Abstract
Chemokines and their receptors control the emigration of leukocytes during inflammation. The role of the RANTES (regulated on activation normal T-cell expressed and secreted) receptors CCR1 and CCR5 in the selective recruitment of monocytes, TH1-like T-cell clones, and peripheral T cells enriched for CD45RO+“memory” cells were tested in a system in which arrest under flow conditions is triggered by RANTES immobilized to activated endothelium. With the use of selective nonpeptide receptor antagonists or blocking antibodies, it was found that the RANTES-induced arrest of these cells was mediated predominantly by CCR1. In contrast, CCR5 mainly contributed to the spreading in shear flow, and both CCR1 and CCR5 supported transendothelial chemotaxis toward RANTES. The data in this study reveal specialized roles of apparently redundant receptors in distinct steps of leukocyte trafficking and suggest that not all receptors currently used to define mononuclear cell subsets are involved in their direct recruitment from the circulation.
Introduction
Leukocyte trafficking involves the sequential and coordinated activation of multiple adhesion and signal molecules.1 The expression of chemokines and the presence of specific chemokine receptors on different leukocyte subsets control selective recruitment of immune effector cells from the peripheral circulation.2,3 By virtue of their differential immobilization, a hierarchical involvement of chemokines and their receptors has been defined for distinct steps of the extravasation cascade, eg, arrest versus diapedesis.4-8 The CC chemokine RANTES (regulated on activation normal T-cell expressed and secreted) is produced by inflamed tissue or released by stimulated platelets; it triggers firm monocyte arrest in flow when bound to activated human microvascular endothelial cells (HMVECs),3,8 illustrating how the mode of endothelial presentation may determine chemokine function. Specific chemokine receptor expression is associated with different leukocyte subsets, including effector T-cell subpopulations.9-11 Moreover, chemokines can act via multiple receptors; eg, RANTES activates CCR1, CCR3, and CCR5.3 We investigated the significance of this redundancy with regard to the role of CCR1 and CCR5 in the recruitment of monocytes and TH1-like T cells. A specialized involvement of these receptors in distinct steps of the leukocyte cascade is shown.
Study design
Cell culture, monoclonal antibodies, reagents
HMVECs and TH1-like T-cell clones 305 and 411 were cultured as described.8,12 Monocytes and peripheral blood mononuclear cells (PBMCs) were isolated by NycoPrep 1.068 (Nycomed, Asker, Norway) and Ficoll hypaque density gradient centrifugation.13,14CD45RO+CD4+ T lymphocytes were purified by negative selection by means of magnetic separation (Miltenyi Biotec, Bergisch Gladbach, Germany). Human recombinant interleukin (IL)–1β and RANTES were from PeproTech (Rocky Hill, NC), CCR1 monoclonal antibody (mAb) and CCR5 mAb 2D7/CCR5 were from R&D Systems (Wiesbaden, Germany) or Pharmingen (Hamburg, Germany). The small molecule antagonists BX471 (to CCR1)15 and TAK-779 (to CCR5)16 (kindly provided by Takeda Chemical Industries) have been described.
Reverse transcription–polymerase chain reaction and flow cytometry
Total RNA was isolated, and reverse transcription-polymerase chain reaction (RT-PCR) was performed for transcripts encoding CCR1, CCR3, CCR4, and CCR5 by means of Cytoxpress Multiplex PCR system protocols (Biosource International, Nivelles, Belgium). Flow cytometric analysis, receptor internalization, and recycling were performed as described.17-19
Leukocyte adhesion under flow conditions
Laminar flow assays were performed as described.7,20 21 Confluent HMVECs activated with IL-1β (10 ng/mL) were preincubated with RANTES or macrophage inflammatory protein (MIP)–1β (1 nM for monocytes, 10 nM for T cells). Leukocytes (5 × 105/mL) in assay buffer (Hanks' balanced salt solution, 10 mM Hepes, 0.5% bovine serum albumin [BSA], 1 mM Mg2+/Ca++) were perfused at 1.5 dyne/cm2 after pretreatment with dimethyl sulfoxide (DMSO), nonpeptide antagonists (10 μM), blocking CCR5 mAb, immunoglobulin (Ig)–G2a (10 μg/mL), or 9-76MCP-1 (1 μM) for 20 minutes. These concentrations were maintained during assays. Cell arrest and spreading were analyzed in multiple high-power fields recorded by video microscopy.
Transmigration and static adhesion assays
Assays were performed as described.13,14 HMVECs were grown on Transwell-filter inserts (Co-star, Corning, Wiesbaden, Germany). PMBCs or T cells in RPMI-1640/0.5% BSA were allowed to transmigrate at 37°C for 90 minutes toward RANTES (100 ng/mL) in the presence of blocking CCR5 mAb, IgG2a (10 μg/mL), or antagonists (100 nM). Transmigrated monocytes or T cells and input were counted by fluorescence-activated cell sorting (FACS) with standard beads. The blocking efficiency of CCR5 mAb was confirmed in assays using filters coated with intercellular adhesion molecule 1 (ICAM-1) (data not shown). Static adhesion assays on purified ICAM-1 were performed as described.17
Results and discussion
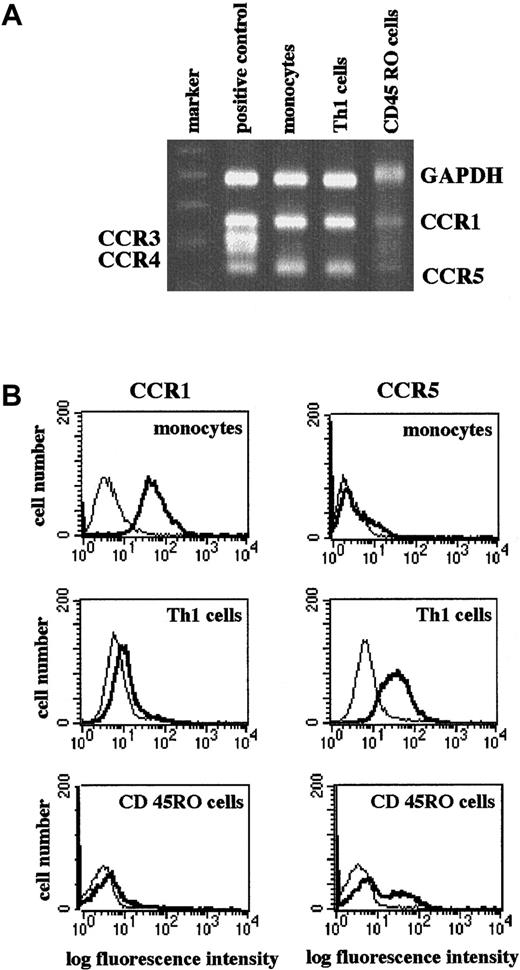
The RANTES receptors CCR1 and CCR5 are differentially expressed on leukocyte subsets.9-11,18 Human blood monocytes express high levels of CCR1 and low levels of CCR5 (Figure1A-B). By contrast, CD45RO+CD4+ T-cell clones of the TH1-subset and freshly isolated CD45RO+“memory” T cells in general express low levels of CCR1 and high levels of CCR5 (Figure 1A-B). As shown previously,22 CCR3, a RANTES receptor associated with TH2-like cells or eosinophils, was not expressed by monocytes or the TH1-like clones. A slightly detectable expression of CCR3 was seen in the CD45RO+-enriched T cells (Figure 1A). The marked differences in CCR1 and CCR5 expression on monocytes and TH1-like/CD45RO+ T cells suggest a selective use of these receptors during inflammatory recruitment of these leukocyte subtypes.
Differential expression of CCR1 and CCR5 on monocytes, TH1 cells, and memory T cells.
RNA was extracted from monocytes, TH1-like cells (clone 305), or purified CD45RO+CD4+ T cells and subjected to RT-PCR. PCR products were analyzed by agarose gel electrophoresis with positive control. Complementary DNA (cDNA) for CCR1-5 was provided in the CytoXpress Multiplex-PCR kit. Shown is a representative gel (A). Cells were stained with isotype control (dotted line) or specific CCR mAbs (solid line) and analyzed in a FACScan. Shown are representative histograms (B).
Differential expression of CCR1 and CCR5 on monocytes, TH1 cells, and memory T cells.
RNA was extracted from monocytes, TH1-like cells (clone 305), or purified CD45RO+CD4+ T cells and subjected to RT-PCR. PCR products were analyzed by agarose gel electrophoresis with positive control. Complementary DNA (cDNA) for CCR1-5 was provided in the CytoXpress Multiplex-PCR kit. Shown is a representative gel (A). Cells were stained with isotype control (dotted line) or specific CCR mAbs (solid line) and analyzed in a FACScan. Shown are representative histograms (B).
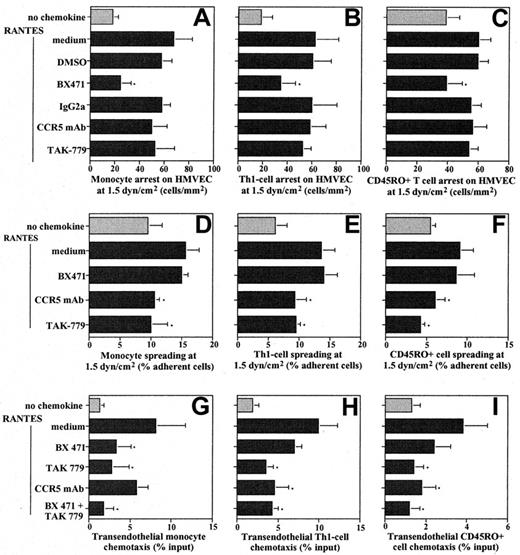
Since the redundancy of receptors for one chemokine implies a specialized involvement in leukocyte emigration, we studied the functional contributions of CCR1 and CCR5 in recruitment of human blood monocytes and TH1-like/CD45RO+ T cells mediated by RANTES bound to inflamed microvascular endothelium under flow conditions. Preincubation of IL-1β–activated HMVECs with RANTES increased shear-resistant monocyte arrest at 1.5 dyne/cm2, which was significantly and dose-dependently inhibited by pretreatment of monocytes with BX471, a nonpeptide CCR1 antagonist15(Figure 2A, not shown). By contrast, TAK-779, a nonpeptide CCR5 antagonist,16 or a blocking CCR5 mAb did not inhibit arrest (Figure 2A). To test whether the predominance of CCR1 in arrest also prevails in cells with abundant CCR5 expression, contributions of these receptors were determined in TH1-like cells and peripheral blood CD45RO+ T cells. As for monocytes, blocking CCR1 but not CCR5 resulted in a significant inhibition of RANTES-induced arrest of these cells in flow (Figure 2B-C). Static assays confirmed that the adhesion of CD45RO+ T cells to ICAM-1 rapidly induced by RANTES co-immobilized at 100 nM (29.5% ± 4.2% of input, n = 6) was significantly blocked by the CCR1 antagonist (13.8% ± 4.5% of input, P ≤ .01) but not the CCR5 antagonist (22.3% ± 5.5% of input), while resting adhesion was unaffected (not shown). Thus, RANTES triggers firm arrest of monocytes and TH1-cells/CD45RO+ cells primarily by engaging CCR1.
Roles of CCR1 and CCR5 in leukocyte recruitment.
Leukocytes were preincubated with CCR5 mAb or IgG2a isotype control (10 μg/mL), CCR1 antagonist BX471,13 CCR5 antagonist TAK-779 at 10 μM in flow assays or at 100 nM in chemotaxis assays, or DMSO (0.1% vol/vol). Monocytes (panels A, D), TH1-cells (clone 305, panels B, E), or CD45RO+CD4+ memory T cells (panels C, F) were perfused on IL-1β–activated HMVECs left untreated (open bars) or pretreated with RANTES (solid bars) in a flow chamber at 1.5 dyne/cm2. After 5 minutes, firmly adherent cells were counted and expressed as cells per square millimeter (panels A-C). Cells undergoing shape change were analyzed and expressed as a percentage of adherent cells (panels D-F). Data represent mean ± SD of 6 to 8 separate experiments. * indicates P < .05 vs RANTES control (nonparametric signed-rank tests). Both CCR1 and CCR5 mediate transendothelial chemotaxis of leukocytes. Monocytes (panel G), TH1-cells (panel H), or CD45RO+CD4+ memory T cells (panel I) were subjected to chemotaxis assays for 90 minutes with RANTES (100 ng/mL) in the lower chamber. Transmigrated cells were counted by FACS and expressed as a percentage of input (panels G-I). DMSO or IgG2a had no effect. Data are mean ± SD of 4 to 6 separate experiments.
Roles of CCR1 and CCR5 in leukocyte recruitment.
Leukocytes were preincubated with CCR5 mAb or IgG2a isotype control (10 μg/mL), CCR1 antagonist BX471,13 CCR5 antagonist TAK-779 at 10 μM in flow assays or at 100 nM in chemotaxis assays, or DMSO (0.1% vol/vol). Monocytes (panels A, D), TH1-cells (clone 305, panels B, E), or CD45RO+CD4+ memory T cells (panels C, F) were perfused on IL-1β–activated HMVECs left untreated (open bars) or pretreated with RANTES (solid bars) in a flow chamber at 1.5 dyne/cm2. After 5 minutes, firmly adherent cells were counted and expressed as cells per square millimeter (panels A-C). Cells undergoing shape change were analyzed and expressed as a percentage of adherent cells (panels D-F). Data represent mean ± SD of 6 to 8 separate experiments. * indicates P < .05 vs RANTES control (nonparametric signed-rank tests). Both CCR1 and CCR5 mediate transendothelial chemotaxis of leukocytes. Monocytes (panel G), TH1-cells (panel H), or CD45RO+CD4+ memory T cells (panel I) were subjected to chemotaxis assays for 90 minutes with RANTES (100 ng/mL) in the lower chamber. Transmigrated cells were counted by FACS and expressed as a percentage of input (panels G-I). DMSO or IgG2a had no effect. Data are mean ± SD of 4 to 6 separate experiments.
Expression of CCR5 on mononuclear-cell infiltrates implies a role in events following arrest.3 Shape change of monocytes or TH1-like/CD45RO+ cells, evident as spreading or polarization in flow was consistently reduced by CCR5 mAb or TAK-779 but not by BX471 (Figure 2D-F). Inhibition was incomplete, indicating that additional chemokine-receptor pairs participated. Moreover, blocking CCR1, CCR5, or both inhibited RANTES-induced transendothelial chemotaxis of monocytes, TH1-like T-cell clones, or the CD45RO+-enriched T cells (Figure 2G-I). This implies that after primary exposure to a RANTES gradient, both receptors can support chemotaxis and that blocking either arrest or spreading may be sufficient to inhibit diapedesis, as proposed previously.23 While the less effective inhibition of monocyte chemotaxis with CCR5 mAb may be due to interactions with Fc receptors, the more pronounced inhibition by BX471 may reflect higher CCR1 expression (Figure 2G). Indeed, monocytes express more CCR1 than CCR5, which may contribute to differential roles in arrest vs spreading. However, in T cells that express more CCR5 than CCR1, arrest was also primarily dependent on CCR1, confirming that leukocyte recruitment involves a functional specialization of CCRs. The CD45RO+ T-cell population consists of diverse subtypes, including a minor subset of CCR3+ T cells. Because this subset is relatively small and the effects of blocking CCR1 are so pronounced, CCR3 is unlikely to account for relevant effects in our assays. Hence, shear-resistant arrest is preferentially mediated by CCR1, while CCR5 participates in spreading and transmigration, revealing distinct functions for different RANTES receptors during leukocyte recruitment.
Binding of chemokines can lead to internalization and recycling of their receptors. Consistent with recent findings,18 both CCR1 and CCR5 were internalized after exposure of the above cell types to RANTES, while CCR5 but not CCR1 recycled after removal of RANTES (not shown). A dynamic integrin regulation by chemokines facilitates lateral and transendothelial migration of leukocytes.13 14Hence, recycling of CCR5 may enable it to respond repeatedly to gradients supporting migration, while persistent down-regulation of CCR1 may be consistent with a role in arrest. Thus, differential CCR recycling may be involved in leukocyte trafficking, while CCR5 may also participate in subsequent events, eg, affecting activity or survival of emigrated cells.
Migrating leukocytes sort through multiple chemotactic signals to reach their destination, as proposed by a combinatorial model.24By enabling high receptor occupancy, immobilized chemokines may prefererentially trigger leukocyte arrest on vascular endothelium, while for transmigration, leukocytes may respond to finely tuned receptor engagement supported by chemokine gradients.7Extending this concept, our data reveal specialized roles of CCRs supporting distinct steps of leukocyte extravasation in response to a shared agonist, which may be presented in both an immobilized and a soluble form. Acknowledging specific functions of chemokine receptors may help to identify antagonists selectively targeting inflammatory recruitment of leukocyte subsets.
Acknowledgments
We thank Profs P.C. Weber and D. Schlöndorff for support and Nina Gellert for expert technical assistance.
Supported by Deutsche Forschungsgemeinschaft grants We-1913/2 (C.W.), GrK-438 (K.S.C.W.), and SFB-464/469 (P.J.N.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Christian Weber, Institut für Prophylaxe der Kreislaufkrankheiten, Pettenkoferstrasse 9, D-80336 Munich, Germany; e-mail: christian.weber@klp.med.uni-muenchen.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal