg 121 to Glu alone had no effect on the relative affinity of native IL-4 (data not shown). Thus, the mutation Thr 13 to Asp provides a mean affinity enhancement of 4- to 5-fold.
Competitive binding of native IL-4 and BAY 36-1677.
The relative affinity of native IL-4 (○) and BAY 36-1677 (●) for IL-4Rα was compared using a solid-phase binding assay as described in “Patients, materials, and methods.” Using this assay format, the absolute KI values were calculated to be 2.8 × 10−9 M (95% CI, 2.3 to 3.5 × 10−9 M) and 6.1 × 10−10 M (95% CI, 4.9 to 7.4 × 10−10 M), respectively.
Competitive binding of native IL-4 and BAY 36-1677.
The relative affinity of native IL-4 (○) and BAY 36-1677 (●) for IL-4Rα was compared using a solid-phase binding assay as described in “Patients, materials, and methods.” Using this assay format, the absolute KI values were calculated to be 2.8 × 10−9 M (95% CI, 2.3 to 3.5 × 10−9 M) and 6.1 × 10−10 M (95% CI, 4.9 to 7.4 × 10−10 M), respectively.
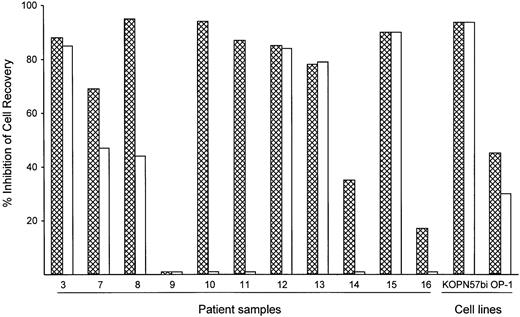
To assess the potency of BAY 36-1677 relative to that of its parent cytokine, we compared the cytotoxicity of BAY 36-1677 and native IL-4 (both at 25 ng/mL) against leukemic cells from 11 patients with ALL and 2 cell lines (KOPN57bi and OP-1). As shown in Figure4, BAY 36-1677 was cytotoxic in 10 of 11 ALL cases; neither BAY 36-1677 nor IL-4 had any detectable toxicity on cells from case 9. Remarkably, IL-4 lacked detectable cytotoxicity in 4 of the 10 cases susceptible to BAY 36-1677, 2 of whom were highly sensitive to BAY 36-1677 (nos. 10 and 11). IL-4 had markedly lower activity in 2 others compared to BAY 36-1677 (47% vs 69% cell kill for no. 7 and 44% vs 95% cell kill for no. 8). Both BAY 36-1677 and IL-4 exhibited similar cytotoxicity on the cell lines KOPN57bi and OP-1. We also tested whether IL-13, which signals through the IL-4Rα/IL-13Rα receptor, would exert a cytotoxic effect on immature B cells, similar to that described in a previous report.35Neither of the 2 BAY 36-1677–sensitive cell lines, KOPN57bi and OP-1, nor any of the resistant lines were affected by IL-13 at 60 ng/mL, a concentration 10-fold higher than the established ED50(data not shown). These results suggest that IL-4–induced cytotoxicity was not mediated by signaling through the IL-4Rα/IL-13Rα receptor.
Comparative cytotoxicity of BAY 36-1677 and native IL-4.
Bars represent the inhibition of recovery of leukemic lymphoblast after 7 days of culture on stroma with BAY 36-1677 (▩) or native IL-4 (■) (both at 25 ng/mL). The results of duplicate cultures with either cytokine were compared to control cultures without cytokines.
Comparative cytotoxicity of BAY 36-1677 and native IL-4.
Bars represent the inhibition of recovery of leukemic lymphoblast after 7 days of culture on stroma with BAY 36-1677 (▩) or native IL-4 (■) (both at 25 ng/mL). The results of duplicate cultures with either cytokine were compared to control cultures without cytokines.
Apoptotic cell death induced by BAY 36-1677
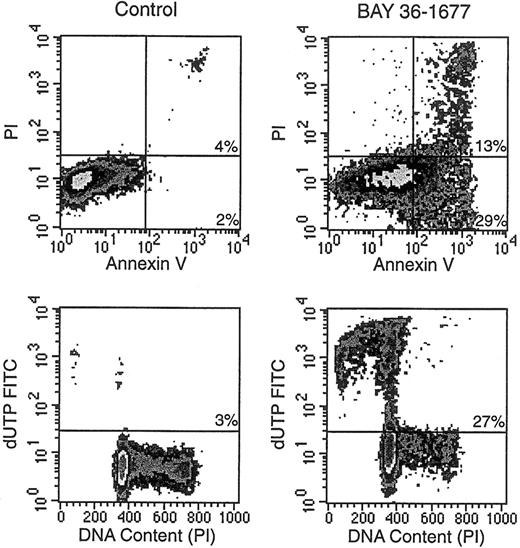
BAY 36-1677 consistently induced changes in the light-scattering properties of leukemic cells. These effects, which resembled those ascribed to antileukemic drugs that act by triggering apoptosis,32 consisted of decreased forward light scatter, indicative of a reduction in cell size, accompanied by increased orthogonal light scatter, indicative of augmented intracellular granularity (not shown). Microscopic changes in cell morphology, such as nuclear fragmentation in cells with apparently intact surface membranes, were also typical of apoptosis.32 Other reliable signs of apoptosis apparent after treatment with BAY 36-1677 included exposure of phosphatidyl serine residues on cell membranes by annexin V binding, massive DNA fragmentation demonstrated by incorporation of dUTP conjugated to FITC, and hypodiploidy detected by DNA content analysis (Figure5).32 33
BAY 36-1677 induces apoptosis in leukemic lymphoblasts.
KOPN57bi cells were exposed for 7 days to BAY 36-1677 (25 ng/mL) and then labeled with annexin V FITC and propidium iodide (PI; top panels). Flow cytometric density plots show binding of annexin V in BAY 36-1677–treated cells, indicating exposure of phosphatidylserine residues on the cell membrane (early stages of apoptosis), and PI labeling, indicating membrane permeabilization (late-stage cell death). Bottom panels illustrate DNA content analysis and staining with dUTP FITC, which labels cells with fragmented DNA. BAY 36-1677 caused DNA fragmentation and hypodiploidy, both characteristic of apoptosis.
BAY 36-1677 induces apoptosis in leukemic lymphoblasts.
KOPN57bi cells were exposed for 7 days to BAY 36-1677 (25 ng/mL) and then labeled with annexin V FITC and propidium iodide (PI; top panels). Flow cytometric density plots show binding of annexin V in BAY 36-1677–treated cells, indicating exposure of phosphatidylserine residues on the cell membrane (early stages of apoptosis), and PI labeling, indicating membrane permeabilization (late-stage cell death). Bottom panels illustrate DNA content analysis and staining with dUTP FITC, which labels cells with fragmented DNA. BAY 36-1677 caused DNA fragmentation and hypodiploidy, both characteristic of apoptosis.
Effects of BAY 36-1677 on the growth of normal hematopoietic cells
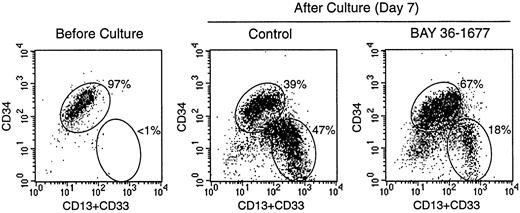
To test the effects of BAY 36-1677 on normal hematopoietic cells, we cultured CD34+ cells purified from cord blood on bone marrow stromal layers. Under these culture conditions, the cells expand and differentiate into myeloid cells at discrete stages of maturation.24 In 3 experiments, the total numbers of viable cells recovered after 7 days of culture in control conditions were 377%, 565%, and 654% of those originally seeded. Cell recovery in cultures containing BAY 36-1677 (25 ng/mL) was 103.3% ± 15.3% of control values. Although it did not substantially affect the total number of hematopoietic cells recovered, BAY 36-1677 appeared to have contrasting effects on different subpopulations of myeloid differentiation (Figure 6). Recovery of the most mature myeloid cells (strong expression of CD13 and CD33 but weak expression or absence of CD34) was reduced in cultures with BAY 36-1677 (52.1% ± 17.1%; n = 3), whereas recovery of the more immature cells (strong expression of CD34) was enhanced (158.4% ± 19.5%; n = 3) in control cultures.
Effect of BAY 36-1677 (25 ng/mL) on stroma-supported cultures of normal CD34+ cells from cord blood.
At the beginning of the culture (left panel), most cells had high CD34 expression. After 7 days of culture, maturing myeloid cells with CD13 and CD33 expression and low or absent CD34 appeared (center and right panels). The number of cells recovered in cultures with and without the variant IL-4 was identical, but immature cells with high CD34 expression were predominant with BAY 36-1677.
Effect of BAY 36-1677 (25 ng/mL) on stroma-supported cultures of normal CD34+ cells from cord blood.
At the beginning of the culture (left panel), most cells had high CD34 expression. After 7 days of culture, maturing myeloid cells with CD13 and CD33 expression and low or absent CD34 appeared (center and right panels). The number of cells recovered in cultures with and without the variant IL-4 was identical, but immature cells with high CD34 expression were predominant with BAY 36-1677.
The lack of suppressive effects on normal in vitro hematopoiesis was corroborated by results of colony-forming assays (Table2). BAY 36-1677 (25 ng/mL) did not suppress the formation of erythroid, myeloid, or mixed colonies derived from cord blood mononuclear cells. By contrast, in one experiment, erythroid colony formation was slightly but significantly enhanced by the variant IL-4 (Table 2).
Number of hematopoietic colonies formed from cord blood cells after 14 days of culture without and with BAY 36-1677 (25 ng/mL)
| Colony type* . | Experiment 1 . | Experiment 2 . | ||
|---|---|---|---|---|
| Control . | BAY 36-1677 . | Control . | BAY 36-1677 . | |
| Erythroid | 55.7 ± 5.4† | 66.0 ± 3.7 | 19.3 ± 2.6 | 26.7 ± 2.5 |
| Myeloid | 50.3 ± 4.5 | 40.0 ± 3.6 | 22.7 ± 4.2 | 23.7 ± 6.6 |
| Mixed | 6.7 ± 1.9 | 5.0 ± 0.8 | 2.0 ± 1.4 | 1.3 ± 0.5 |
| Total | 112.7 ± 6.0 | 111.0 ± 4.9 | 44.0 ± 6.4 | 51.7 ± 8.7 |
| Colony type* . | Experiment 1 . | Experiment 2 . | ||
|---|---|---|---|---|
| Control . | BAY 36-1677 . | Control . | BAY 36-1677 . | |
| Erythroid | 55.7 ± 5.4† | 66.0 ± 3.7 | 19.3 ± 2.6 | 26.7 ± 2.5 |
| Myeloid | 50.3 ± 4.5 | 40.0 ± 3.6 | 22.7 ± 4.2 | 23.7 ± 6.6 |
| Mixed | 6.7 ± 1.9 | 5.0 ± 0.8 | 2.0 ± 1.4 | 1.3 ± 0.5 |
| Total | 112.7 ± 6.0 | 111.0 ± 4.9 | 44.0 ± 6.4 | 51.7 ± 8.7 |
In experiment 1, cells were plated at a concentration of 2 × 104/mL; in experiment 2, they were plated at a concentration of 5 × 103/mL.
BFU-E indicates burst-forming unit-erythroid; CFU-E, colony-forming unit-erythroid; -G, granulocyte; -M, monocyte; -GM, granulocyte/monocyte; -GEMM, granulocyte/erythroid/monocyte/megakaryocyte.
Erythroid indicates BFU-E and CFU-E; myeloid, CFU-G, CFU-M, and CFU-GM; mixed, CFU-GEMM.
Mean (± SD) number of colonies from 3 culture dishes. Differences are not statistically significant, except for measurement of erythroid colonies in experiment 2 (P < .05 by ttest).
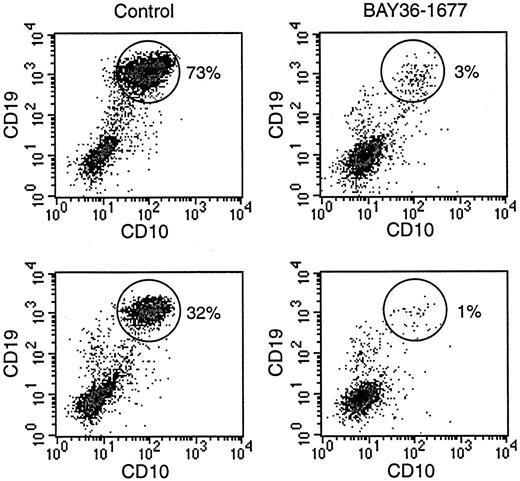
To directly compare the effects of BAY 36-1677 on normal and leukemic cells, we prepared mixtures of the cell line KOPN57bi and normal CD34+ cells (Figure 7). With mixtures containing 20% leukemic cells and 80% normal cells, the proportion of leukemic cells increased to 73% after 7 days of culture. The addition of BAY 36-1677 (25 ng/mL) markedly changed the outcome of the cultures: the percentage of leukemic cells was reduced to 3%. In 2 experiments with a lower starting proportion of leukemic cells (5%), this value increased to 32% and 20% after 7 days of culture in the absence of BAY 36-1677, but it dropped to 1% and 0.8% when the cytokine was present. These experiments were repeated with slower-growing primary ALL cells (no. 5, Table 1). Results were similar. In experiments with a starting population of 35% leukemic cells, these represented 40% of cells after 7 days of culture without BAY 36-1677 and 5% in cultures with the variant IL-4. With a lower starting proportion of leukemic cells (15%), this value increased to 25% without BAY 36-1677 and decreased to 2% with BAY 36-1677.
BAY 36-1677 confers a growth advantage to normal hematopoietic cells.
Normal CD34+ cells were mixed with leukemic KOPN57bi cells (20%, top; 5%, bottom) and cultures for 7 days on stroma. Flow cytometric density plots illustrate that in control cultures (left panels), proportions of ALL cells (identified by their high CD19 and CD10 expression; circles) increased, whereas these decreased in the presence of BAY 36-1677 (25 ng/mL; right panels).
BAY 36-1677 confers a growth advantage to normal hematopoietic cells.
Normal CD34+ cells were mixed with leukemic KOPN57bi cells (20%, top; 5%, bottom) and cultures for 7 days on stroma. Flow cytometric density plots illustrate that in control cultures (left panels), proportions of ALL cells (identified by their high CD19 and CD10 expression; circles) increased, whereas these decreased in the presence of BAY 36-1677 (25 ng/mL; right panels).
Effects of BAY 36-1677 on endothelial cells and fibroblasts
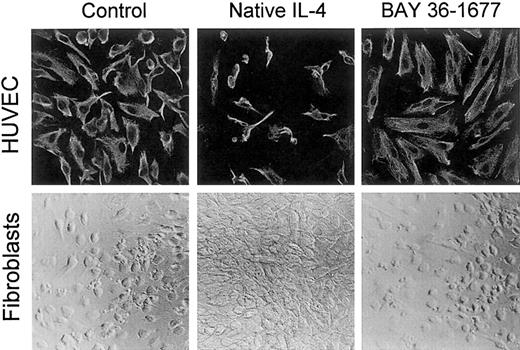
In contrast to its toxic effects on leukemic cells, BAY 36-1677 did not affect the growth or the appearance of umbilical cord–derived endothelial cells, even when added to the cultures at 100 ng/mL. Native IL-4, on the other hand, markedly suppressed cell growth and altered cell appearance (Figure 8), as previously described.36 A similar effect was produced by IL-13 (not shown).36 This suggests that, in endothelial cells, IL-4Rα/IL-13Rα receptors likely mediate the inhibitory signals of IL-4.
BAY 36-1677 does not affect the growth of endothelial cells and fibroblasts.
Native IL-4 and BAY 36-1677, both at 100 ng/mL, were added to cultures of HUVECs (top panels) for 1 week and skin fibroblasts (bottom panels) for 3 weeks. HUVECs were labeled with a mixture of antivimentin antibody and actin-binding phalloidin after culture. Native IL-4 impaired the growth of endothelial cells and stimulated fibroblast proliferation.
BAY 36-1677 does not affect the growth of endothelial cells and fibroblasts.
Native IL-4 and BAY 36-1677, both at 100 ng/mL, were added to cultures of HUVECs (top panels) for 1 week and skin fibroblasts (bottom panels) for 3 weeks. HUVECs were labeled with a mixture of antivimentin antibody and actin-binding phalloidin after culture. Native IL-4 impaired the growth of endothelial cells and stimulated fibroblast proliferation.
Both native IL-4 and IL-13 stimulated fibroblast proliferation, whereas BAY 36-1677 (100 ng/mL) lacked any discernible effect on fibroblast proliferation or morphology (Figure 8). These results are consistent with the ability of IL-4 and IL-13 to induce signal through the IL-4Rα/IL-13Rα receptor complex,37 38 whereas BAY 36-1677 does not promote a signal through this receptor.
Discussion
One of the important objectives of modern cancer research is to develop molecules capable of suppressing cancer cell growth while sparing normal cells. In this study, we tested the cytotoxicity of a molecularly engineered IL-4 variant, BAY 36-1677, against cells from patients with ALL, the most common form of cancer in children. The cytokine variant induced apoptosis in leukemic cells from patients with high-risk leukemia but did not suppress the growth of normal hematopoietic cells. Consequently, BAY 36-1677 conferred a growth advantage to normal hematopoietic cells over leukemic lymphoblasts in cultures designed to test the growth potential of each cell type. By contrast, in control cultures lacking BAY 36-1677, the leukemic cells prevailed, recapitulating the fate of patients with residual aggressive leukemia. BAY 36-1677 was considerably more cytotoxic than native IL-4. We speculate that this is owing to its higher affinity for the IL-4Rα receptor, resulting in a more potent signal.
Early findings of IL-4–mediated killing of leukemic lymphoblasts12 raised the possibility that this cytokine could be a useful addition to ALL treatment protocols. When administered in tolerable doses to patients with solid tumors to boost tumor immune surveillance, IL-4 reached serum levels that would be expected to trigger apoptosis in leukemic cells,14,16supporting a therapeutic role for this agent in patients with ALL. There were, however, several side effects that could prevent the use of IL-4 at higher, potentially more effective, doses.14-19Capillary leak syndrome, presumably caused by IL-4 signaling to endothelial cells, was among the most severe toxicities observed.14,16 IL-4 also induces the release of proinflammatory cytokines from fibroblasts,38 which could contribute to multi-organ toxicity. The amino acid substitutions introduced in the IL-4 molecule to generate BAY 36-1677 were specifically chosen to eliminate activation of the IL-4Rα/IL-13Rα receptor, which is expressed by both endothelial cells and fibroblasts.21 22 The lack of any discernible effect of BAY 36-1677 on endothelial cells and fibroblasts in the current study substantiates the predicted outcome of the amino acid change and suggests that clinical testing of BAY 36-1677 would not be limited by serious toxicities involving these cell types.
IL-4 is toxic not only for leukemic immature B cells but also for their normal human and murine counterparts.12,39 The toxicity toward murine immature B cells was previously thought to be the indirect outcome of IL-4–induced secretion of unknown factors from stroma.39 Although leukemic cells from patients were maintained on bone marrow stroma in the current study, we doubt that they were killed through indirect actions of the cytokine. First, BAY 36-1677 does not activate the IL-4Rα/IL-13Rα receptor expressed on stromal fibroblasts and did not cause detectable cellular changes in the stromal layers. Second, in previous studies, we found no indication that stroma treated with native IL-4 produced factors with antileukemic activity.12 Finally, the proapoptotic effects of BAY 36-1677 and IL-4 were also seen in ALL cells growing without stromal cells. The relation between susceptibility to the suppressive effects of BAY 36-1677 and the amounts of and affinity for IL-4Rα/IL-2Rγ receptors warrants further investigation. It is possible that the complete lack of BAY 36-1677 (and IL-4) cytotoxicity in 2 of the 16 cases studied resulted simply from a lack of functional IL-4Rα/IL-2Rγ receptors. Indeed, we found that REH cells, which were insensitive to BAY 36-1677, lacked IL-4Rα (CD124) expression. However, 2 other insensitive cell lines expressed IL-4Rα (CD124) and IL-2Rγ (CD132). Conceivably, in these cells, the 2 receptor chains could be expressed separately without forming a functional IL-4Rα/IL-2Rγ receptor. Alternatively, additional signaling components beyond the surface expression of the IL-4 receptor complex may determine the susceptibility to the cytokine.40
Our findings demonstrate that molecular manipulation can be used to increase the specificity and potentially improve the therapeutic index of anticancer compounds. Corticosteroids, microtubule poisons, topoisomerase inhibitors, and DNA synthesis inhibitors are the most commonly used classes of antileukemic drugs.1 With the possible exception of corticosteroids, none of these agents are selective for leukemic cells, and their toxicity extends to diverse types of normal hematopoietic cells and to nonhematopoietic cells. The results reported here suggest that BAY 36-1677 might be a useful addition to contemporary treatment regimens for ALL. With its strong selectivity toward leukemic cells, BAY 36-1677 could be safely introduced in patients with minimal residual disease after remission induction therapy to suppress leukemia cell growth and to aid the expansion of normal hematopoietic cells.
Acknowledgments
We thank the Roczniak Lab (Bayer Biotechnology) for technical contributions to the development of the IL-4 binding assay, and we thank Elaine Coustan-Smith for flow cytometric analysis of apoptosis.
Supported by National Cancer Institute grants RO1-CA58297 and P30-CA21765 and by the American Lebanese Syrian Associated Charities.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Dario Campana, Department of Hematology-Oncology, St Jude Children's Research Hospital, 332 North Lauderdale, Memphis, TN 38105-2794; e-mail: dario.campana@stjude.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal