Abstract
Macrothrombocytopenia with leukocyte inclusions is a rare autosomal dominant platelet disorder characterized by a triad of giant platelets, thrombocytopenia, and characteristic Döhle body-like leukocyte inclusions. A previous study mapped a locus for the disease on chromosome 22q12.3-q13.2 by genome-wide linkage analysis. In addition, the complete DNA sequence of human chromosome 22 allowed a positional candidate approach, and results here indicate that the gene encoding nonmuscle myosin heavy chain-A, NMMHC-A, is mutated in this disorder. Mutations were found in 6 of 7 Japanese families studied: 3 missense mutations, a nonsense mutation, and a one-base deletion resulting in a premature termination. Immunofluorescence studies revealed that NMMHC-A distribution in neutrophils appeared to mimic the inclusion bodies. These results provide evidence for the involvement of abnormal NMMHC-A in the formation of leukocyte inclusions and also in platelet morphogenesis.
Introduction
May-Hegglin anomaly (MHA; MIM 155100) is a rare autosomal dominant platelet disorder with normal biochemical features of platelets content.1-6 Basophilic leukocyte inclusion body is another feature of MHA and Sebastian syndrome appears to be differentiated from MHA by ultrastructural features of leukocyte inclusions.7 We have taken a positional cloning approach and mapped a locus for MHA on chromosome 22q12.3-q13.2 by genome-wide linkage analysis.8 Subsequently, the critical region was defined as the interval between D22S683 and D22S1177 (0.7 Mb).9,10 Finally the complete genomic DNA sequence of human chromosome 2211 allows us to identify that the gene encoding nonmuscle myosin heavy chain-A, NMMHC-A(MYH9), is mutated. After our study was completed, 2 papers describing the identification of NMMHC-A mutations in this disorder have recently been reported.12 13 We further identify that the cellular distribution of NMMHC-A is abnormal in these patients.
Study design
Families
This study included 7 Japanese families with autosomal dominant macrothrombocytopenia with leukocyte inclusions (MHA/Sebastian syndrome). Families IN and WA have been described elsewhere.8 14 The diagnosis was made by a triad of giant platelets, thrombocytopenia, and leukocyte inclusions, in hematomorphologic examinations. In addition, family members were screened for the presence or absence of abnormal hemostasis, renal disease, or hearing disability. None had a clinical history of a renal insufficiency or deafness. Each individual gave informed consent for the study.
Mutation detection
Genomic DNA was extracted from peripheral blood leukocytes.8 Based on the NMMHC-A genomic DNA sequence (GenBank AL022302 and Z82215),11 we designed intronic primers to amplify the entire coding sequence and exon-intron boundaries (sequences available on request). The NMMHC-Acomplementary DNA (cDNA) sequence numbered as the first ATG is +1. Polymerase chain reaction (PCR) was performed and the amplified DNA was subjected to direct sequence analysis as described.15 We performed restriction analysis to confirm the presence of mutations in the other family members and to examine 170 unrelated control subjects (methods available on request).
Immunofluorescence and immunoblot analysis
For immunofluorescence staining, peripheral blood samples were smeared on glass slides and air dried. After being permeabilized with acetone, the cells were hydrated and incubated with anti-NMMHC-A polyclonal antibody (Biomedical Technologies, Stoughton, MA). Slides were then incubated with rhodamine-labeled swine antirabbit IgG (DAKO, Glostrup, Denmark). The stained cells were analyzed by confocal laser scanning microscopy (MRC-1024, BioRad, Richmond, CA). Immunoblot analysis was performed on solubilized platelet lysates from affected patients in families IN, MA and MU as described.16
Results and discussion
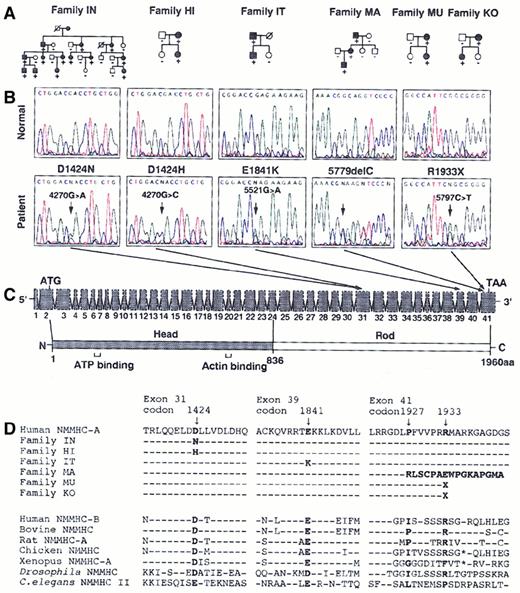
According to the chromosome 22 sequence, a number of candidate genes are expressed within the MHA critical region, includingCACNG2, DNAL4, EIF3S7, HPS,NMMHC-A, NCF4, PVALB, andTXN2.11 We assumed that NMMHC-A is a strong candidate because it is exclusively expressed in platelets and granulocytes and its transcription is up-regulated in the course of hematopoietic differentiation.17-19NMMHC-Acontains 41 exons with a predicted open reading frame of 5883 bp. We sequenced the entire coding regions and exon-intron boundaries from 7 families. In 6 families, we found 5 heterozygous mutations inNMMHC-A that cosegregated with the disease phenotype in each of the families (Figure 1A,B). Three missense mutations, D1424N, D1424H, and E1841K were found in families IN, HI, and IT, respectively (Figure 1B). In family MA, there is a one-base deletion (5779delC), which would result in a frameshift and premature termination. In 2 unrelated families, MU and KO, we found a nonsense mutation (R1933X).
NMMHC-A mutational analysis.
(A) Affected or unaffected individuals are represented by filled or open symbols, respectively. Mutation status indicated by −, wild-type homozygote; +, mutant heterozygote. (B) The portions of the representative electropherograms illustrate the 5 heterozygous nucleotide changes. Forward sequence is shown from families IN, HI, and IT, and reverse sequence from families MA, MU, and KO. In each panel, the normal sequence is shown at the top, and the mutated sequence is shown at the bottom. The mutated position is indicated by arrows. (C) Schematic representations of 41 exons of NMMHC-A are shown at the top, and the predicted amino acid of NMMHC-A at the bottom. The amino terminal globular head domain is shaded and the carboxyl terminal rod domain is not. The transcription initiation codon (ATG), natural stop codon (TAA), ATP-binding domain and actin-binding domain are indicated. (D) NMMHC-A sequence alignment. Amino acid sequence alignment is shown from the 2 human NMMHC isoforms and the known NMMHC from the other species. The amino acid alterations in each of the 6 families are indicated in bold. D1424N, D1424H, E1841K, and R1933X mutations occur at highly conserved residues of the protein. In family MA, 5779delC mutation causes a frameshift and a premature termination at 20 amino acids downstream of the mutation.
NMMHC-A mutational analysis.
(A) Affected or unaffected individuals are represented by filled or open symbols, respectively. Mutation status indicated by −, wild-type homozygote; +, mutant heterozygote. (B) The portions of the representative electropherograms illustrate the 5 heterozygous nucleotide changes. Forward sequence is shown from families IN, HI, and IT, and reverse sequence from families MA, MU, and KO. In each panel, the normal sequence is shown at the top, and the mutated sequence is shown at the bottom. The mutated position is indicated by arrows. (C) Schematic representations of 41 exons of NMMHC-A are shown at the top, and the predicted amino acid of NMMHC-A at the bottom. The amino terminal globular head domain is shaded and the carboxyl terminal rod domain is not. The transcription initiation codon (ATG), natural stop codon (TAA), ATP-binding domain and actin-binding domain are indicated. (D) NMMHC-A sequence alignment. Amino acid sequence alignment is shown from the 2 human NMMHC isoforms and the known NMMHC from the other species. The amino acid alterations in each of the 6 families are indicated in bold. D1424N, D1424H, E1841K, and R1933X mutations occur at highly conserved residues of the protein. In family MA, 5779delC mutation causes a frameshift and a premature termination at 20 amino acids downstream of the mutation.
Each mutant allele was expressed at the messenger RNA (mRNA) level as demonstrated by reverse transcription-PCR and subsequent sequence analysis or restriction analysis on platelet mRNA (not shown). Neither of these sequence alterations was found in 170 unrelated control subjects. The coding sequences of the other 7 candidate genes in affected patients in families IN and MA were normal. Sequence analysis did not reveal nonsynonymous sequence alterations in NMMHC-Ain family WA, suggesting a genetic heterogeneity for this disorder. This family is composed of 11 members and a maximum 2-point lod score of 1.81 was obtained for markers D22S278, D22S277, D22S283, and D22S272, at a recombination fraction of 0.00.
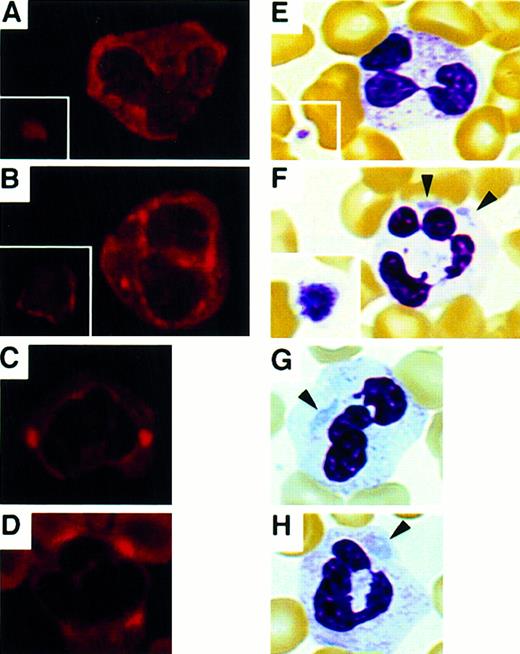
Immunoblot analysis of the platelet lysates showed NMMHC-A is present in the patients' platelets, but no abnormal migrating band was observed (not shown). We then performed immunofluorescence analysis and studied NMMHC-A localization. Normal platelets and leukocytes contain NMMHC-A, and Maupin and colleagues have shown that it is diffusely distributed in the cytoplasm of leukocytes.19 In our study, Figure 2A indicated a similar intracellular localization of NMMHC-A from control subjects. In the patients, NMMHC-A was localized circumferentially as a ring and in punctuated spots at the cell periphery in platelets and neutrophils, respectively (Figure 2B-D). This pattern appeared to mimic the leukocyte inclusion bodies observed in the patients (Figure 2F-H). In lymphocytes, however, NMMHC-A was diffusely localized in the cytoplasm both in the controls and patients (not shown). These findings strongly suggest that the aberrant NMMHC-A localization is related to the formation of neutrophil inclusions. Normal NMMHC distribution in the patients' lymphocytes is consistent with the finding that inclusion bodies are not found in lymphocytes. Although bone marrow specimens could not be obtained, a similar approach to patients' megakaryocytes might reveal the role of NMMHC-A for the formation and release of large platelets.
NMMHC-A localization in platelets and neutrophils.
Immunofluorescence micrographs of peripheral blood smears stained with anti-NMMHC-A antibody (A-D) and May-Grünwald-Giemsa-stained smears (E-H). (A,E) Control platelets (inset) and neutrophils. (B,F) Platelets (inset) and neutrophils of a patient from family IN. (C,G) Neutrophils of a patient from family MA. (D,H) Neutrophils of a patient from family MU. Inclusion bodies in neutrophils are indicated by arrowheads. (Original magnification × 400)
NMMHC-A localization in platelets and neutrophils.
Immunofluorescence micrographs of peripheral blood smears stained with anti-NMMHC-A antibody (A-D) and May-Grünwald-Giemsa-stained smears (E-H). (A,E) Control platelets (inset) and neutrophils. (B,F) Platelets (inset) and neutrophils of a patient from family IN. (C,G) Neutrophils of a patient from family MA. (D,H) Neutrophils of a patient from family MU. Inclusion bodies in neutrophils are indicated by arrowheads. (Original magnification × 400)
NMMHC-A is one of the members of a large myosin heavy-chain gene family and proteins coded by this gene family are the actin-based molecular motors that hydrolyze adenosine triphosphate (ATP) and propel actin filaments.20 By self-association in its carboxyl terminal domain, MHC forms the backbone of the thick myosin filament.20 The random association of wild-type and mutant polypeptides would suggest that the mutations in the rod domain have a dominant negative effect by disturbing contractile function without completely destroying the functionally important myosin heads (Figure1C). Indeed, the 5 NMMHC-A mutations found in our study were all located in the C-terminal domain and residues D1424, E1841, and R1933 are highly conserved (Figure 1C,D).
In humans, 2 different genes for NMMHC,NMMHC-A17,18 and NMMHC-B(MYH10),21 have been identified but the only naturally occurring mutations of NMMHC-A were documented by us and others.12,13 D1424H, E1841K, and R1933X were also found in other ethnic groups, suggesting that the mutations appear to be common within the worldwide population.12,13Surprisingly, D1424H was also found in the patients with Fechtner syndrome, which is characterized by macrothrombocytopenia with leukocyte inclusions, nephritis, hearing loss, and cataract formation.22 However, our patients in family HI with D1424H did not develop other clinical manifestations. Indeed, Rocca and coworkers have reported that not all affected individuals show the full-blown phenotype even in the same family of Fechtner syndrome.22 Further examinations are required to interpret the discrepancy between genotype and variable expression of clinical symptoms.
Acknowledgments
We thank N. Enomoto for providing family MA, K. Hoshi for family KO, and T. Maeda and M. Tanaka for their technical assistance of confocal microscopy. We thank C. Inoue and K. Ozawa for their help.
Supported by Grants-in Aid for Scientific Research 10557090,11470209, and 12670981 from the Ministry of Education, Science, Sports and Culture, and for Research on Specific Diseases from the Ministry of Health and Welfare.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hidehiko Saito, First Department of Internal Medicine, Nagoya University School of Medicine, 65 Tsurumai, Showa-ku, Nagoya 466-8550, Japan; e-mail: hsaito@med.nagoya-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal