Abstract
Platelet activation is normally induced by primary agonists such as adenosine diphosphate (ADP), thrombin, and collagen, whereas other agonists, such as epinephrine, can play important accessory roles. It is now reported that the macrophage-derived chemokine (MDC), thymus activation–regulated chemokine (TARC), and stromal cell–derived factor one (SDF-1) are highly effective activators of platelet function under a variety of conditions, stimulating platelet shape change, aggregation, and adhesion to collagen or fibrinogen. Chemokine-mediated platelet activation was rapid and maximal (less than 5 seconds) under arterial flow conditions and depended strongly on the presence of low levels of primary agonists such as ADP or thrombin. Concentrations of ADP (0.05-0.25 μM) or thrombin (0.005-0.02 U/mL) that induced minimal aggregation caused major aggregation acting in combination with the chemokines. The ability of apyrase to block chemokine-dependent aggregation or adhesion was consistent with an important role for ADP. Chemokine-stimulated aggregation was also insensitive to indomethacin, suggesting that the activation of cyclo-oxygenase is not involved. TARC, MDC, and SDF-1 increased intracellular calcium concentrations [Ca2+]iwhen combined with low levels of ADP. The MDC and TARC receptor CCR4 was expressed on platelets, and an anti-CCR4 antibody blocked aggregation induced by TARC or MDC. Treatment of platelets with SDF-1 and MDC rapidly exposed P-selectin (CD62P) on the cell surface but did not induce the secretion of serotonin. These findings suggest that the chemokines MDC, TARC, and SDF-1, which may be produced during inflammatory responses, coupled with low levels of ADP or thrombin, can serve as strong stimuli for activating platelet function.
Introduction
Efficient hemostasis depends on a complicated series of events that actively involve blood platelets. Platelets also contribute significantly to the initial phases of hemostasis by their rapid adhesion to matrix proteins such as collagen, by aggregation to form thrombi, and by secretion of vasoconstrictor compounds such as serotonin. Much is known about activation and signaling mechanisms initiated by the specific receptors that lead to these functional responses. Adenosine diphosphate (ADP), a potent platelet-aggregating agent, targets 2 main heterotrimeric guanosine triphosphate-binding protein-coupled receptors—P2TAC which interacts with Gαi to regulate adenylyl cyclase, and P2Y1, linked to Gαq and to the activation of phospholipase C-β.1,2 Thrombin also interacts with 2 main receptors on human platelets, the proteolytically activated receptors PAR1 and PAR4.3 4 A continuing and intriguing area of research has been the ability of co-agonists, typified by epinephrine, to potentiate platelet function initiated by the primary agonists ADP and thrombin.
Several recent reports have suggested that chemokines may be able to activate platelets. The α-chemokine receptor CXCR45-7 is expressed in a range of cell types,8 including platelets and precursor megakaryocytes,9-11 leading to the possibility that the CXCR4 agonist SDF-1 may influence platelet adhesion9 or aggregation.11 No evidence for SDF-1–stimulating platelet function or increasing intracellular [Ca2+]i was reported in these earlier studies.9-11 However, there is earlier evidence that platelet factor 4 (PF4),12 now recognized as a chemokine,6,7 may induce platelet aggregation and secretion13 even though a specific receptor for PF4 has yet to be identified. More recent observations suggest that the chemokine IL-8 may activate platelet function.14 After completion of most of the studies reported here, Abi-Younes et al15 have reported that SDF-1 is an effective activator of platelet aggregation in a plasma environment.14
The aim of our studies was to examine whether SDF-1 or other chemokines could activate platelet function under flow conditions ranging from those in arterial-to-venous circulation. We demonstrated that macrophage-derived chemokine (MDC), thymus activation–regulated chemokine (TARC), and stromal cell–derived factor one (SDF-1) were strong agonists that rapidly stimulated platelet aggregation and adhesion, an ability largely dependent on the presence of low levels of the primary platelet agonist ADP. These chemokines also became effective in the presence of low levels of thrombin, which in itself caused minimal platelet aggregation. Platelets express CXCR4, the SDF-1–specific chemokine receptor,9-11 and we now show that platelets also express the MDC and TARC receptor, CCR4, on their surfaces. Together with the recent report that SDF-1 can induce aggregation and calcium fluxes in platelets,15 our findings provide new evidence that MDC, TARC, and SDF-1 can combine with primary platelet agonists such as ADP to serve as important activators of platelet function. Such activation may serve as a dynamic link between chemokines produced during inflammation and activation of hemostatic events.
Materials and methods
Materials
Sepharose 4B, fibrinogen from human plasma, collagen type IV from human placenta, CNBr-activated Sepharose 4B beads, fatty acid-free bovine serum albumin, indomethacin, prostaglandin I2(PGI2), apyrase (grade VII), and other specialty chemicals used were obtained from Sigma (St Louis, MO). Chemokines (IL-8, MIP-1β, RANTES, TARC, and SDF-1α) were from R&D Systems (Minneapolis, MN). TARC, ELC, SLC, and MDC were also kindly provided by Drs D. Chantry and P. Gray (ICOS, Bothell, WA). INDO-1/AM was obtained from Molecular Probes (Eugene OR). Fluorescein isothiocyanate (FITC)-conjugated goat antimouse IgG and purified mouse IgG1 were from Sigma. Purified IgG2a and phycoerythrin (PE)-conjugated IgG2a were from BioSource International (Camarillo CA). The P-selectin monoclonal antibody (mAb; S12) was a gift from R. McEver. The mAbs were directed against the CCR4 receptor and were provided by D. Chantry and P. Gray (ICOS). The PE-conjugated mAb against CXCR4 receptor 12G5 was from BD Pharmingen (Los Angeles, CA).
Preparation of platelet-rich plasma and washed platelets
After informed consent was obtained, platelet-rich plasma (PRP) was prepared from human venous blood anticoagulated with acid–citrate–dextrose (ACD; 120 mM sodium citrate, 110 mM glucose, 80 mM citric acid) by 3 short centrifugation steps at 350g.16 Washed platelets were isolated from PRP by centrifugation at 620g for 20 minutes in the presence of 0.05 vol ACD, apyrase (7.5 U/mL ADPase activity), indomethacin (1 mg/mL), and PGI2 (0.3 mg/mL). The pellet was suspended in half the original PRP volume of ACD containing albumin (1 mg/mL), apyrase, and indomethacin as above. After centrifuging for 20 minutes at 620g, the supernatant was discarded and the pellet was gently suspended in an appropriate volume of warm HEPES-based suspension buffer containing 5 mM glucose, 2 mg/mL fibrinogen, and 1 mg/mL albumin, when aggregation was to be assessed. These procedures usually yield platelet preparations that are fully responsive to low levels of ADP, an important consideration with regard to detecting platelet sensitivity to the chemokines TARC, SDF-1, and MDC. Slightly different solutions were used when carrying out adhesion experiments; for adhesion to type IV collagen, a Mg2+-based medium was used because the α2β1 integrin collagen receptor is most active with this cation17 For adhesion assays involving fibrinogen, a Ca2+/Mg2+ medium was used.18 Before testing the effects of chemokines on platelet function as aggregation or adhesion, the suspensions were warmed for 15 minutes at 37°C to restore platelet discoid shape and to minimize the platelet activation that can occur at room temperature.19 All assays were completed within 5 hours of platelet isolation. Final yields of platelets were from 80% to 95% of the count in PRP, and the average platelet count in the suspensions was 3.41 ± 0.20 × 108 platelets/mL.
Platelet aggregation assays
High-shear aggregation.
To assess whether chemokines might rapidly activate aggregation under arterial flow conditions, we used the quenched-flow approach developed in our laboratory.16,20 The basic principle involves pumping platelets through small-diameter tubing, typically 0.25 to 0.30 mm internal diameter, at flow rates of approximately 5 to 10 μL/sec, providing shear stresses from approximately 10 to 50 dyn/cm2. Chemokines, agonists, or both were mixed with prewarmed platelets at a T junction leading to the reaction tubing. At the outlet T junction, a quenching solution of 0.1% glutaraldehyde from a third syringe was added to rapidly quench the extent of aggregation.20 Reaction times from 0.15 seconds to 10 or more seconds could be readily analyzed in terms of aggregation and signal transduction kinetics.20-22 The extent of platelet aggregation was determined from decreases in the singlet particle count using a resistive particle counter.16
Low-shear aggregation.
Three similar procedures in small test tubes were used; the first used stir bars rotated at approximately 1000 rpm at 37°C. Two hundred and fifty microliters of prewarmed, washed platelets was exposed to 5 μL buffer as a control, chemokine, or primary agonist such as ADP. After 10-second stirring, 250 μL 2% glutaraldehyde in saline was added to quench the reaction. The second method used only 35 μL washed platelets, with the addition of chemokines or agonists giving a total incubation volume of 50 μL. This modification was instituted to lower the cost of chemokine usage. No magnetic stirrers were used, and shear forces were generated by placing the 1.5-mL Eppendorf test tubes on a variable-speed vortex mixer, causing vortex formation without frothing. Quenching was achieved by the addition of 50 μL 2% glutaraldehyde. The rotational rate of the vortex mixer was adjusted to provide similar amounts of platelet aggregation as achieved in the stir-bar system. Because this approach proved functionally effective, we then used a commercial rotational shaker (Eppendorf; Thermomixer R, Brinkmann, Westbury, NY) as a third system. A rotational rate of 1200 rpm and the use of 50 μL total reaction volume induced similar extents of aggregation as with the other low-shear systems. Treating the system as generating a back-and-forth “sloshing” motion with waves enabled calculation of a median shear stress of 1 to 5 dyn/cm2. Aggregation was evaluated with reference to changes in the control platelet singlet count, as in the high-shear experiments.
We did not adjust the platelet count to a fixed number but instead preferred to carry out the experiments as quickly as possible. For platelet adhesion under flow, adhesion efficiency was independent of the platelet count,18 and for aggregation experiments, t tests for statistical significance of chemokine effects were always performed. It should be noted that the actual platelet count in the reaction tubing, in which equal volumes of platelets and agonist solutions were mixed at the inlet,16was half that used for the low-shear experiments. Therefore, the high-shear experiments enabled ready detection of chemokine effects above baseline aggregation efficiencies. If the higher counts had been used, the relative stimulations would have been lower. The higher count used in the low-shear conditions when aggregation efficiency is inherently lower16 provided aggregation kinetics more favorable for detecting chemokine effects.
Estimation of EC50 values for the activation of platelet aggregation by chemokines
The extent of platelet aggregation was evaluated over a range of chemokine concentrations (0, 0.005, up to 5 μg/mL) in the presence of low levels of ADP to induce significant aggregation at the highest chemokine levels of 60 to 80%, depending on the donor. Data as percentage aggregation were then plotted against chemokine concentration and sigmoidal Boltzman fits generated using the Origin graphing program (MicroCal Software, Northampton, MA). The concentration of chemokine causing one half the maximal physiologic response (EC50) was obtained for each platelet preparation, and mean values with standard errors were finally calculated.
Platelet adhesion assay
The continuous flow adhesion approach was essentially as described previously.18 CNBr-activated Sepharose beads were coated with type IV collagen human or fibrinogen and used as adhesive surfaces.23 Platelets were prewarmed for 15 minutes at 37°C, and runs were conducted at 37°C. One syringe containing washed platelets and another containing isotonic saline for the control or chemokines were connected to the micro-adhesion column.23 Their contents were mixed and pumped through the protein-coated beads. To determine the influence of SDF-1 and MDC on adhesion, we usually used 50 μL beads at a pumping speed of 3.4 μL/s, providing shear rates of approximately 1700 seconds−1 and an adhesion or bead contact time of 0.67 seconds for collagen and 1.7 μL/s (850 seconds−1 or 1.34 seconds) for fibrinogen to achieve comparable amounts of adhesion before chemokine testing. Platelets (3 × 108/mL) were pumped over the beads at this shear rate for approximately 30 seconds. Adhesion to collagen or fibrinogen was determined by counting single platelets in the suspension before exposure to the beads and in the effluent emerging from the column, and it is expressed as the percentage of platelets bound to collagen or fibrinogen. In some experiments, adhesion studies were performed after an initial pre-incubation of platelets for 10 minutes with apyrase (7.5 U/mL) or indomethacin (1 μg/mL), without stirring.
Platelet secretion assay of serotonin
Platelets were labeled by incubating PRP with 0.2 μCi [2-14C]serotonin for 20 minutes at 37°C, then following the procedures outlined above for preparing washed preparations. Subsequent procedures and estimation of the extent of secretion were as described previously.24
Intracellular platelet [Ca2+]i
PRP was incubated at 37°C for 30 minutes with 8 U/mL apyrase, 0.3 μg/mL prostacyclin, 1 μg/mL indomethacin, extra ACD (1:20 initial PRP volume), and 2.5 μM INDO-AM. After centrifugation for 20 minutes at 350g, the platelets were suspended in half their original PRP volume with 8 U/mL apyrase and 3 mg/mL albumin. This was followed by a similar centrifugation and a final resuspension in Eagle minimal essential medium containing 5 mM HEPES, 21 mM sodium bicarbonate, and 3 mg/mL albumin. Fibrinogen was omitted, and the [Ca2+] was 1.8 mM. The INDO-loaded platelets were prewarmed for 15 minutes at 37°C before they were added to a 3 mL fluorescence cuvette with magnetic stirrer, all at 37°C. Agonists (MDC, SDF-1, or ADP) were added to the stirred suspensions, and changes in fluorescence emission intensity at 400 and 480 nm were continuously analyzed in an Aminco-Bowman SLM 8000 spectrofluorometer (Urbana, IL). When appropriate, 8 U/mL apyrase was added 3 minutes before the agonist was added. Excitation was at 335 nm, and internal free calcium ([Ca2+]i) was calculated from the 400:480 ratio and standard curves as described previously.21
Scanning electron microscopy
Prewarmed platelets were normally exposed to chemokines for 10 seconds in the absence or presence of the primary agonist (ADP) under low-shear conditions as described above, before they were quenched in 1% glutaraldehyde. Subsequent procedures, dehydration, and critical-point drying were standard,19 25 and coated specimens were examined in a JEOL 6400 scanning electron microscope with automated image digitization and archiving.
Flow cytometry analysis
Washed platelets were resuspended in Eagle buffer without fibrinogen at 2 × 108 platelets/mL and stimulated for 10 seconds under stirring conditions with control buffer, SDF-1, MDC, ADP, or thrombin. Activated platelets were immediately fixed with prewarmed 1% paraformaldehyde in PBS and incubated for 1 hour at 37°C. After washing, the suspensions of fixed platelets (5 × 106platelets/100 μL) were incubated with the appropriate primary antibody. This was followed by staining with FITC- or PE-conjugated secondary antibodies. Cell-associated immunofluorescence was analyzed with a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA) using CellQuest software.
Statistical analyses
When appropriate, the Student t test for paired samples was used to evaluate whether there was a significant difference between experimental situations. In general, data are expressed as mean ± SE.
Results
MDC, TARC, and SDF-1 rapidly stimulate platelet aggregation under arterial flow conditions
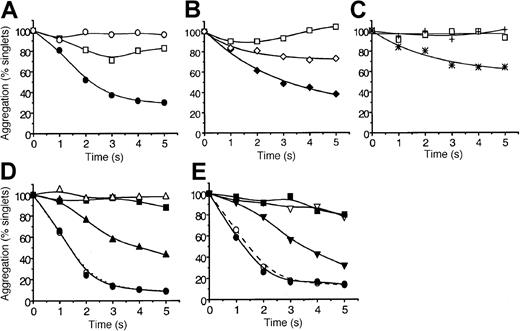
The ability of MDC, TARC, SDF-1, MIP-1β, RANTES, IL-8, SLC, and ELC to induce platelet aggregation under arterial high-shear conditions (10-35 dyn/cm2) was tested. We used a quenched-flow approach coupled to single-particle counting as a sensitive index of aggregation16 20 The data in Figure1A show that SDF-1 alone failed to induce significant aggregation within 5 seconds, but, when combined with a low dose of ADP (0.1 μM), it caused approximately 70% aggregation. This dose of ADP by itself induced a low level of aggregation that was reversible after 3 seconds. Similar to SDF-1, the chemokine MDC caused strong platelet aggregation (approximately 60% loss of singlets at 5 seconds) in the presence of low levels of ADP (Figure 1B). However, unlike SDF-1, MDC induced significant aggregation in the absence of ADP (approximately 25% by 5 seconds), whereas ADP by itself was essentially inactive. In Figure 1C we show that the chemokine TARC also effectively stimulated aggregation in the presence of low levels of ADP. The traces presented in Figure 1D and 1E reveal that SDF-1, at even lower levels of ADP (0.05 μM) or with very low levels of thrombin (0.005 U/mL, approximately 0.5 nM), was able to induce aggregation at rates that began to approach those caused by maximal doses of the primary agonists. In the absence of SDF-1, ADP and thrombin at these very low levels were essentially inactive. Pumping saline or buffer did not elicit significant aggregation within the 5-second exposure to high shear. These “flowing control” traces are not shown to help the clarity of the figures.
The chemokines MDC, TARC, and SDF-1 rapidly potentiate platelet aggregation under high-shear flow conditions.
Washed platelets were exposed in the quenched-flow system (10 to 35 dyn/cm2, “Materials and methods”16 20) to: (A) SDF-1 alone (0.5 μg/mL, ○), ADP alone (0.1 μM, ■), or SDF-1 combined with ADP (●); (B) MDC alone (0.5 μg/mL, ⋄), ADP alone (0.05 μM, ■) MDC combined with ADP (♦); (C) TARC alone (0.5 μg/mL, +), ADP alone (0.05 μM, ■), TARC combined with ADP (*); (D) SDF-1 (0.5 μg/mL) potentiation at low (0.05 μM) and high (10 μM) ADP levels; SDF-1 alone (▪), low ADP alone (▵), and low ADP combined with SDF-1 (▴); high ADP alone (○) and high ADP combined with SDF-1 (●). (E) SDF-1 potentiation at low (0.005 U/mL) and high (5 U/mL) thrombin levels. SDF-1 alone (▪); low thrombin (▿), and low thrombin combined with SDF-1 (▾); high thrombin alone (○), and high thrombin combined with SDF-1 (●). Aggregation is expressed as percentage platelet singlets remaining at each reaction time, as detected by resistive-particle counting. Data are typical for 11 experiments with SDF-1 and for 3 to 4 experiments with MDC and TARC. Experiments were carried out at 37°C as described in “Materials and methods.”
The chemokines MDC, TARC, and SDF-1 rapidly potentiate platelet aggregation under high-shear flow conditions.
Washed platelets were exposed in the quenched-flow system (10 to 35 dyn/cm2, “Materials and methods”16 20) to: (A) SDF-1 alone (0.5 μg/mL, ○), ADP alone (0.1 μM, ■), or SDF-1 combined with ADP (●); (B) MDC alone (0.5 μg/mL, ⋄), ADP alone (0.05 μM, ■) MDC combined with ADP (♦); (C) TARC alone (0.5 μg/mL, +), ADP alone (0.05 μM, ■), TARC combined with ADP (*); (D) SDF-1 (0.5 μg/mL) potentiation at low (0.05 μM) and high (10 μM) ADP levels; SDF-1 alone (▪), low ADP alone (▵), and low ADP combined with SDF-1 (▴); high ADP alone (○) and high ADP combined with SDF-1 (●). (E) SDF-1 potentiation at low (0.005 U/mL) and high (5 U/mL) thrombin levels. SDF-1 alone (▪); low thrombin (▿), and low thrombin combined with SDF-1 (▾); high thrombin alone (○), and high thrombin combined with SDF-1 (●). Aggregation is expressed as percentage platelet singlets remaining at each reaction time, as detected by resistive-particle counting. Data are typical for 11 experiments with SDF-1 and for 3 to 4 experiments with MDC and TARC. Experiments were carried out at 37°C as described in “Materials and methods.”
Of the additional chemokines tested on different platelet preparations under high-shear conditions in the presence of low ADP levels, MIP-1β, ELC, and SLC were inactive (tested at 0.5 μg/mL; data not shown). However, RANTES and IL-8 were weakly active, causing approximately 10% to 20% greater aggregation than ADP alone (data not shown), with variability between donors, and some platelet preparations were inactive under the conditions tested. The aggregates formed after RANTES or IL-8 treatment were unstable and tended to disaggregate by 5 seconds.
MDC and TARC are more active than SDF-1 under low-shear conditions
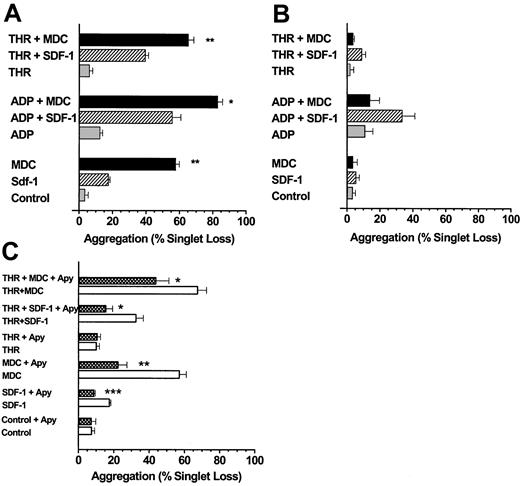
We next examined the ability of MDC, TARC, and SDF-1 to induce platelet aggregation under low-shear stresses approximating those in the venous circulation.20 MDC and TARC were more potent than SDF-1 at inducing aggregation within 10 seconds (Figures2A, 3A) when tested in the absence of added ADP or thrombin. Very low doses of ADP or thrombin significantly increased the extent of platelet aggregation mediated by MDC, TARC, or SDF-1 (P < .001). When platelets were first exposed to SDF-1, MDC, and TARC but were left unstirred for 10 minutes at 37°C, the aggregation induced by the subsequent addition of ADP or thrombin and stirring for 10 seconds was almost completely lost (Figures 2B, 3B), particularly with MDC.
MDC and SDF-1 stimulate platelet aggregation under low-shear conditions.
(A) The chemokine MDC is a stronger activator of platelet aggregation than SDF-1, and both chemokines are more effective in the presence of low levels of ADP or thrombin. Washed platelets stirred in a test tube were exposed to SDF-1 or MDC at 0.5 μg/mL in the presence or absence of ADP (0.05 μM) or thrombin (0.01 U/mL) for 10 seconds. (B) MDC and SDF-1 stimulation of platelet aggregation is labile, particularly for MDC. Additions were the same as in panel A, except that the chemokines were first mixed with the platelet suspensions, which were left at 37°C and were not stirred for 10 minutes before initiating low-shear conditions (stirring) and the addition of an agonist (ADP or thrombin). (C) Extracellular ADP is a major factor responsible for the ability of MDC and SDF-1 to stimulate platelet aggregation. Platelet suspensions were pre-incubated at 37°C with apyrase (5 U/mL ADPase activity) for 10 minutes under static conditions before an agonist, a chemokine, or both were added and low-shear stirring was initiated. Values are means ± SEM from 3 platelet preparations for panels A to C. The significance of the effects of MDC compared to SDF-1 (A) and of apyrase in each experimental situation (C) is *P < .05, **P < .01, ***P < .001.
MDC and SDF-1 stimulate platelet aggregation under low-shear conditions.
(A) The chemokine MDC is a stronger activator of platelet aggregation than SDF-1, and both chemokines are more effective in the presence of low levels of ADP or thrombin. Washed platelets stirred in a test tube were exposed to SDF-1 or MDC at 0.5 μg/mL in the presence or absence of ADP (0.05 μM) or thrombin (0.01 U/mL) for 10 seconds. (B) MDC and SDF-1 stimulation of platelet aggregation is labile, particularly for MDC. Additions were the same as in panel A, except that the chemokines were first mixed with the platelet suspensions, which were left at 37°C and were not stirred for 10 minutes before initiating low-shear conditions (stirring) and the addition of an agonist (ADP or thrombin). (C) Extracellular ADP is a major factor responsible for the ability of MDC and SDF-1 to stimulate platelet aggregation. Platelet suspensions were pre-incubated at 37°C with apyrase (5 U/mL ADPase activity) for 10 minutes under static conditions before an agonist, a chemokine, or both were added and low-shear stirring was initiated. Values are means ± SEM from 3 platelet preparations for panels A to C. The significance of the effects of MDC compared to SDF-1 (A) and of apyrase in each experimental situation (C) is *P < .05, **P < .01, ***P < .001.
TARC stimulates platelet aggregation under low-shear conditions.
(A) Low levels of ADP (0.05 μM) stimulate TARC-induced platelet aggregation. The TARC concentration was 0.5 μg/mL, and the alternative minimal-volume aggregation assay using the Eppendorf orbital shaker was used to minimize chemokine usage (“Materials and methods”). (B) Down-regulation of the TARC response due to exposure of platelets to TARC before adding ADP (conditions as in Figure 2B). (C) Influence of prior platelet exposure to apyrase before challenge by TARC (conditions as in Figure 2C). After 10 seconds of exposure to low shear, all reactions were quenched with glutaraldehyde, and aggregation was expressed as the percentage loss in platelet singlets. Values are from 7 preparations for panels A and B and from 9 to 13 preparations for panel C. Appropriate paired or unpaired t tests were used for tests of statistical significance for the influence of apyrase. **P < .01; ***P < .001.
TARC stimulates platelet aggregation under low-shear conditions.
(A) Low levels of ADP (0.05 μM) stimulate TARC-induced platelet aggregation. The TARC concentration was 0.5 μg/mL, and the alternative minimal-volume aggregation assay using the Eppendorf orbital shaker was used to minimize chemokine usage (“Materials and methods”). (B) Down-regulation of the TARC response due to exposure of platelets to TARC before adding ADP (conditions as in Figure 2B). (C) Influence of prior platelet exposure to apyrase before challenge by TARC (conditions as in Figure 2C). After 10 seconds of exposure to low shear, all reactions were quenched with glutaraldehyde, and aggregation was expressed as the percentage loss in platelet singlets. Values are from 7 preparations for panels A and B and from 9 to 13 preparations for panel C. Appropriate paired or unpaired t tests were used for tests of statistical significance for the influence of apyrase. **P < .01; ***P < .001.
The ability of an anti-CCR4 antibody (305L) to block MDC- or TARC-induced aggregation was tested. Platelets were exposed to apyrase before chemokine was added to provide CCR4-dependent aggregation independent of ADP. The aggregation caused by MDC or TARC was nearly completely prevented by the antibody (data not shown).
Extracellular ADP involvement in platelet aggregation induced by MDC, TARC, and SDF-1
The observation that the chemokines MDC, TARC, and SDF-1 may require the presence of the primary agonist ADP for effective stimulation of platelet aggregation was confirmed by an alternative approach. Pretreatment of platelets with apyrase to hydrolyze any ADP in the platelet suspension prevented the stimulation of platelet aggregation seen with SDF-1 (Figure 2C). Apyrase, however, only partially prevented MDC- (Figure 2C) or TARC-mediated aggregation (Figure 3C), and the resultant stimulations were significantly above control values (P < .05). The combination of MDC or TARC with low levels of thrombin in the presence of apyrase still induced significant stimulation (P < .01) of platelet aggregation above that of the thrombin controls (Figures 2C, 3C). These data show that unlike SDF-1, MDC and TARC can induce considerable aggregation in the absence of extracellular ADP.
The use of apyrase to remove external ADP was also tested for platelets exposed to SDF-1 or MDC under the high-shear conditions used for the experiments in Figure 1. Platelet aggregation with SDF-1 was completely prevented by apyrase treatment, even in the presence of low levels of ADP in the quenched-flow system16 (data not shown). With MDC, platelet exposure to apyrase caused similar results as seen under low-shear conditions (Figure 2C), only partially preventing the stimulation caused by the chemokine. We next examined whether the ability of SDF-1 and MDC to stimulate platelet aggregation might involve the generation of thromboxane A2(TxA2) by cyclooxygenase. This pathway is critical for the ability of many agonists, especially collagen, to induce irreversible platelet aggregation.17 26 Therefore, platelets were exposed to indomethacin to block cyclo-oxygenase activity and then were analyzed for their responsiveness to SDF-1 or MDC in the presence of low levels of ADP and high shear. No influence of indomethacin was seen on platelet aggregation induced by the chemokines (data not shown).
Sensitivity of platelet aggregation to MDC, TARC, and SDF-1
A series of experiments was performed to evaluate the EC50 for the chemokines shown above to be capable of inducing significant and rapid platelet aggregation, especially in the presence of low levels of ADP. The data in Table1 reveal that MDC and TARC were similar in their ability to stimulate aggregation, with EC50 values of approximately 8 nM. The EC50 values did not appear to be influenced by the different shear conditions. On the other hand, SDF-1α was approximately 4 times less potent than MDC or TARC, with EC50 values of approximately 29 nM. These values are comparable to a Kd of 22 nM for SDF-1 binding to platelets11 but greater than the EC50 of 0.7 nM for MDC-mediated chemotaxis or by MDC binding to the murine pre-B cell line L1.2.27
Sensitivity of platelet aggregation to SDF-1α, MDC, and TARC under different shear conditions
| . | SDF-1α . | MDC . | TARC . |
|---|---|---|---|
| (EC50; nmol/L chemokine) | |||
| High shear (n = 3) | 28.1 ± 4.1 | 8.5 ± 2.1 | 7.9 ± 0.1 |
| Low shear (n = 4) | 29.7 ± 7.9 | 6.7 ± 1.3 | 7.8 ± 1.2 |
| . | SDF-1α . | MDC . | TARC . |
|---|---|---|---|
| (EC50; nmol/L chemokine) | |||
| High shear (n = 3) | 28.1 ± 4.1 | 8.5 ± 2.1 | 7.9 ± 0.1 |
| Low shear (n = 4) | 29.7 ± 7.9 | 6.7 ± 1.3 | 7.8 ± 1.2 |
Washed platelets were aggregated under either high-shear conditions in the quenched-flow system or under low-shear in stirred tubes as described in “Materials and methods.” Low levels of ADP (0.05 to 0.25 μM) were used to provide minimal aggregation in the absence of chemokine. EC50 values are means ± SE for different platelet preparations (n) and were calculated as given in “Materials and methods.”
Platelet adhesion to collagen and fibrinogen under high shear is stimulated by MDC, SDF-1, and TARC
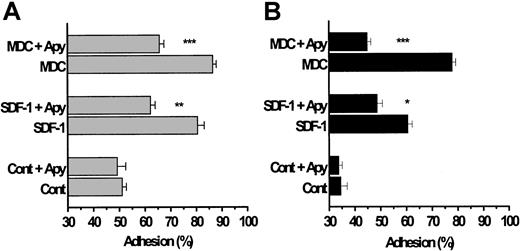
We initially considered whether MDC or SDF-1 might enhance platelet adhesion under arterial flow conditions. Washed platelets were pumped over beads coated with basement-membrane type IV collagen or fibrinogen, for an adhesion time of 0.67 seconds, using a continuous-flow adhesion chamber under shear stresses ranging from 15 to 50 dyn/cm2.18 Approximately 40% to 50% of unstimulated platelets normally adhere to these proteins within this time,23 as assessed by measuring the loss of single particles in the effluent emerging from the column. The results illustrated in Figure 4A reveal that SDF-1 and MDC potentiated platelet adhesion to type IV collagen (P < .001) and to fibrinogen (Figure 4B;P < .001). Subsequent experiments with TARC showed that this chemokine stimulated adhesion to immobilized collagen type IV and to fibrinogen to an extent similar to that observed with MDC (data not shown).
The chemokines SDF-1 and MDC enhance platelet adhesion to collagen and fibrinogen under high-shear flow conditions.
Washed platelets were pumped through a continuous-flow adhesion system18;20 and mixed with SDF-1 and MDC (0.5 μg/mL) just before they entered the adhesion column, which provided a contact (adhesion) time of 0.67 seconds. The beads in the adhesion column were coated with either basement-membrane type IV collagen (A) or with fibrinogen (B). As described in the “Materials and methods,” adhesion to collagen was studied in a Mg2+-based medium and that to fibrinogen was studied in a Ca2+/Mg2+-based medium, all in the absence of added soluble fibrinogen. In some of the experiments, the platelets were exposed to apyrase for 10 minutes at 37°C before they were pumped through the column. Results are expressed as means ± SEM from 3 donors and represent the percentage of platelets adhering to the beads. The significance of the decreases in platelet adhesion caused by apyrase is *P < .02, **P < .01, and ***P < .001.
The chemokines SDF-1 and MDC enhance platelet adhesion to collagen and fibrinogen under high-shear flow conditions.
Washed platelets were pumped through a continuous-flow adhesion system18;20 and mixed with SDF-1 and MDC (0.5 μg/mL) just before they entered the adhesion column, which provided a contact (adhesion) time of 0.67 seconds. The beads in the adhesion column were coated with either basement-membrane type IV collagen (A) or with fibrinogen (B). As described in the “Materials and methods,” adhesion to collagen was studied in a Mg2+-based medium and that to fibrinogen was studied in a Ca2+/Mg2+-based medium, all in the absence of added soluble fibrinogen. In some of the experiments, the platelets were exposed to apyrase for 10 minutes at 37°C before they were pumped through the column. Results are expressed as means ± SEM from 3 donors and represent the percentage of platelets adhering to the beads. The significance of the decreases in platelet adhesion caused by apyrase is *P < .02, **P < .01, and ***P < .001.
Pre-exposure of the platelets to apyrase significantly blunted the subsequent ability of the chemokines to enhance adhesion to collagen or fibrinogen. Although these blocking effects of apyrase were generally similar to those evident during the low-shear experiments on platelet aggregation (Figure 2C), apyrase treatment did not completely prevent the stimulation of adhesion elicited by SDF-1 or MDC to collagen or fibrinogen SDF-1 (Figure 4A-B; P < .01). It should be emphasized that platelet adhesion to collagen was carried out in the absence of any fibrinogen and with Mg2+ as divalent cation in the medium, a condition in which the α2β1 integrin is the dominant receptor.18 In addition, the peptide GRGDSP was included in the incubation medium, when adhesion to collagen was studied, to block any fibrinogen-dependent aggregation by the αIIBβ3 integrin. Consequently, the ability of the chemokines to enhance adhesion is unlikely to reflect the occurrence of platelet aggregation.
Platelet intracellular calcium levels are increased by SDF-1, TARC, and MDC
Because the activation of platelet function is usually associated with increased levels of intracellular [Ca2+]i, we examined whether the exposure of platelets to MDC, TARC, or SDF-1 under the low-shear conditions described above (Figures 2, 3), would do the same. The data presented in Figure 5 reveal the chemokines MDC, TARC, and SDF-1, when acting in combination with low levels of ADP, caused modest transient increases in [Ca2+]iof some 100 to 300 nM above typical resting platelet levels of approximately 200 nM. This behavior was consistently observed for the 3 chemokines and the various donors tested. Low levels of ADP (0.05-0.10 μM) by themselves, or the individual chemokines, caused minimal increases in [Ca2+]i (less than 50 nM, Figure5A-C). However, this characteristic appeared to vary depending on the donor; most were poorly responsive. For preparations that were responsive, MDC and SDF-1 induced increases similar to those seen in Figure 5A-C, and preincubation of the platelet suspensions with apyrase completely eliminated the SDF-1 increase while only partially blocking the increase caused by MDC (data not shown).
MDC, TARC, and SDF-1 cause increases in intracellular platelet [Ca2+]i in the presence of low levels of ADP.
INDO-1–loaded platelets21 were exposed to the chemokines (0.5 μg/mL) under low-shear conditions at 37°C, and fluorescence changes were monitored for several minutes. (A) MDC added to low levels of ADP (0.10 μM); second trace, MDC by itself; third trace, ADP by itself. (B) TARC added to ADP (0.10 μM); second trace TARC by itself. (C) SDF-1 added to ADP (0.10 μM); second trace SDF-1 by itself. The x-axis represents relative changes in [Ca2+]i,, and a 1-minute y-axis is also indicated.
MDC, TARC, and SDF-1 cause increases in intracellular platelet [Ca2+]i in the presence of low levels of ADP.
INDO-1–loaded platelets21 were exposed to the chemokines (0.5 μg/mL) under low-shear conditions at 37°C, and fluorescence changes were monitored for several minutes. (A) MDC added to low levels of ADP (0.10 μM); second trace, MDC by itself; third trace, ADP by itself. (B) TARC added to ADP (0.10 μM); second trace TARC by itself. (C) SDF-1 added to ADP (0.10 μM); second trace SDF-1 by itself. The x-axis represents relative changes in [Ca2+]i,, and a 1-minute y-axis is also indicated.
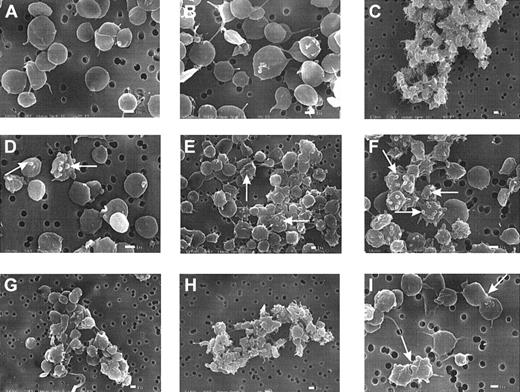
Platelet morphology is altered by MDC and SDF-1
When platelets are exposed to ADP or thrombin, they initially undergo a shape change, usually detected through scanning electron microscopy either as enhanced light scattering of cell suspensions and increased particle volume19 or as a striking extension of pseudopodia and loss of discoid.25,28 These changes are rapid and are complete within 2 seconds of exposure to the agonist.25 Experiments (Figure 6A-I) were carried out to evaluate whether MDC and SDF-1 might affect platelet morphology consistent with their ability to stimulate aggregation (Figures 1, 2). The electron micrographs reveal that a number of morphologic changes were associated with exposure to the chemokines. The formation of blebs (Figure 6D-F), rather than the thinner and longer pseudopodia typically seen with ADP, was common.25 28 Also shown is the generation of small aggregates when low levels of ADP were combined with SDF-1 or MDC (Figure 6G-H). As with our experiments on aggregation, prior exposure of the platelets to apyrase blocked the effects caused by the chemokines, especially the formation of blebs (images not shown). The images in Figure 6 B and C compare the influence of low ADP by itself (50 nM), which induced minimal shape change or aggregation, with a maximal dose (10 μM) that caused massive clumping or aggregation and shape change. Complementary parts of the figure (Figure 6D/G and E/H) compare the effects of the chemokines MDC and SDF-1 alone and in the presence of low levels of ADP (50 nM).
SDF-1 and MDC induce bleb formation in platelets and cause microaggregation.
Washed platelets were subjected to SDF-1 or MDC at 0.5 μg/mL or to ADP under low-shear stresses for 10 seconds at 37°C, as described for Figure 2, and then fixed with glutaraldehyde for subsequent processing and examination by scanning electron microscopy.25 (A) Control. (B) Low ADP level (0.05 μM). (C) High ADP level (10 μM). (D) SDF-1. (E) MDC. (F) MDC–SDF-1 combination. (G) Low ADP and SDF-1. (H) Low ADP and MDC. (i) MDC. The white bar indicates 1 μm. The micrographs are representative of results with platelet preparations from 3 donors.
SDF-1 and MDC induce bleb formation in platelets and cause microaggregation.
Washed platelets were subjected to SDF-1 or MDC at 0.5 μg/mL or to ADP under low-shear stresses for 10 seconds at 37°C, as described for Figure 2, and then fixed with glutaraldehyde for subsequent processing and examination by scanning electron microscopy.25 (A) Control. (B) Low ADP level (0.05 μM). (C) High ADP level (10 μM). (D) SDF-1. (E) MDC. (F) MDC–SDF-1 combination. (G) Low ADP and SDF-1. (H) Low ADP and MDC. (i) MDC. The white bar indicates 1 μm. The micrographs are representative of results with platelet preparations from 3 donors.
CCR4 is expressed on the platelet surface, the and exposure of P-selectin (CD62P) occurs after stimulation by SDF-1 and MDC, but secretion of dense-granule contents does not occur. The flow cytometry traces illustrated in Figure 7A demonstrate that isolated human platelets express the chemokine receptor CCR4 on their surfaces, consistent with our findings that MDC and TARC effectively stimulated aggregation (Figures 1, 2). We also detected the presence of CXCR4 (data not shown), confirming earlier reports.9-11 Platelet exposure to SDF-1 or MDC for 10 seconds in the presence of low levels of ADP and under low-shear conditions caused the appearance of surface-associated P-selectin (approximately 60% positive cells), at levels similar to those induced by thrombin (Figure 7B). The presence of ADP or SDF-1 alone did not stimulate the expression of P-selectin, whereas MDC alone caused limited exposure (data not shown). In addition, because SDF-1 or MDC in the presence or absence of low levels of ADP did not induce the secretion of dense-granule serotonin above control values (0.4% ± 0.3%; n = 3; P < .05), it is unlikely that any dense-granule ADP contributed to chemokine-induced platelet aggregation. A high dose of thrombin (1 U/mL) caused a typical secretion of 72.4% ± 2.7% in the same 10-second period.
CCR4 on resting human platelets and P selectin after stimulation.
(A) Presence of CCR4 on resting human platelets detected by flow cytometry and (B) expression of P selectin after stimulation with SDF-1 (0.5 μg/mL) and MDC (0.5 μg/mL) and low doses of ADP (0.1 μM), compared with thrombin (1 U/mL). (A) Washed, resting platelets fixed with paraformaldehyde were stained with anti-CCR4 mAb or the same concentration of an isotype control IgG followed by the addition of FITC-labeled goat–anti-mouse IgG. The staining with control antibody is shown as the solid profile, and the staining with the anti-CCR4 antibody is shown as the open profile. Percentage positive expression is shown above. (B) Platelets were stimulated in the absence of fibrinogen with SDF-1 and ADP for 10 seconds, MDC and ADP for 10 seconds, thrombin for 10 seconds (middle distribution), and for 10 minutes (right distribution) at 37°C and immediately fixed. Fixed platelets were incubated with anti-P selectin mAb S12 or control nonspecific mouse IgG1 for 1 hour at room temperature, followed by the addition of FITC-labeled goat-antimouse IgG. Treated platelets are shown in the open profiles.
CCR4 on resting human platelets and P selectin after stimulation.
(A) Presence of CCR4 on resting human platelets detected by flow cytometry and (B) expression of P selectin after stimulation with SDF-1 (0.5 μg/mL) and MDC (0.5 μg/mL) and low doses of ADP (0.1 μM), compared with thrombin (1 U/mL). (A) Washed, resting platelets fixed with paraformaldehyde were stained with anti-CCR4 mAb or the same concentration of an isotype control IgG followed by the addition of FITC-labeled goat–anti-mouse IgG. The staining with control antibody is shown as the solid profile, and the staining with the anti-CCR4 antibody is shown as the open profile. Percentage positive expression is shown above. (B) Platelets were stimulated in the absence of fibrinogen with SDF-1 and ADP for 10 seconds, MDC and ADP for 10 seconds, thrombin for 10 seconds (middle distribution), and for 10 minutes (right distribution) at 37°C and immediately fixed. Fixed platelets were incubated with anti-P selectin mAb S12 or control nonspecific mouse IgG1 for 1 hour at room temperature, followed by the addition of FITC-labeled goat-antimouse IgG. Treated platelets are shown in the open profiles.
Discussion
We have found that 3 chemokines, TARC, MDC, and SDF-1, were highly effective at stimulating platelet function under arterial and venous flow conditions. This occurred rapidly, causing up to 80% adhesion to type IV collagen or to fibrinogen within 1 second and 70% platelet aggregation by 5 seconds. RANTES and IL-8 were weakly active, whereas MIP-1β, ELC, and SLC were inactive. CCR4, the receptor for MDC and TARC,7 is now shown to be expressed on the platelet surface, as is the SDF-1 receptor CXCR4 previously reported.9,10 In view of these earlier studies, it was likely that SDF-1 should activate platelets, but an initial study indicated that SDF-1 was inactive.11 The recent work of Abi-Younes et al15 subsequently provided good evidence that SDF-1 can induce platelet aggregation in a plasma environment either in its α (our study) or β forms. In addition, SDF-1 was able to increase platelet [Ca2+]i, and evidence was presented that inhibitors such as genistein and of PI-3 kinase could prevent the SDF-1–induced aggregation. This study did not, however, reveal the ability of other chemokines to induce aggregation, particularly the involvement of low levels of primary agonists to trigger productive chemokine-induced aggregation or adhesion under flow conditions.
An important finding in our aggregation and adhesion studies (Figures1-4) for MDC, TARC, and SDF-1 relates to the critical role of primary platelet agonists in chemokine function. Very low levels of ADP (50-100 nM) or thrombin (0.005-0.01 U/mL, approximately 1 nM), which alone induced little aggregation by 5 seconds under high shear (Figure 1A-E) or by 10 seconds under low shear (Figure 2A), became highly effective when combined with TARC, SDF-1, or MDC. The ability of apyrase to block or reduce chemokine-induced aggregation or adhesion and increases in [Ca2+]i also supports a role for ADP. There is precedence that ADP can play important activating roles for other receptors. A useful earlier study29 using an ADP-scavenging system shows that the ability of epinephrine to stimulate aggregation requires the simultaneous presence of ADP and that concentrations of ADP in platelet suspensions range from 50 to approximately 600 nM. This range covers the approximately 130-nM concentration reported in 1978 by Jabs et al.30Several recent reports point to a role of ADP in platelet function beyond its immediate activity as a primary agonist. Thus, the ability of the thrombin receptor-activating peptide, PAR1, to induce irreversible aggregation via stimulation of PI-3 kinase is prevented by apyrase pretreatment of platelets.31 In addition, low levels (40 nM) of ADP are required for thrombopoietin to mobilize Ca2+ in platelets.32
The likelihood that chemokines may be responsible for the local stimulation of platelet function is supported by our observations about the pre-exposure of platelets to MDC, TARC, or SDF-1 in the absence of primary agonists and shear forces. This situation almost totally prevented subsequent agonist and shear-dependent aggregation (Figure2B; Figure 3B). Consequently, the simultaneous presence of primary agonist, chemokine, and shear forces provides the optimal local environment for productive aggregation or adhesion (Figure 4). Reasons for the down-regulation of the platelet chemokine receptors during the pre-exposure period may involve inactivation by phosphorylation and internalization, for which there is strong evidence in other cell types for SDF-1 and CXCR4.33-38
Blebs occur on a variety of cells in response to a range of stimuli and often reflect the surface exposure of phosphatidyl serine as detected by annexin V binding.39 In platelets, this exposure may lead to enhanced procoagulant activity.40 Therefore, chemokine-induced blebs on platelets are likely to enhance blood coagulation in addition to direct stimulation of aggregation, as reported here. Signaling mechanisms leading to bleb formation may require disruption of the tethering forces maintaining stable plasma membrane structure and interactions with the actin-rich cytoskeleton.41
The speed of platelet activation (Figure 1A-E and Figure 4A-B; less than 1 second) under arterial flow conditions suggests that chemokines or other factors, such as ADP, emanating locally from endothelial cells and inflammatory cells, such as macrophages, could significantly enhance the efficiency of hemostasis. This is likely even in the presence of an endothelial cell-associated ecto-ADPase (CD39),42,43 which has relatively high EC50values of approximately 10 μM ADP.44 There is also the likelihood that suboptimal combinations of different chemokines produced locally at sites of inflammation will prove effective for activating platelet function, even at extremely low levels of ADP (less than 50 nM). The presence of functioning CXCR4 and CCR4 chemokine receptors on platelets9-11 and the results of our study are consistent with platelet activation and consumption into aggregates and at sites of adhesion. These events may be linked to the thrombocytopenia45-47 to increased exposure of platelet α-granule P-selectin (CD62P) and to release of the α-granule chemokines RANTES48 and PF4 associated with HIV infection.12 Vascular endothelium represents a significant source of SDF-1 that can enhance integrin-mediated arrest of CD34+ cells under shear conditions.49 The possibility, therefore, exists that chemokine or HIV-1 activated platelets will adhere to endothelial cells and to exposed basement-membrane proteins such as type IV collagen. Chemokine-induced exposure of P-selectin also provides the opportunity for platelet interaction with P-selectin glycoprotein ligand 1 on leukocytes.50 Overall, our results are consistent with a dynamic relation between inflammation and hemostasis. Thus, chemokines that may be released from endothelial cells (SDF-1) or leukocytes (MDC, TARC) are effective at rapidly stimulating platelet function under arterial and venous blood flow conditions.
Since the submission of our work, a report by Kowalska et al51 has described the ability of SDF-1 and MDC to induce platelet aggregation and changes in intracellular calcium under a variety of conditions. As with their earlier report,11 they were unable to show in washed platelet suspensions that SDF-1 could induce aggregation in the presence of low levels of ADP or increase [Ca2+]i. These results are in contrast to those reported in our current study, whereas their data and the ability of apyrase to blunt increases in [Ca2+]i caused by MDC parallel some of our observations (Figure 5, and discussed in “Platelet intracellular calcium levels are increased by SDF-1, TARC, and MDC).
Acknowledgments
We thank Bill Ross of the University of Virginia FACS Core Laboratories for help with the flow cytometric analyses, Mike Lawrence of the Department of Biomedical Engineering for help in shear-stress calculations, Patrick Gray and David Chantry of ICOS Corporation for supplying some of the chemokines, and Carol Raport of ICOS Corporation for generating the anti-CCR4 antibody.
Supported by the Carman Trust (A.R.L.G., R.K.P.-G., D.V., S.S.) and by grant AI 39943 from the National Institutes of Health/National Institute of Allergy and Infectious Diseases (D.C.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Adrian R. L. Gear, Department of Biochemistry and Molecular Genetics, University of Virginia Health Sciences Center, 1300 Jefferson Park Ave, Charlottesville, VA 22908; e-mail: alg4p@virginia.edu.


![Fig. 5. MDC, TARC, and SDF-1 cause increases in intracellular platelet [Ca2+]i in the presence of low levels of ADP. / INDO-1–loaded platelets21 were exposed to the chemokines (0.5 μg/mL) under low-shear conditions at 37°C, and fluorescence changes were monitored for several minutes. (A) MDC added to low levels of ADP (0.10 μM); second trace, MDC by itself; third trace, ADP by itself. (B) TARC added to ADP (0.10 μM); second trace TARC by itself. (C) SDF-1 added to ADP (0.10 μM); second trace SDF-1 by itself. The x-axis represents relative changes in [Ca2+]i,, and a 1-minute y-axis is also indicated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/4/10.1182_blood.v97.4.937/5/m_h80410676005.jpeg?Expires=1770474131&Signature=carV-KulKl4GJ52J~QTKip60GvGUc~KUIdx8niD0h9cO84jHJC-WSHh91orCH5qSWXbeTO1i0k80lvvBNk0X2WJ414AL8rBExYwrCS3npPeSmQ2d8814aKYWuxo1WgOhE4J--NiMsztDwGfzus5gUPFqwSOczfoChOGDH3ndwkQxFS26d~WzF65QfIveAjvSrLK7-UJt0zJJCPV4v1B2LB2cZ7W-yMml2KokPcj5Zaq~KYQbgTg8c~FXS-IeOaQWOvo-jxcguhYqduRiVAxYnZtI~qz3nHVJeTcWA2hdtQ19nyhiONYbLfN8qZ9EvD8AkphWvZz4AvS7vR1YXh3rNA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal