Tyrosine kinase fusion oncogenes that occur as a result of chromosomal translocations have been shown to activate proliferative and antiapoptotic pathways in leukemic cells, but the importance of autocrine and paracrine expression of hematopoietic cytokines in leukemia pathogenesis is not understood. Evidence that leukemic transformation may be, at least in part, cytokine dependent includes data from primary human leukemia cells, cell culture experiments, and murine models of leukemia. This report demonstrates that interleukin (IL)-3 plasma levels are elevated in myeloproliferative disease (MPD) caused by the TEL/tyrosine kinase fusions TEL/platelet-derived growth factor beta receptor (PDGFβR), TEL/Janus kinase 2 (JAK2), and TEL/neurotrophin-3 receptor (TRKC). Plasma granulocyte-macrophage colony-stimulating factor (GM-CSF) levels were elevated by TEL/PDGFβR and TEL/JAK2. However, all of the fusions tested efficiently induced MPD in mice genetically deficient for both GM-CSF and IL-3, demonstrating that these cytokines are not necessary for the development of disease in this model system. Furthermore, in experiments using normal marrow transduced with TEL/PDGFβR retrovirus mixed with marrow transduced with an enhanced green fluorescent protein (EGFP) retrovirus, the MPD induced in these mice demonstrated minimal stimulation of normal myelopoiesis by the TEL/PDGFβR-expressing cells. In contrast, recipients of mixed GM-CSF–transduced and EGFP-transduced marrow exhibited significant paracrine expansion of EGFP-expressing cells. Collectively, these data demonstrate that, although cytokine levels are elevated in murine bone marrow transplant models of leukemia using tyrosine kinase fusion oncogenes, GM-CSF and IL-3 are not required for myeloproliferation by any of the oncogenes tested.
Introduction
Despite extensive study, the precise role of hematopoietic cytokines in the development of leukemia is not fully understood. However, there is evidence to suggest that leukemogenesis is a cell autonomous process. Murine leukemias induced by the Abelson virus do not require cytokines,1,2 and cytokine messenger RNA (mRNA) levels are not elevated in cultured chronic myelogenous leukemia (CML) cells compared with normal bone marrow cells.3 Furthermore, BCR/ABL and other leukemia-associated tyrosine kinase fusions activate proliferative4 and antiapoptotic pathways5,6in leukemia cells. Antibody blocking studies have shown that BCR/ABL cell lines do not require granulocyte-macrophage colony-stimulating factor (GM-CSF) to enter the cell cycle,7 nor do they require interleukin (IL)-3 or GM-CSF to activate downstream cytokine activation pathways, including the activation of signal transducer and activator of transcription (STAT) 5.8
However, several lines of evidence suggest that hematopoietic growth factors might play a critical role in the development of leukemia. Studies on primary human leukemia cells have shown that they produce bioactive cytokines9-16 and usually require cytokines for growth in vitro.17-20 Autocrine production of IL-3 and granulocyte colony-stimulating factor has also been demonstrated in CD34+ leukemic blasts from some patients with chronic-phase CML,10 and cytokines may facilitate the differentiation of CML blasts.21
Also, it is clear that GM-CSF regulates the growth of leukemic cells from patients with juvenile myelomonocytic leukemia (JMML).22-25 The central role that GM-CSF plays has recently been confirmed in a murine model of neurofibromin (Nf1)-associated JMML.25 However, the relevance of this finding to other forms of myeloid leukemia is not known.
Data from cell line transfection studies and murine leukemia models also suggest a role for cytokines in leukemogenesis. BCR/ABL activates signal transduction pathways that overlap with those activated by cytokines,26 and hematopoietic cells transfected with BCR/ABL are growth factor independent27,28 and secrete hematopoietic growth factors.29 Furthermore, BCR/ABL+ acute lymphoblastic leukemia cell lines produce GM-CSF and express the GM-CSF receptor,30 and acute myeloid leukemia (AML) blast cells are responsive to recombinant GM-CSF.31
Growth factor levels are elevated in several murine models of leukemia.32-37 Although murine bone marrow transplantation (mBMT) assays have demonstrated the leukemogenic potential of the tyrosine kinase fusions BCR/ABL,38 TEL/Janus kinase 2 (JAK2),39 TEL/platelet-derived growth factor beta receptor (PDGFβR),40 and TEL/neurotrophin-3 receptor (TRKC)41 by using primary murine hematopoietic cells, ectopic expression of IL-3,42 GM-CSF,43,44 or IL-645 46 by themselves induce myeloproliferative phenotypes in mice.
Furthermore, certain observations in the mBMT experiments have supported the hypothesis that paracrine stimulation of hematopoietic cells might contribute to the disease phenotype. Zhang and Ren33 have reported high levels of GM-CSF and IL-3 mRNA and protein in mice transplanted with bone marrow cells retrovirally transduced by BCR/ABL.33 In addition, when EGFP is used as a marker for retroviral infection in mBMT experiments, a significant proportion of myeloid cells in leukemic mice are EGFP− and may not express the tyrosine kinase fusion oncogene.33,39 These data suggest that nontransduced hematopoietic cells may be proliferating in response to paracrine stimuli such as hematopoietic cytokines. Hematopoietic growth factors and their receptors would be potential targets for pharmacologic intervention in these diseases.47
We sought to conclusively determine the role that the cytokines GM-CSF and IL-3 play in the development of MPD mediated by tyrosine kinase fusion proteins. The fusion oncogenes TEL/PDGFβR, TEL/JAK2, and TEL/TRKC induced elevated levels of GM-CSF and IL-3 in mBMT models of leukemia. With the use of mice genetically devoid of these cytokines, we have demonstrated that GM-CSF and IL-3 are not required for induction of MPD by any of these constitutively activated tyrosine kinases.
Materials and methods
Constructs
Construction of retroviral vectors expressing TEL/PDGFβR, TEL/JAK2, and TEL/TRKC has been described previously.39-41Each vector used in the double knockout animal experiments expresses the respective fusion oncogene from the viral long terminal repeat (LTR), and EGFP simultaneously from the internal ribosomal entry site (IRES). All retroviral supernatants used were produced by transient transfection of 293T cells as previously described.48 For bone marrow mixing studies, the GM-CSF complementary DNA (cDNA; provided by Frank Lee, DNAX, Palo Alto, CA) was subcloned into the multicloning site of murine stem cell virus (MSCV)-neo (Clontech, Palo Alto, CA). Construction of a retroviral vector-expressing TEL/PDGFβR and the neomycin-resistance gene has been described previously.40 All viral supernatants had equivalent titers as assessed by EGFP expression in transduced Ba/F3 cells.
Mouse strains
Mice with homozygous null mutations in both the Il-3and Gm-csf genes (S.G. et al, manuscript submitted) were backcrossed more than 9 generations into a Balb/c background. Wild-type Balb/c mice were purchased from Taconic Farms (Germantown, NY).
Murine bone marrow transplantation
Transplant assays were performed as previously described.49 Briefly, donor mice were prepared by a single intraperitoneal dose of 5-fluorouracil (5-FU; 150 mg/kg; Sigma, St Louis, MO) on day −8. Six days later, on day −2, 5-FU–primed mice were killed, and bone marrow cells were isolated by flushing femurs and tibias with RPMI media supplemented with 10% fetal bovine serum (GibcoBRL, Rockville, MD). Cells were incubated at 37°C overnight in media supplemented with IL-3, stem cell factor, and IL-6 (R&D Systems, Minneapolis, MN). On day −1 the media was changed, 1 mL of retroviral supernatant and 2 μg/μL Polybrene (Sigma) was added, and cells were centrifuged at 1000g for 90 minutes in a 6-well tissue culture plate. On day 0, the above centrifuge infection procedure was repeated. Cells were then resuspended in Hanks buffered salt solution (GibcoBRL), at a concentration of 106 cells/mL, and 0.5 to 1.0 mL was intravenously injected into BALB/c mice, lethally irradiated with 900 cGy by lateral tail vein. Mice were monitored thrice weekly for the development of disease.
Cytokine quantitation
Mice were anesthetized using Metofane (Medical Developments, Springvale, Victoria, Australia), and plasma was obtained by retro-orbital phlebotomy by using heparinized capillary tubes (VWR, McGaw Park, IL). Blood was anticoagulated by using 10 mM EDTA and subjected to centrifugation of 1000g for 5 minutes. Plasma was stored at −80°C until analysis. Analysis was performed by using the commercially available enzyme-linked immunosorbent assay (ELISA; Cytimmune Sciences, College Park, MD) according to the manufacturer's instructions.
Histopathology and flow cytometry
Histopathologic sections were obtained as previously described.40 Spleen cell suspensions were obtained and processed for flow cytometry as described previously.50 In addition, propidium iodide (PI; Sigma) was added to stained and washed cells in some experiments at a final concentration of 100 ng/mL 5 minutes before analysis on the cytometer. Antibodies used in the current analysis were allophycocyanin conjugated anti-Gr-1, phycoerythrin-conjugated anti-Mac-1, biotin-conjugated anti-CD19, and phycoerythrin-conjugated anti-Thy-1.2 (Pharmingen, San Diego, CA). Allophycocyanin-conjugated streptavidin (Caltag, South San Francisco, CA) was used as a secondary reagent to detect biotinylated anti-CD19. Acquisition and data analysis were performed as previously described.40 For high-volume flow cytometry, peripheral blood mononuclear cells were isolated by incubating whole blood from affected animals in red blood cell lysis solution (150 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA, pH 7.4) for 5 minutes and washing cells once in phosphate-buffered saline. Unstained cells were then analyzed for EGFP fluorescence, using a MoFlo flow cytometer and CyCLOPS Summit software (Cytomation, Fort Collins, CO).
Results
Cytokine levels are elevated in mice by leukemia-associated tyrosine kinase fusion proteins
Murine BMT experiments using wild-type Balb/c donor and recipient mice and TEL/JAK2, TEL/PDGFβR, and TEL/TRKC were performed. As has been described previously, 100% of mice developed MPD (data not shown). At 3 to 5 weeks following transplant, plasma samples from Balb/c mice transplanted with nontransduced marrow (n = 3) and mice transplanted with vector-transduced marrow (n = 6), TEL/PDGFβR (n = 6), TEL/TRKC (n = 3), or TEL/JAK2 (n = 3) were analyzed for the cytokines GM-CSF and IL-3 by the ELISA immune assay (Figure1). In related studies,51 we found that mice with BCR/ABL-induced MPD had increased IL-3 levels but normal levels of GM-CSF. The mean levels of IL-3 were significantly elevated in mice transplanted with TEL/PDGFβR (14-fold) and TEL/JAK2 (11-fold) compared to control mice. Levels of IL-3 were also elevated in TEL/TRKC mice (4.5-fold) but did not achieve statistical significance.
Elevated GM-CSF and IL-3 levels in mice transplanted with tyrosine kinase fusions.
Plasma from wild-type Balb/c mice transplanted with fusion oncogenes indicated was assayed independently for IL-3 and GM-CSF. Control BMT denotes mice transplanted with untransduced marrow; MSCVneo BMT denotes mice transplanted with bone marrow transduced with vector alone. Values that are significantly elevated (P < .05) over vector only controls are indicated by an asterisk.
Elevated GM-CSF and IL-3 levels in mice transplanted with tyrosine kinase fusions.
Plasma from wild-type Balb/c mice transplanted with fusion oncogenes indicated was assayed independently for IL-3 and GM-CSF. Control BMT denotes mice transplanted with untransduced marrow; MSCVneo BMT denotes mice transplanted with bone marrow transduced with vector alone. Values that are significantly elevated (P < .05) over vector only controls are indicated by an asterisk.
The same samples were also quantitatively assayed for GM-CSF (Figure1). Like BCR/ABL, transplantation with TEL/TRKC did not substantially elevate GM-CSF levels. The mean GM-CSF level in mice transplanted with TEL/JAK2 was elevated (34%) but not significantly (Figure 1). In contrast, TEL/PDGFβR caused a modest (51%) but significant increase in GM-CSF levels. These data are consistent with the possibility that the leukemic phenotype induced by tyrosine kinase fusion oncogenes was due in part to enhanced expression of hematopoietic cytokines.
Tyrosine kinase fusions cause fatal myeloproliferation in mice deficient for GM-CSF and IL-3
To directly determine the role of GM-CSF and IL-3 in the development of tyrosine kinase–induced MPD, mBMT experiments were performed using bone marrow derived from mice genetically deficient in both GM-CSF and IL-3 (double knockout mice) (Gillessen et al, manuscript submitted). Mice transplanted with TEL/PDGFβR, TEL/JAK2, or with TEL/TRKC (Table 1) all (100%) developed fatal MPD with the same latency as seen in experiments with wild-type donors (Table 1 and data not shown). To exclude the possibility that GM-CSF or IL-3 secreted from recipient stromal cells, residual hematopoietic cells, or other tissue sources contributed to leukemogenesis, we repeated a set of transplant experiments by using mice deficient in GM-CSF and IL-3 both as bone marrow donors and as transplant recipients. All of these mice also developed MPD (Table 1and Figure 2). The results described below refer to data obtained from mice transplanted using GM-CSF/IL-3 deficient marrow into GM-CSF/IL-3 deficient recipients.
Results of murine bone marrow transplants using mice deficient in GM-CSF and IL-3
| Oncogene . | Donor . | Recipient . | Mice (n) . | Median survival (wk) . |
|---|---|---|---|---|
| TEL/PDGFR | KO | Wild type | 4 | 2.7 |
| TEL/PDGFR | KO | KO | 8 | 3.3 |
| TEL/JAK2 | KO | Wild type | 8 | 4.7 |
| TEL/JAK2 | KO | KO | 4 | 5.3 |
| TEL/TRKC | KO | Wild type | 4 | 1.8 |
| TEL/TRKC | KO | KO | 8 | 6.8* |
| Oncogene . | Donor . | Recipient . | Mice (n) . | Median survival (wk) . |
|---|---|---|---|---|
| TEL/PDGFR | KO | Wild type | 4 | 2.7 |
| TEL/PDGFR | KO | KO | 8 | 3.3 |
| TEL/JAK2 | KO | Wild type | 8 | 4.7 |
| TEL/JAK2 | KO | KO | 4 | 5.3 |
| TEL/TRKC | KO | Wild type | 4 | 1.8 |
| TEL/TRKC | KO | KO | 8 | 6.8* |
GM-CSF indicates granulocyte-macrophage colony-stimulating factor; IL-3, interleukin 3; KO, Gmcsf−/− and Il3−/− doubly deficient mice.
Statistically significant (P < .003) difference between survival of mice transplanted into doubly deficient versus wild-type recipients.
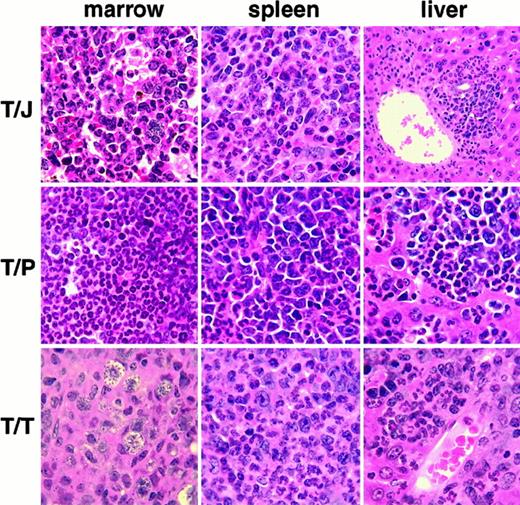
Histopathology of MPD induced by tyrosine kinase fusions in the absence of GM-CSF and IL-3.
TEL/JAK2 (T/J), TEL/PDGFβR (T/P), TEL/TRKC (T/T). MPD is manifested in each case by destructive infiltration of the liver, and replacement of normal hematopoietic elements in the bone marrow and spleen by myeloid lineage cells, including many mature granulocytes. The T/J liver panel is magnified at low power (10×), all other panels are magnified at high power (100×). All panels are views of tissues stained with hematoxylin and eosin.
Histopathology of MPD induced by tyrosine kinase fusions in the absence of GM-CSF and IL-3.
TEL/JAK2 (T/J), TEL/PDGFβR (T/P), TEL/TRKC (T/T). MPD is manifested in each case by destructive infiltration of the liver, and replacement of normal hematopoietic elements in the bone marrow and spleen by myeloid lineage cells, including many mature granulocytes. The T/J liver panel is magnified at low power (10×), all other panels are magnified at high power (100×). All panels are views of tissues stained with hematoxylin and eosin.
The latency of disease in TEL/JAK2 and TEL/PDGFβR transplanted GM-CSF/IL-3 deficient mice was not significantly different from wild-type mice receiving the same transduced marrow (Table 1). However, the latency of disease in TEL/TRKC mice was significantly delayed (P = .003) when double knockout mice were used both as marrow donors and as recipients (Table 1). The disease phenotype in mice transplanted without GM-CSF or IL-3 was similar to that seen in the corresponding wild-type experiments and included extramedullary hematopoiesis, splenomegaly with effacement of the spleen by myeloproliferation, granulocytic leukocytosis, and replacement of the bone marrow by myeloproliferation (Figure 2).
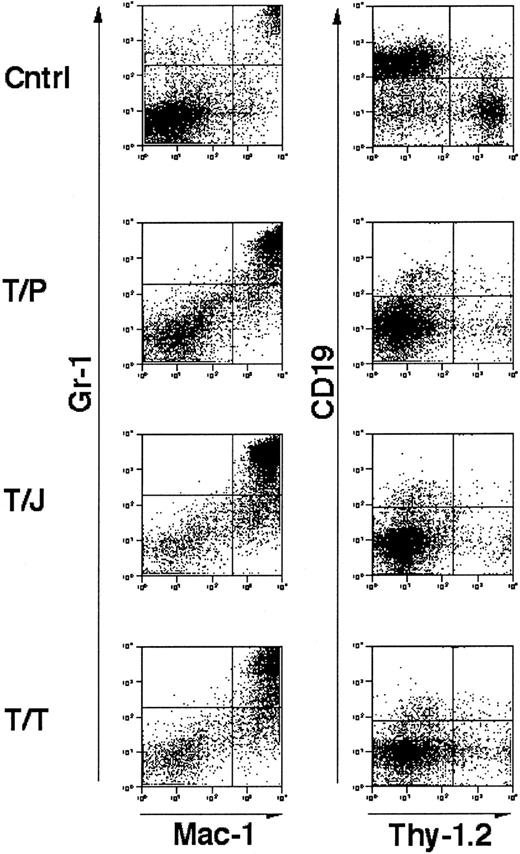
Flow cytometric analysis of cells isolated from the spleens of affected animals demonstrated an abnormal population of Gr-1+, Mac-1+ mature granulocytes (Figure3). These results are similar to those obtained previously in mBMTs with wild-type marrow and wild-type recipients.39-41 These data indicate that mice that are genetically deficient in GM-CSF and IL-3 develop fatal MPD in response to TEL/PDGFβR, TEL/JAK2, and TEL/TRKC tyrosine kinase fusion oncogenes.
Immunophenotype of splenocytes from GM-CSF−/−, IL-3−/− doubly deficient mice transplanted with tyrosine kinase fusion oncogenes.
Unfractionated splenocytes stained for mature granulocyte markers Gr-1 and Mac-1 (left panels) and lymphocyte markers CD19 and Thy-1.2 (right panels). “Cntrl” indicates a negative control comprised of spleen cells from a mouse transplanted with a kinase inactive mutant of TEL/TRKC.41 T/P, TEL/PDGFβR; T/J, TEL/JAK2, T/T, TEL/TRKC. Cells were analyzed from mice 3 to 4 weeks following transplant. In all cases, splenic involvement is characterized by pathologic myeloproliferation. Negative control cells demonstrate minimal myeloproliferation and normal lymphocyte populations.
Immunophenotype of splenocytes from GM-CSF−/−, IL-3−/− doubly deficient mice transplanted with tyrosine kinase fusion oncogenes.
Unfractionated splenocytes stained for mature granulocyte markers Gr-1 and Mac-1 (left panels) and lymphocyte markers CD19 and Thy-1.2 (right panels). “Cntrl” indicates a negative control comprised of spleen cells from a mouse transplanted with a kinase inactive mutant of TEL/TRKC.41 T/P, TEL/PDGFβR; T/J, TEL/JAK2, T/T, TEL/TRKC. Cells were analyzed from mice 3 to 4 weeks following transplant. In all cases, splenic involvement is characterized by pathologic myeloproliferation. Negative control cells demonstrate minimal myeloproliferation and normal lymphocyte populations.
A large proportion of EGFP− myeloid cells from TEL/PDGFβR-IRES-EGFP-induced MPD are nonviable
A significant percentage of myeloid cells in mice with MPD induced by BCR/ABL33 TEL/JAK2,39 and TEL/PDGFβR40 with the IRES-EGFP vector are EGFP−, and it was suggested that this represented paracrine stimulation of nontransduced myeloid cells by IL-3 and/or GM-CSF.33 However, we found a similar population of GR-1+/EGFP− myeloid cells in mice transplanted with TEL/PDGFβR or TEL/TRKC when both donor and recipient were IL-3/GM-CSF double knockout mice (Figure4A). To further assess potential paracrine contributions to disease, we performed propidium iodide (PI) staining to assess viability of EGFP+ and EGFP− myeloid lineage cells in leukemic mice. We found that in mice transplanted with TEL/PDGFβR or TEL/TRKC, 46% to 78% of Gr-1+/EGFP− cells did not exclude PI, indicating that they were not viable (Figure 4B). In contrast, only 1% to 2% of Gr-1+/EGFP+ cells were PI positive, indicating that nearly all of the EGFP+ cells were viable at the time of analysis. A similar effect was seen in the TEL/JAK2-transplanted mice (data not shown). PI gating to exclude nonviable cells thus substantially decreased the population of EGFP− cells and correspondingly decreased the magnitude of the putative paracrine effect as manifested by EGFP−cells. To further assess this phenomenon, fresh peripheral blood cells from 2 animals with TEL/PDGFβR-induced MPD were analyzed for EGFP expression without subjecting the cells to the prolonged antibody incubation necessary for immunophenotype analysis. EGFP−cells comprised only 5% to 8% of total cells analyzed in this fashion (Figure 4A). Taken together, these data indicated that the EGFP− cell population was not the consequence of paracrine stimulation of nontransduced marrow. Rather, these data suggest that a significant proportion of the EGFP− myeloid cells are nonviable and that the time and/or washing steps required for processing cells for immunophenotype analysis increases the number of EGFP− myeloid cells.
Nonviable myeloid cells lose GFP staining.
(A) Unstained peripheral blood leukocytes from 2 animals with TEL/PDGFβR-induced MPD were analyzed for EGFP expression, using high-volume flow cytometry. (B) Spleen cells from GM-CSF−/− and IL-3−/− double-deficient mice transplanted with tyrosine kinase fusion oncogenes (as in Figure 3) were analyzed for Gr-1 and EGFP positivity. Gr-1+ cells were gated into EGFP− (R1) and EGFP+ (R2) populations and were assessed for PI staining. Nonviable cells uptake PI and stain positively. EGFP− cells (R1 gate, left panels) are comprised of both PI-positive and -negative populations. EFGP+ cells (R2 gate, right panels) are comprised predominantly of a single population of PI-negative cells.
Nonviable myeloid cells lose GFP staining.
(A) Unstained peripheral blood leukocytes from 2 animals with TEL/PDGFβR-induced MPD were analyzed for EGFP expression, using high-volume flow cytometry. (B) Spleen cells from GM-CSF−/− and IL-3−/− double-deficient mice transplanted with tyrosine kinase fusion oncogenes (as in Figure 3) were analyzed for Gr-1 and EGFP positivity. Gr-1+ cells were gated into EGFP− (R1) and EGFP+ (R2) populations and were assessed for PI staining. Nonviable cells uptake PI and stain positively. EGFP− cells (R1 gate, left panels) are comprised of both PI-positive and -negative populations. EFGP+ cells (R2 gate, right panels) are comprised predominantly of a single population of PI-negative cells.
Direct assessment of paracrine stimulation of bone marrow cells in mixing experiments
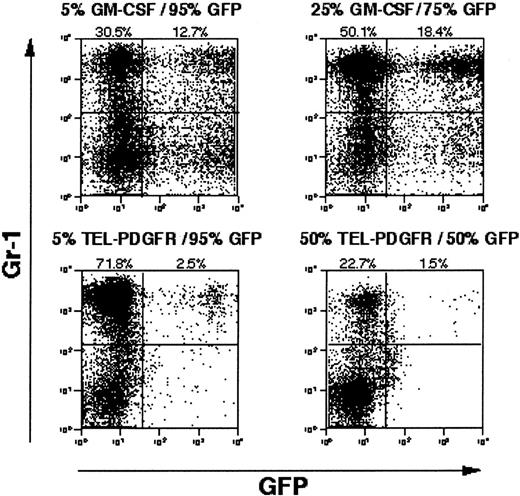
Although PI gating decreased the magnitude of the EGFP− cell population, there was still a significant proportion of Gr-1+/EGFP− cells present in MPD spleens (10% to 18%). To more directly assess a paracrine contribution to the disease phenotype, we performed experiments in which bone marrow cells transduced with TEL/PDGFβR retrovirus lacking EGFP were admixed with bone marrow cells transduced with a retrovirus containing EGFP but no tyrosine kinase fusion oncogenes (Figure5A). A significant paracrine contribution to the leukemia phenotype would be expected to result in expansion of the EGFP+ population of cells. As a positive control for a paracrine effect, bone marrow cells transduced with a retrovirus containing GM-CSF were also admixed with EGFP-transduced bone marrow cells. In MPD induced by retroviral transduction of GM-CSF, a significant proportion of cells in the spleen of disease animals are Gr-1+/EGFP+ (15%), consistent with GM-CSF–mediated paracrine stimulation of cells. In contrast, when TEL/PDGFβR-transduced cells were admixed with EGFP-expressing cells, only 2% of the Gr-1+ population was EGFP+(Figure 5B). These data indicated that the MDP induced by TEL/PDGFβR did not rely to any significant extent on paracrine mechanisms.
Competitive repopulation with GM-CSF versus TEL/PDGFβR.
Wild-type Balb/c bone marrow transduced with GM-CSF or TEL/PDGFβR was combined with wild-type Balb/c bone marrow transduced with EGFP alone and was transplanted into lethally irradiated syngeneic mice. After development of MPD evidenced by splenomegaly, splenocytes from GM-CSF plus EGFP mice (top panels) and TEL/PDGFβR plus EGFP mice (bottom panels) were assayed for expression of EGFP. Input populations of cells were 5% GM-CSF with 95% EGFP (upper left), 25% GM-CSF with 75% EGFP (upper right), 5% TEL/PDGFβR with 95% EGFP (lower left), and 50% TEL/PDGFβR with 50% EGFP (lower right).
Competitive repopulation with GM-CSF versus TEL/PDGFβR.
Wild-type Balb/c bone marrow transduced with GM-CSF or TEL/PDGFβR was combined with wild-type Balb/c bone marrow transduced with EGFP alone and was transplanted into lethally irradiated syngeneic mice. After development of MPD evidenced by splenomegaly, splenocytes from GM-CSF plus EGFP mice (top panels) and TEL/PDGFβR plus EGFP mice (bottom panels) were assayed for expression of EGFP. Input populations of cells were 5% GM-CSF with 95% EGFP (upper left), 25% GM-CSF with 75% EGFP (upper right), 5% TEL/PDGFβR with 95% EGFP (lower left), and 50% TEL/PDGFβR with 50% EGFP (lower right).
Discussion
Recent findings have suggested that cell nonautonomous factors, such as hematopoietic cytokines, might contribute to disease pathogenesis in human MPD and in mBMT models of MPD induced by tyrosine kinase fusions.33 Indeed, we have observed elevated IL-3 protein levels in the plasma of mice with MPD induced by all of the tyrosine kinase fusions examined. Elevations in levels of GM-CSF were more modest. We have demonstrated that GM-CSF and IL-3 are not required for development of MPD induced by BCR/ABL.51 Furthermore, in the experiments described above, we have shown that TEL/PDGFβR, TEL/JAK2, and TEL/TRKC also do not require GM-CSF or IL-3.
To definitively test the role of both IL-3 and GM-CSF in the development of disease in mice, we used a strategy whereby the respective tyrosine kinase fusion protein associated with human leukemia is retrovirally transduced into bone marrow that is deficient for GM-CSF and IL-3. We observed no significant differences in histopathology, immunophenotype, or disease latency when comparing GM-CSF/IL-3 double-deficient hematopoietic progenitors with wild-type progenitors. Furthermore, there were also no differences in the histopathology or immunophenotype when double-deficient mice were used both as marrow donors and as recipients, eliminating the possibility that stromal cells elaborate GM-CSF or IL-3 necessary for disease pathogenesis. These data demonstrate that these cytokines are not required for development of disease in this model.
Although all animals eventually succumbed to disease, when IL-3– and GM-CSF–deficient cells expressing TEL/TRKC were transplanted into doubly deficient recipient mice, 2 of 4 animals survived longer than expected. This phenomenon was not seen when deficient donor cells were transplanted into wild-type recipients. Although we cannot exclude the possibility that there is a subtle effect on disease latency in TEL/TRKC mice, the fact that the other mice in this experiment died rapidly from MPD suggests that there may be a recipient-related effect on survival besides the development of disease per se. For example, the accumulation in the lungs of surfactant lipids reported in GM-CSF–deficient mice52-54 may have protected these mice from death due to pulmonary infiltration and/or hemorrhage. This hypothesis is consistent with data indicating that TEL/TRKC activates signal transduction pathways distinct from those activated by the other tyrosine kinase fusions tested.41
In mice transplanted with hematopoietic progenitors transduced with TEL/JAK2, TEL/PDGFβR, TEL/TRKC, or BCR/ABL and EGFP, there is a significant population of Gr-1+ cells that do not express EGFP. Our data demonstrate that a significant proportion of EGFP− myeloid cells from MPD mice are nonviable, as judged by PI staining, whereas EGFP+ cells are nearly all viable. These data indicate that rapid processing of cells and PI gating provide a more accurate assessment of EGFP expression in this context. This phenomenon could be potentially explained by egress of EGFP from nonviable cells.
However, not all of the EGFP− myeloid cells excluded PI. To further assess a possible contribution of paracrine disease mechanisms to disease pathogenesis, we performed mixing experiments with cells transduced with TEL/PDGFβR that lacked EGFP and with cells transduced with EGFP retrovirus without TEL/PDGFβR. TEL/PDGFβR-transduced cells caused the expected myeloproliferative phenotype but did not demonstrate any ability to stimulate proliferation of EGFP+ cells through cell nonautonomous mechanisms. In control experiments using GM-CSF retrovirus rather than TEL/PDGFβR, there was a significant paracrine effect as expected. In addition, freshly derived TEL/PDGFβR cells sorted to exclude PI-positive cells showed only a small percentage of cells that were Gr-1+ and EGFP−. Taken together, these data indicate minimal cell nonautonomous contribution to TEL/PDGFβR-induced MPD.
The lack of evidence for a contribution of GM-CSF and IL-3 to the disease phenotype in the murine model of leukemia should be interpreted with caution. We have excluded a central role for GM-CSF and IL-3 in disease pathogenesis induced by each of the tyrosine kinase fusion oncogenes tested in our model, and we find no evidence for paracrine stimulation in the case of TEL/PDGFβR. However, other cytokines may still play a role in disease mediated by BCR/ABL, TEL/JAK2, or TEL/TRKC. Granulocyte colony-stimulating factor has been shown to play a role in autocrine stimulation of BCR/ABL+ human leukemia cells,10 and oncostatin M has recently been identified as a possible mediator of TEL/JAK2 disease.55 Further experiments will be required to determine the precise role of these cytokines in disease pathogenesis. Also, it should be noted that these experiments have focused on the myeloproliferative phenotype induced by these fusion proteins. They do not exclude the possibility that the phenotype associated with blast crisis of CML for example, or lymphoid neoplasms caused by tyrosine kinase fusions such as nucleophosmin anaplastic lymphoma kinase (NPM/ALK), might be due in part to noncell autonomous mechanisms. It is also possible that therapies directed at specific hematopoietic cytokines in humans might ameliorate disease phenotype, even if there is not an absolute requirement for that cytokine in murine models of disease.
In summary, analysis of mice deficient in GM-CSF and IL-3 has demonstrated that these cytokines are not required in an mBMT assay for the development of MPD induced by a spectrum of tyrosine kinase fusions associated with human hematologic malignancy.
We thank Francesca Garcia for secretarial assistance, and Dr Katherine Weilbaecher and Dr Timothy Ley for critical reading of the manuscript.
G.D. is a Clinical Scholar of the Leukemia and Lymphoma Society. R.A.V.E. is a Scholar of the Leukemia and Lymphoma Society and the Carl and Margaret Walter Scholar in Blood Research at Harvard Medical School.
Supported in part by National Institute Health grants CA81197-01 (M.H.T.), AR44628 (I.R.W.), CA57593 (R.A.V.E.), CA74886, CA39542 (G.D.), DK50654, and CA66996 (D.G.G.) and the MarJo Foundation. Support was also received from the Swiss National Science Foundation, the Swiss Cancer League (S.G.), and the Cancer Research Institute/Partridge Foundation (G.D.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michael H. Tomasson, Division of Oncology Services, Section of Bone Marrow Transplantation, 660 South Euclid Ave, Campus Box 8007, St Louis, MO 63110.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal