Abstract
Elevated plasma lipoprotein (a) (Lp[a]) and cardiac events show a modest but significant association in various clinical studies. However, the influence of high Lp(a) on the gene expression in blood monocytes as a major cell involved in atherogenesis is poorly described. To identify genes influenced by elevated serum Lp(a), the gene expression was analyzed on a complementary DNA microarray comparing monocytes from a patient with isolated Lp(a) hyperlipidemia and coronary heart disease with monocytes from a healthy blood donor with low Lp(a). By using this approach, numerous genes were found differentially expressed in patient-versus-control monocytes. Verification of these candidates by Northern blot analysis or semiquantitative polymerase chain reaction in monocytes from additional patients with Lp(a) hyperlipidemia and healthy blood donors with elevated Lp(a) confirmed a significant induction of plasminogen activator inhibitor type 2 (PAI-2) messenger RNA (mRNA) in monocytes from male, but not from female, individuals with high Lp(a), indicating that this observation is gender specific. This led also to increased intracellular and secreted PAI-2 protein in monocytes from male probands with Lp(a) hyperlipidemia. Plasminogen activator inhibitor type 1 (PAI-1) mRNA was found suppressed only in the patients′ monocytes and not in healthy probands with high Lp(a) levels. Purified Lp(a) induced PAI-2 mRNA and protein and reduced PAI-1 expression in monocytes isolated from various controls. The finding that PAI-2 is elevated in monocytes from male patients with isolated Lp(a) hyperlipidemia and male healthy probands with high Lp(a) and that purified Lp(a) up-regulates PAI-2 in control monocytes in vitro indicate a direct, but gender-specific, effect of Lp(a) for the induction of PAI-2 expression.
Introduction
Lipoprotein (a) (Lp[a]) has been recognized as a risk factor for atherosclerosis1,2and the structural similarity of apolipoprotein (a) (apo[a]) with plasminogen3 may explain some of the atherogenic properties of this unusual lipoprotein. Lp(a) binds to fibrin and cell surface receptors because of its affinity to lysine4 and may inhibit the generation of plasmin,5 thereby promoting fibrin deposition. Generation of plasmin by proteolysis of its zymogen is catalyzed by the urokinase plasminogen activator (uPA) bound to the uPA receptor6 (CD87) or tissue-type plasminogen activator (t-PA). PA activity is inhibited by plasminogen activator inhibitor types 1 (PAI-1) and 2 (PAI-2).7,8 Plasmin is primarily responsible for the proteolysis of the fibrin clot but can also activate or degrade a variety of other proteins such as collagen, metalloproteinases, or tumor growth factor β (TGF-β).9The binding of CD87 to vitronectin is enhanced by uPA and inhibited by PAIs, and therefore the adhesion and migration of different cells requires a coordinated regulation of the plasminogen system.10 The interference of Lp(a) with this regulatory network because of its homology to plasminogen may disturb a variety of physiologic processes. Lp(a) influences the gene expression in different cells and an enhanced expression of PAI-1 was reported for endothelial cells.11 The production of vascular adhesion molecule 1 (VCAM-1) and E-selectin in cultured coronary artery endothelial cells is increased by Lp(a).12 In mesangial cells, Lp(a) up-regulates the transcription factors c-mycand c- fos.13
Most of the studies focused on the development of the atherosclerotic plaque and the interference of Lp(a) with this complex process. However, differential gene expression in blood monocytes because of elevated Lp(a) serum concentrations has received comparatively little attention, although blood monocytes are influenced by dyslipidemia.14,15 Functionally abnormal monocytes have been reported in patients with hypercholesterolemia16,17and more mature monocytes are described in patients with xanthomatosis.18 An increased expression of uPA and uPAR by native and oxidized Lp(a) in monocytes was demonstrated.19 However, this up-regulation was also induced by LDL to a similar extent, and significant data were only obtained by using high amounts of the lipoproteins. In addition, an enhanced expression of the integrin CD11b was reported20but could not be confirmed by other investigators.19
Differentially expressed genes can be identified by several strategies such as subtractive hybridization, differential display reverse transcription–polymerase chain reaction (DDRT-PCR), or the serial analysis of gene expression (SAGE)21 technique. We have used the recently developed complementary DNA (cDNA) microarray technology that enables us to investigate the expression of thousands of genes simultaneously. A microarray approach was successful in the detection of genes related to rheumatoid arthritis and inflammatory bowel disease.22 The aim of this study was to identify genes that are differentially expressed in monocytes from patients with isolated Lp(a) hyperlipidemia compared with control cells. In addition to the identification of several genes related to interindividual variations and genes preferentially dysregulated in patients monocytes, a high expression of PAI-2 messenger RNA (mRNA) was detected in monocytes from male individuals with elevated Lp(a) but not in female donors with high Lp(a). We assume that the up-regulation of PAI-2 in men is directly mediated by elevated Lp(a) and may be associated with the development of atherosclerosis. The findings indicate that Lp(a) probably interferes with the plasminogen system and linked pathways, not only because of the homology of apo(a) to plasminogen, but also because of the induction of PAI-2 expression.
Patients, materials, and methods
Patients and probands with elevated lipoprotein (a)
Data of the patients with history of myocardial infarction (P1, P2, P3) and healthy blood donors with elevated Lp(a) are listed in Table 1. L1, L3, L4 are male donors and L2, L5, L6, L7, L8, L9, and L10 are female blood donors. Lp(a) was determined by a nephelometric test (Immuno AG, Vienna, Austria). High density lipoprotein cholesterol (HDL-C) was measured by a homogenous enzymetric assay and low density lipoprotein cholesterol (LDL-C) by a direct enzymetric assay (Roche Diagnostics, Mannheim, Germany). The patients have a history of myocardial infarction.23 Blood donors with normal lipid status and Lp(a) plasma levels of less than 5 mg/dL were used as controls (C1 to C20) and were 25 to 45 years of age (12 men, 8 women). The isolation of blood monocytes was approved by the Medical Ethics Committee at the University Hospital of Regensburg, Germany.
Description of patients and probands with elevated lipoprotein (a)
| Patient/proband . | Sex . | Age . | LDL-C (mg/dL) . | HDL-C (mg/dL) . | Lp(a) (mg/dL) . |
|---|---|---|---|---|---|
| P1 | Male | 59 | 150 | 45 | 150 |
| P2 | Male | 47 | 124 | 31 | 96 |
| P3 | Male | 43 | 140 | 43 | 110 |
| L1 | Male | 29 | 129 | 49 | 115 |
| L2 | Female | 31 | 178 | 66 | 90 |
| L3 | Male | 20 | 106 | 39 | 117 |
| L4 | Male | 31 | 129 | 55 | 134 |
| L5 | Female | 20 | 82 | 71 | 73 |
| L6 | Female | 22 | 199 | 80 | 163 |
| L7 | Female | 24 | 158 | 92 | 75 |
| L8 | Female | 37 | 158 | 73 | 128 |
| L9 | Female | 77 | 104 | 99 | 95 |
| L10 | Female | 55 | 151 | 101 | 132 |
| Patient/proband . | Sex . | Age . | LDL-C (mg/dL) . | HDL-C (mg/dL) . | Lp(a) (mg/dL) . |
|---|---|---|---|---|---|
| P1 | Male | 59 | 150 | 45 | 150 |
| P2 | Male | 47 | 124 | 31 | 96 |
| P3 | Male | 43 | 140 | 43 | 110 |
| L1 | Male | 29 | 129 | 49 | 115 |
| L2 | Female | 31 | 178 | 66 | 90 |
| L3 | Male | 20 | 106 | 39 | 117 |
| L4 | Male | 31 | 129 | 55 | 134 |
| L5 | Female | 20 | 82 | 71 | 73 |
| L6 | Female | 22 | 199 | 80 | 163 |
| L7 | Female | 24 | 158 | 92 | 75 |
| L8 | Female | 37 | 158 | 73 | 128 |
| L9 | Female | 77 | 104 | 99 | 95 |
| L10 | Female | 55 | 151 | 101 | 132 |
P1 to P3 are patients with isolated Lp(a) hyperlipidemia and a history of myocardial infarction. L1 to L10 are healthy controls with high Lp(a) levels.
Controls (C1 to C20) had normal LDL-C and HDL-C levels and Lp(a) was less than 5 mg/dL (not listed). LDL-C indicates low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; Lp(a), lipoprotein (a).
Culture media and reagents
RPMI medium was from Gibco BRL (Karlsruhe, Germany). PAI-1 and PAI-2 enzyme-linked immunosorbent assays (ELISAs) were from Diagnostics International (Schriesheim, Germany). Other laboratory reagents and chemicals were purchased from Sigma Chemical (Deisenhofen, Germany), unless noted otherwise. Nylon membranes (Genescreen) for Northern blotting were from NEN Life Science (Boston, MA) and α[32P]dCTP from Amersham Pharmacia Biotech (Braunschweig, Germany). Oligonucleotides were synthesized by MWG (Ebersberg, Germany).
Isolation and culture of monocytes
Peripheral blood monocytes from healthy blood donors with elevated Lp(a) (L1, L2), patients with Lp(a) hyperlipidemia (P1, P2), and controls were isolated by leukapheresis, followed by counterflow elutriation. Fractions containing more than 95% monocytes were pooled and cultured on plastic petri dishes (3 × 106 cells/mL) in RPMI medium with 20% autologous serum for 18 hours. For PCR analysis and determination of secreted and intracellular PAI-2 protein, peripheral blood leukocytes were isolated from whole blood with a Vacutainer CPT (Becton Dickinson, Franklin Lakes, NJ) and monocytes were further purified by magnetic separation with CD14 beads (Miltenyi Biotec, Bergisch Gladbach, Germany).
Isolation of low density lipoprotein and lipoprotein (a)
LDL and Lp(a) were purified from citrated plasma as described by Kostner et al.24 Protein concentrations were determined according to Lowry et al.25 Endotoxin in the isolated lipoproteins was determined by an assay from Sigma Chemical (Deisenhofen, Germany) and was less than 0.01 ng/mL in the culture medium.
Primers and complementary DNA probes
The oligonucleotides listed in Table2 were used for semiquantitative PCR and for cDNA probe synthesis for Northern blot analysis. The corresponding PCR fragments were cloned (TOPO TA Cloning, Invitrogen, De Schelp, The Netherlands) and sequenced.
Oligonucleotides/probes used for semiquantitative polymerase chain reaction or Northern blot analysis
| Gene . | Primer sequence . | Length of PCR product . |
|---|---|---|
| CD87 | uni 5′GCTGCTCCACACCTGCGTCCCAGCCTC | 1005 bp |
| rev 5′CCAGCCAGGGCAGAGAGGGGGATTCT | ||
| PAI-2 | uni 5′AAACAATGGAGGATCTTTGTGTGG | 707 bp |
| rev 5′CATCTGTACAGGTGTGCGCTGAG | ||
| PAI-1 | uni 5′CTTGTCTTTGGTGAAGGGTCTGCTG | 887 bp |
| rev 5′CTTCCTGAGGTCGTCGACTTCAGTCTCC | ||
| MCP-1 | uni 5′CTGCTGCTCATAGCAGCCAC | 725 bp |
| rev 5′ACATCCCAGGGGTAGAACTG | ||
| Defensin | uni 5′GGCATTTATTTGAGATGAGG | 426 bp |
| rev 5′CCTCTCTGGTCACCCTGCCT | ||
| IP-10 | uni 5′CATGCAGAGCATATATCTATCTG | 656 bp |
| rev 5′CAAGGATGGACCACACAGAGGCTG | ||
| Adipophilin | uni 5′ATGATGCAGCTCGTGAGCAGT | 213 bp |
| rev 5′CTGCTGAGAGCCTGCTGGTAG | ||
| C3aR | uni 5′ACAACCATAATAGATGTGGC | 400 bp |
| rev 5′CGTAGAAGGAATTGCTAGAA | ||
| CD16 | uni 5′CAGCTGGCATGCGGACTGAAGATCTCC | 750 bp |
| rev 5′GGGTGACTTAAGAGATCTAGGGGTCGT | ||
| GAPDH | uni 5′GAAGGTGAAGGTCGGAGTCA | 505 bp |
| rev 5′GATACCAAAGTTGTCATGGA |
| Gene . | Primer sequence . | Length of PCR product . |
|---|---|---|
| CD87 | uni 5′GCTGCTCCACACCTGCGTCCCAGCCTC | 1005 bp |
| rev 5′CCAGCCAGGGCAGAGAGGGGGATTCT | ||
| PAI-2 | uni 5′AAACAATGGAGGATCTTTGTGTGG | 707 bp |
| rev 5′CATCTGTACAGGTGTGCGCTGAG | ||
| PAI-1 | uni 5′CTTGTCTTTGGTGAAGGGTCTGCTG | 887 bp |
| rev 5′CTTCCTGAGGTCGTCGACTTCAGTCTCC | ||
| MCP-1 | uni 5′CTGCTGCTCATAGCAGCCAC | 725 bp |
| rev 5′ACATCCCAGGGGTAGAACTG | ||
| Defensin | uni 5′GGCATTTATTTGAGATGAGG | 426 bp |
| rev 5′CCTCTCTGGTCACCCTGCCT | ||
| IP-10 | uni 5′CATGCAGAGCATATATCTATCTG | 656 bp |
| rev 5′CAAGGATGGACCACACAGAGGCTG | ||
| Adipophilin | uni 5′ATGATGCAGCTCGTGAGCAGT | 213 bp |
| rev 5′CTGCTGAGAGCCTGCTGGTAG | ||
| C3aR | uni 5′ACAACCATAATAGATGTGGC | 400 bp |
| rev 5′CGTAGAAGGAATTGCTAGAA | ||
| CD16 | uni 5′CAGCTGGCATGCGGACTGAAGATCTCC | 750 bp |
| rev 5′GGGTGACTTAAGAGATCTAGGGGTCGT | ||
| GAPDH | uni 5′GAAGGTGAAGGTCGGAGTCA | 505 bp |
| rev 5′GATACCAAAGTTGTCATGGA |
PCR indicates polymerase chain reaction; uni, forward primer; rev, reverse primer; bp, base pair.
Isolation of RNA, Northern blot analysis, and reverse transcriptase–polymerase chain reaction
Total cellular RNA from elutriation purified monocytes was isolated by the guanidine isothiocyanate–cesium chloride technique.26 The 10 μg total RNA was separated through a 1.2% agarose gel containing 6% formaldehyde and blotted onto nylon membranes. After cross-linking with ultraviolet-irradiation (Stratalinker model 1800, Stratagene, La Jolla, CA), the membranes were hybridized with the cDNA probes listed in Table 2, stripped, and subsequently hybridized with a human GAPDH probe (Clontech, Palo Alto, CA). The probes were radiolabeled with α-[32P]dCTP using the Prime-It II Kit from Stratagene (Amsterdam Zuidoost, The Netherlands). Hybridization and washing conditions were performed as recommended by the manufacturer of the membrane.
The RNA from cells isolated by magnetic beads was purified by the RNA isolation kit (Roche, Penzberg, Germany). Reverse transcriptase– PCR (RT-PCR) of total RNA (100 ng) was performed by using the RT-PCR kit from PerkinElmer according to the instructions of the kit. For the PCR reaction, 100 pmol of each primer (Table 1) were used in a 100 μL reaction. PCR conditions were as follows: initial denaturation at 5 minutes 94°C, cycling at 44 seconds 92.3°C, 40 seconds 60.8°C, 46 seconds 71.5°C, and final extension at 72°C for 5 minutes in a Perkin Elmer 9600 thermal cycler. Eighteen cycles were performed to amplify GAPDH, adipophilin, and IP-10 cDNA, and 20 cycles for defensins, PAI-1, PAI-2, CCR-2, and MCP1. The fragments were analyzed on a 2% agarose gel and ethidium bromide–stained DNA was visualized on the Lumi Imager (Roche, Penzberg, Germany).
Microarray hybridization
Messenger RNA was purified from total RNA by oligodT beads (Roche). Hybridization, signal detection, and data analysis were performed by Incyte Genomics (Palo Alto, CA). (For detailed information refer to http://www.incyte.com.)
The 200 ng mRNA isolated from the monocytes of patient 1 and control 1 were independently reverse transcribed using Cy5- or Cy3-labeled nucleotides, respectively, mixed, and simultaneously hybridized to the UniGEM V 2.0 microarray (Incyte Genomics, Fremont, CA) with 8556 human cDNAs spotted. The array was rinsed and scanned for the 2 fluorescent colors independently. Data were analyzed using GemTools software (Incyte Genomics) and expressed as ratios of patient to control mRNA.
Immunologic assays
PAI-1 and PAI-2 protein determinations were performed as recommended by the distributor of the ELISA. For intracellular determination, cells were frozen, thawed, and sonified. Twenty microliters, 10 μL, and 5 μL of a 0.5 mg protein per milliliter to 3 mg protein per milliliter cell-lysate were used for the ELISAs. Secreted PAI-2 was analyzed by using 20 μL culture supernatant. To determine PAI-1, the supernatant was concentrated 7-fold and dialyzed against phosphate-buffered saline (PBS).
Results
Identification of differentially expressed genes in monocytes from a patient with isolated Lp(a) hyperlipidemia
Monocytes were purified by elutriation and cultivated for 18 hours in 20% autologous serum before RNA isolation. The gene expression in monocytes from a patient (P1, male) with elevated Lp(a) as the only risk factor for premature vascular disease23 was compared with control monocytes (C1, male) by hybridization of labeled cDNAs to a microarray. In total, 114 genes with a difference in expression ranging from 19.5 to 2 were identified. Although 80 transcripts were induced, 34 mRNAs were suppressed in patient cells. From these candidates, 8 genes with known function and the highest difference in expression between patients and control monocytes were selected for further investigations (Table 3). The expression of these candidates was examined by Northern blot analysis and/or semiquantitative PCR in 2 additional patients (P2, P3) with isolated Lp(a) hyperlipidemia, 4 healthy probands with elevated Lp(a) (L1 to L4), and 12 different controls (C1 to C12).
Selected candidate genes identified by the microarray approach
| Balanced difference factor . | Gene . | Abbreviation . |
|---|---|---|
| +19.5 | Plasminogen activator inhibitor 2 | PAI-2 |
| +7.9 | Interferon-induced cell line protein 10 | IP-10 |
| +4.0 | Neutrophil defensins 1, 2, and 3 | nd |
| +3.8 | FcγRIII | CD16 |
| +3.2 | Monocyte chemotactic protein 1 | MCP-1 |
| −3.0 | C-C chemokine receptor type 2 | CCR2 |
| +3.0 | C3a anaphylatoxin receptor | C3aR |
| +2.7 | Adipophilin | nd |
| Balanced difference factor . | Gene . | Abbreviation . |
|---|---|---|
| +19.5 | Plasminogen activator inhibitor 2 | PAI-2 |
| +7.9 | Interferon-induced cell line protein 10 | IP-10 |
| +4.0 | Neutrophil defensins 1, 2, and 3 | nd |
| +3.8 | FcγRIII | CD16 |
| +3.2 | Monocyte chemotactic protein 1 | MCP-1 |
| −3.0 | C-C chemokine receptor type 2 | CCR2 |
| +3.0 | C3a anaphylatoxin receptor | C3aR |
| +2.7 | Adipophilin | nd |
Known genes with a high-balanced difference factor were selected for further investigations. The plus sign for the balanced difference factor indicates a higher expression in patient monocytes when compared with the control. A minus sign indicates suppression of this gene in the patient monocytes.
nd indicates not defined.
Analysis of the expression of the identified candidate genes in controls and additional patients or probands with elevated Lp(a)
Further investigations of the genes listed in Table 3 by Northern blot analysis in patients (P1, P2, P3), healthy probands with high Lp(a) (L1, L2, L3, L4), and 12 controls with low Lp(a) revealed that the genes could be classified in 3 groups.
Variation of gene expression within different donors.
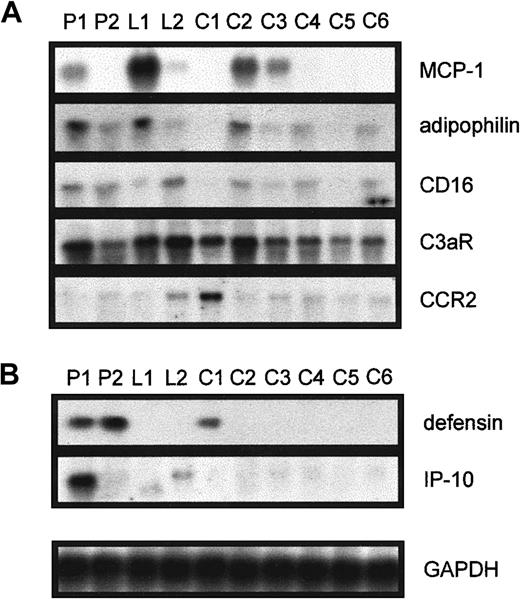
The expression of MCP-1, adipophilin, CD16, C3aR, and CCR-2 (Figure1A) was investigated in monocytes from patient 1 (used for the microarray analysis), patient 2, and the healthy probands L1 and L2 with elevated Lp(a) by Northern blot analysis and compared with the mRNA expression of 6 controls. We found that the mRNA expression of these identified candidate genes simply varies between different individuals and was not related to elevated serum Lp(a). Nevertheless, these results show that monocytes from individuals with Lp(a) hyperlipidemia are not generally activated.
Northern blot analysis in various individuals for the genes found to be differentially expressed between P1 and C1.
Elutriation-purified monocytes from patients with isolated Lp(a) hyperlipidemia (P1, P2), healthy controls with elevated Lp(a) (L1, L2), and 6 controls with low Lp(a) (C1 to C6) were incubated in 20% autologous serum for 18 hours, RNA was isolated, and 10 μg total RNA was used to investigate the expression of (A) MCP-1, adipophilin, CD16, C3aR, and CCR2 and (B) defensin and IP-10. Equal RNA loading was confirmed by reprobing the blots with GAPDH.
Northern blot analysis in various individuals for the genes found to be differentially expressed between P1 and C1.
Elutriation-purified monocytes from patients with isolated Lp(a) hyperlipidemia (P1, P2), healthy controls with elevated Lp(a) (L1, L2), and 6 controls with low Lp(a) (C1 to C6) were incubated in 20% autologous serum for 18 hours, RNA was isolated, and 10 μg total RNA was used to investigate the expression of (A) MCP-1, adipophilin, CD16, C3aR, and CCR2 and (B) defensin and IP-10. Equal RNA loading was confirmed by reprobing the blots with GAPDH.
Up-regulation of genes in patients with elevated Lp(a).
Although IP-10 mRNA was up-regulated in monocytes isolated from patient 1 (Figure 1B) and patient 3 (not shown), defensin expression was induced in P1 and P2 (Figure 1B). The IP-10 mRNA was not induced in any of the 9 controls investigated, and defensin mRNA was only detected in one control. However, the expression of IP-10 or defensins was not elevated in healthy probands with similar serum Lp(a) concentrations to the patients.
Up-regulation of PAI-2 in patients and probands with elevated Lp(a).
PAI-2 mRNA was elevated in patient 1 and patient 2 and one healthy proband (L1) with high Lp(a) (Figure 2A) by Northern blot analysis in comparison to controls with low Lp(a). Monocytes from the female donor (L2) with high Lp(a) showed no increased expression of PAI-2 mRNA (Figure 2A). Semiquantitative PCR revealed an up-regulation of PAI-2 in monocytes from the male donors P1, P3, L3, and the proband L4 (Figure 2B) when compared with 6 additional controls. In contrast, monocytes isolated from premenopausal (L2, L5 to L8) and postmenopausal (L9, L10) women with high Lp(a) did not show increased PAI-2 mRNA levels (not shown). Furthermore, PAI-2 mRNA was analyzed in monocytes from P3, L1, and controls isolated from Ficoll purified leukocytes by CD14-coupled magnetic beads without cultivation (not shown). We obtained identical results concerning the induction of PAI-2 mRNA levels in donors with high Lp(a) and therefore can exclude an effect of different isolation protocols or cultivation of monocytes on the expression of this gene.
Northern blot and semiquantitative PCR analysis of PAI-2 expression.
(A) Elutriation-purified monocytes from patients with isolated Lp(a) hyperlipidemia (P1, P2), healthy controls with elevated Lp(a) (L1, L2), and 6 controls with low Lp(a) (C1 to C6) were incubated in 20% autologous serum for 18 hours. RNA was isolated and 10 μg total RNA was used to investigate the expression of PAI-2. The film was exposed for 4 hours at −80°C to emphasize the difference in PAI-2 expression in the first 3 lanes. The blot was stripped and hybridized with a GAPDH probe. (B) RNA was prepared from monocytes of patients with isolated Lp(a) hyperlipidemia (P1, P3), healthy probands with elevated Lp(a) (L3, L4), and controls with low Lp(a) (C1, C2, C7, C8, C9, C10, C11, C12) as described in “Materials and methods.” The 100 ng RNA was used to amplify PAI-2 cDNA (20 cycles) or GAPDH cDNA (18 cycles).
Northern blot and semiquantitative PCR analysis of PAI-2 expression.
(A) Elutriation-purified monocytes from patients with isolated Lp(a) hyperlipidemia (P1, P2), healthy controls with elevated Lp(a) (L1, L2), and 6 controls with low Lp(a) (C1 to C6) were incubated in 20% autologous serum for 18 hours. RNA was isolated and 10 μg total RNA was used to investigate the expression of PAI-2. The film was exposed for 4 hours at −80°C to emphasize the difference in PAI-2 expression in the first 3 lanes. The blot was stripped and hybridized with a GAPDH probe. (B) RNA was prepared from monocytes of patients with isolated Lp(a) hyperlipidemia (P1, P3), healthy probands with elevated Lp(a) (L3, L4), and controls with low Lp(a) (C1, C2, C7, C8, C9, C10, C11, C12) as described in “Materials and methods.” The 100 ng RNA was used to amplify PAI-2 cDNA (20 cycles) or GAPDH cDNA (18 cycles).
Expression of PAI-1 and CD87
The high expression of PAI-2 mRNA found in all male probands with elevated Lp(a) stimulated the analysis of the mRNA levels of the second plasminogen activator inhibitor, namely, PAI-1, and the urokinase plasminogen activator receptor (CD87). Although PAI-1 cDNA was not part of the microarray, the differential expression of CD87 was less than 2. Northern blot analysis revealed a down-regulation of PAI-1 mRNA in P1, P2, and P3 (not shown). The mRNA of the CD87 was slightly reduced in P1, P2, and L2. Confirmatory analysis of protein expression for CD87 by FACS analysis in whole blood did not show significant differences between patient, control cells with high Lp(a) or controls with low Lp(a) (not shown).
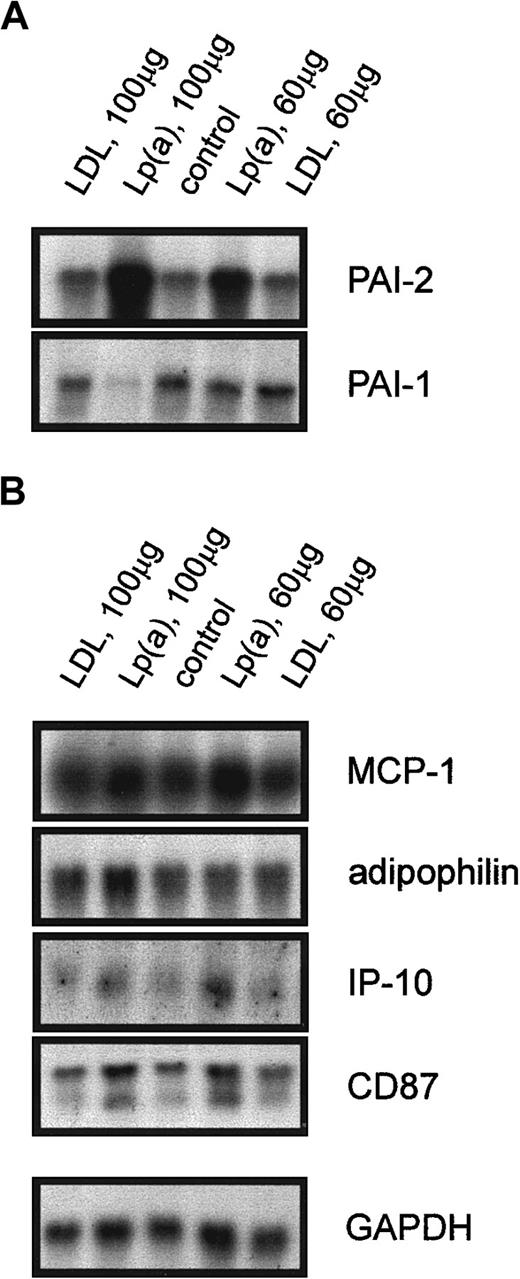
Regulation of the identified candidate genes by purified Lp(a)
To find out whether these ex vivo results can be reproduced in vitro, we incubated monocytes from 3 healthy probands in 20% autologous serum alone, in serum with purified LDL as a control for the lipoprotein storage buffer, or with purified Lp(a). Northern blot analysis shown for one representative experiment in Figure3A revealed a significant induction of PAI-2 mRNA when the cells were incubated with 100 μg/mL Lp(a) or 60 μg/mL Lp(a) for 18 hours. The expression of PAI-1 mRNA was reduced in monocytes treated with 100 μg/mL Lp(a), whereas 60 μg/mL did not influence PAI-1 mRNA levels (Figure 3A). These effects were specific for Lp(a) because incubation with purified LDL had no influence on the expression of PAI-1 and PAI-2. As expected from the ex vivo experiments, purified Lp(a) did not alter the expression of MCP-1, adipophilin, IP-10, and CD87 (Figure 3B), or defensins (not shown). We obtained identical results when monocytes from male or female donors were used for the in vitro incubation with purified Lp(a). Therefore, the lack of PAI-2 induction in female probands with high Lp(a) is not a property of the isolated monocytes.
Northern blot analysis of Lp(a)-sensitive genes and genes not regulated by Lp(a).
Elutriation-purified monocytes from controls with low Lp(a) were incubated with purified LDL (100 μg/mL) and (60 μg/mL), purified Lp(a) (100 μg/mL) and (60 μg/mL) in 20% autologous serum or in 20% serum alone (control) for 18 hours. Total RNA was isolated and the expression of Lp(a)-sensitive genes (A) PAI-2, PAI-1, and genes not regulated by Lp(a) (B) MCP-1, adipophilin, IP-10, CD87, and GAPDH was analyzed.
Northern blot analysis of Lp(a)-sensitive genes and genes not regulated by Lp(a).
Elutriation-purified monocytes from controls with low Lp(a) were incubated with purified LDL (100 μg/mL) and (60 μg/mL), purified Lp(a) (100 μg/mL) and (60 μg/mL) in 20% autologous serum or in 20% serum alone (control) for 18 hours. Total RNA was isolated and the expression of Lp(a)-sensitive genes (A) PAI-2, PAI-1, and genes not regulated by Lp(a) (B) MCP-1, adipophilin, IP-10, CD87, and GAPDH was analyzed.
PAI-1 and PAI-2 protein expression
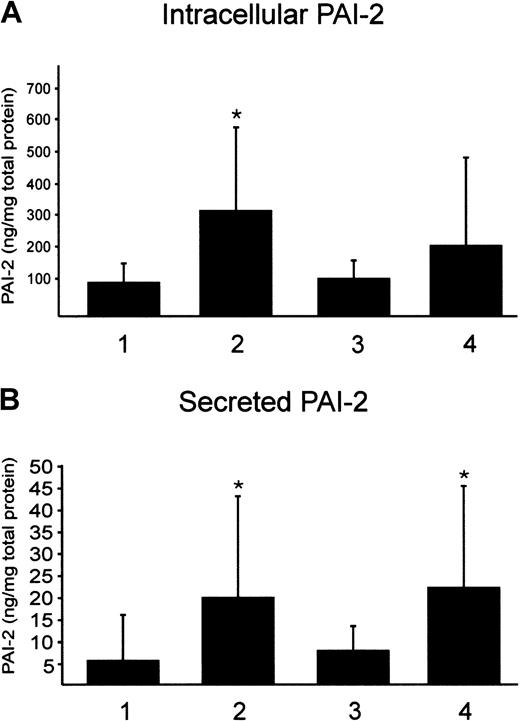
In a first approach, we determined PAI-1 and PAI-2 protein in the plasma of P1 to P3, L1 to L4, and 14 controls. Although PAI-2 is not detectable by ELISA in controls and probands with elevated Lp(a), the PAI-1 protein concentration was highly variable in all study subjects. This result was anticipated because PAI-1 is also produced in endothelial cells and adipose tissue. PAI-2 was shown to be poorly secreted and mainly stored intracellularly. Therefore, monocytes from male donors with low and high Lp(a) were isolated by Ficoll gradient and CD14 beads and cultivated for 18 hours in autologous serum. Intracellular PAI-2 protein was determined by ELISA. We found a significant, nearly 3-fold increase in the intracellular PAI-2 protein expression in probands with elevated Lp(a) compared with controls (Figure 4A). Secreted PAI-2 was found to be 3-fold induced in monocytes from probands with high Lp(a) (Figure4B). Furthermore, we determined intracellular and secreted PAI-2 in our in vitro model. Control monocytes from 6 different donors were incubated with purified Lp(a) in 20% autologous serum for 18 hours or LDL as a control. We found that intracellular PAI-2 was higher in monocytes incubated with Lp(a) but not significantly induced by Lp(a) (Figure 4A). This may be explained by the high variation of intracellular PAI-2 protein and more experiments would be necessary to obtain significant data. Secreted PAI-2 was 3-fold higher when compared with controls (Figure 4B). We found no difference in the induction of PAI-2 whether the monocytes were isolated from female or male donors (not shown). Secreted PAI-1 was found slightly decreased, with control cells secreting 7.5 ± 5.5 ng PAI-1 per milligram total cell protein and monocytes that have been incubated with Lp(a) secreting 4 ± 4.2 ng/mg cell protein.
Determination of intracellular and secreted PAI-2 by ELISA.
Ficoll-purified monocytes were isolated by magnetic beads and incubated in 20% autologous serum for 18 hours. Intracellular PAI-2 (A) and secreted PAI-2 (B) were determined in monocytes from 12 probands with low Lp(a) (1), 6 male donors with high Lp(a) (2), control monocytes from 6 probands incubated with 100 μg/mL LDL (3), or with 100 μg/mL purified Lp(a) (4). PAI-2 is given per milligram of total protein. Mean values obtained from 12 (1, 2) or 6 (3, 4) independent determinations in duplicate ± SD are shown. Significance was determined using t test (*P < .05).
Determination of intracellular and secreted PAI-2 by ELISA.
Ficoll-purified monocytes were isolated by magnetic beads and incubated in 20% autologous serum for 18 hours. Intracellular PAI-2 (A) and secreted PAI-2 (B) were determined in monocytes from 12 probands with low Lp(a) (1), 6 male donors with high Lp(a) (2), control monocytes from 6 probands incubated with 100 μg/mL LDL (3), or with 100 μg/mL purified Lp(a) (4). PAI-2 is given per milligram of total protein. Mean values obtained from 12 (1, 2) or 6 (3, 4) independent determinations in duplicate ± SD are shown. Significance was determined using t test (*P < .05).
Discussion
Elevated levels of plasma Lp(a) have been considered a risk factor for premature cardiovascular disease.1,2,27,28 The structural similarity of apo(a) with plasminogen and a subsequent competition for lysine binding sites may explain several of the atherogenic properties of this lipoprotein.29 Most of the published data examined the role of Lp(a) in the formation of the atherosclerotic plaque and the interaction of this lipoprotein with endothelial cells, smooth muscle cells, and macrophage foam cells.30 However, the influence of Lp(a) on blood monocytes is poorly investigated. There is increasing evidence that blood monocyte function may be changed by dyslipidemia.14More mature monocytes are described in patients with xanthomatosis18 and an increased expression of CCR2 was demonstrated in monocytes from donors with elevated LDL-C.31
We compared the gene expression of monocytes from a patient with isolated Lp(a) hyperlipidemia with control cells using a microarray with 8556 different cDNAs. Although 80 mRNAs were induced in patient cells, 34 were down-regulated. The expression of 8 genes with a high-balanced difference factor and known function was further investigated in additional patients and probands with elevated Lp(a) as well as in controls. We found that the mRNA levels for MCP-1, adipophilin, CD16, C3aR, and CCR2 varied between different donors. Furthermore, the expression of these genes is not changed on incubation of control monocytes with isolated Lp(a). Therefore, these genes are not associated to elevated plasma Lp(a). They were identified by the microarray approach because of interindividual variations in the mRNA levels.
IP-10 and defensin mRNA were induced in 2 of 3 patients with coronary heart disease (CHD) compared with healthy controls. The induction of these genes may be associated with CHD in general and is not an effect of high Lp(a). This interpretation is supported by our in vitro experiments. Defensin and IP-10 were not induced by purified Lp(a). These results further demonstrate that monocytes from patients with Lp(a) hyperlipidemia are not nonspecifically activated. PAI-1 mRNA were found reduced only in patients' monocytes. Healthy probands with Lp(a) levels as high as the patients show no suppression of PAI-1 mRNA. However, PAI-1 mRNA expression and protein secretion was reduced in control cells incubated with purified Lp(a) in vitro. This clearly demonstrates the discrepancy between results obtained from the incubation of monocytes with isolated Lp(a) and the mRNA expression observed in monocytes isolated from probands with elevated Lp(a). One explanation might be that additional factors besides Lp(a) contribute to the regulation of PAI-1 expression in healthy donors with high Lp(a). However, it cannot be excluded that the suppression of PAI-1 in monocytes from patients with isolated Lp(a) hyperlipidemia is not associated with elevated Lp(a) at all, because it may be an effect of the disease.
The mRNA for PAI-2 was highly abundant in 3 male patients and 3 male healthy controls with elevated Lp(a) in comparison to 12 controls without Lp(a) elevation. PAI-2 expression was not induced in monocytes from healthy premenopausal or postmenopausal women with high Lp(a). Therefore, the up-regulation of PAI-2 mRNA is gender specific. Although it is well known that premenopausal women are protected from CHD,32 postmenopausal women have the same risk as men. Whether the elevation of PAI-2 even in young men with high Lp(a) may contribute to the gender gap in CHD needs further investigation. Intracellular PAI-2 protein was significantly induced in monocytes from male probands with high Lp(a) compared with control cells. Furthermore, we found a 3-fold increase of secreted PAI-2. Similar results concerning the mRNA and protein expression of PAI-2 were obtained by using purified Lp(a) in our in vitro system. We obtained identical results whether we used monocytes isolated from male or female donors. Therefore, we suppose that Lp(a) directly influences the expression of PAI-2 in male probands, irrespective of whether the donors are healthy or have CHD (patients). However, PAI-2 mRNA is not induced in premenopausal and postmenopausal female donors with high Lp(a). Therefore, the induction of PAI-2 by elevated Lp(a) is gender specific. The underlying mechanisms are not known yet but cannot simply be explained by the gender gap in CHD because it is not evident in postmenopausal women.32
PAI-2 is the main plasminogen activator inhibitor and uPA is the main plasminogen activator secreted from monocytes. PAI-2 mostly inhibits uPA-dependent processes such as the proteolysis of fibronectin and plasminogen.33 An up-regulation of PAI-2 in monocytes can also be achieved by lipopolysaccharide.34 This indicates an important function of PAI-2 in the host response to invading bacteria. It is clearly shown that PAI-2 inhibits uPA-dependent lysis of fibrin clots and extracellular matrix35,36 and modulates uPA-mediated adhesion and migration of cells.36 PAI-2–transfected melanoma cells show an impaired degradation of extracellular matrix and reduced metastases.37 Therefore, increased PAI-2 secreted from monocytes may promote the generation of atherosclerotic lesions by a decreased proteolysis of fibrin clots and dysregulation of plasmin-dependent processes such as the activation of metalloproteinases or TGF-β. In addition, Lp(a) up-regulates PAI-1 secretion from endothelial cells,11 and PAI-1 was demonstrated to be essential for the invasion of tumor cells38 and may also support the transmigration of monocytes to the subendothelium. However, the plasminogen system is regulated by various factors and is linked to multiple pathways, and therefore consequences of elevated PAI-2 secretion from blood monocytes over years or decades are difficult to analyze. Nevertheless, elevated Lp(a) is a risk factor for premature cardiovascular disease and our data indicate that increased PAI-2 expression in blood monocytes is a direct effect of Lp(a).
Lp(a) modulates the gene expression of blood monocytes thereby inducing a proinflammatory response of these cells with respect to the PAI-2 expression. Additional studies in a large patient group are necessary to address the question whether the induction of PAI-2 may be an early marker related to the onset of atherosclerosis in probands with elevated Lp(a) and contributes to the gender gap in cardiovascular disease.
Acknowledgments
The expert technical assistance of Cornelia Hasenknopf, Barbara Schmitz, Daniela Hant, and Michael Wermers and the technical advice of Harald Grillhofer are greatly appreciated.
Supported by a grant from the Deutsche Forschungsgemeinschaft (no. AN111/6-5) and in part by a grant from the Austrian Foundation (no. SFB 702) to G.M.K.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Gerd Schmitz, Institute for Clinical Chemistry and Laboratory Medicine, University of Regensburg, Franz-Josef-Strauß-Allee 11, D-93053 Regensburg, Germany; e-mail:gerd.schmitz@klinik.uni-regensburg.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal