Posttransplantation lymphoproliferative disease (PTLD) is a major complication of current clinical transplantation regimens. The lack of a reproducible large-animal model of PTLD has limited progress in understanding the pathogenesis of and in developing therapy for this clinically important disease. This study found a high incidence of PTLD in miniature swine undergoing allogeneic hematopoietic stem cell transplantation and characterized this disease in swine. Two days before allogeneic peripheral blood stem cell transplantation, miniature swine were conditioned with thymic irradiation and in vivo T-cell depletion. Animals received cyclosporine daily beginning 1 day before transplantation and continuing for 30 to 60 days. Flow cytometry and histologic examination were performed to determine the cell type involved in lymphoproliferation. Polymerase chain reaction was developed to detect and determine the level of porcine gammaherpesvirus in involved lymph node tissue. PTLD in swine is morphologically and histologically similar to that observed in human allograft recipients. Nine of 21 animals developed a B-cell lymphoproliferation involving peripheral blood (9 of 9), tonsils, and lymph nodes (7 of 9) from 21 to 48 days after transplantation. Six of 9 animals died of PTLD and 3 of 9 recovered after reduction of immunosuppression. A novel porcine gammaherpesvirus was identified in involved tissues. Miniature swine provide a genetically defined large-animal model of PTLD with many characteristics similar to human PTLD. The availability of this reproducible large-animal model of PTLD may facilitate the development and testing of diagnostic and therapeutic approaches for prevention or treatment of PTLD in the clinical setting.
Introduction
Our laboratory has been successful in developing protocols for establishing mixed chimerism and tolerance across major histocompatibility complex (MHC) barriers in miniature swine without the use of whole-body irradiation (WBI).1 In one of our protocols developed for this purpose we have observed a high incidence of posttransplantation lymphoproliferative disorder (PTLD). Because of the importance of PTLD clinically, we have attempted to characterize this phenomenon in the miniature swine model.
The development of lymphoid neoplasms in allograft recipients receiving immunosuppressive therapy has been recognized as a major complication of solid organ and bone marrow transplantation for over 30 years.2,3 PTLD and acquired immunodeficiency syndrome (AIDS)-associated B-cell lymphoma are serious and often lethal complications of immunosuppression. The majority of neoplasms involved in PTLD, including those lacking surface immunoglobulin expression, are of B-cell origin.4,5 A strong correlation has been reported between B-cell neoplasms developing in immunosuppressed patients and the presence of the B-lymphotropic gammaherpesvirus Epstein-Barr virus (EBV).6 In humans, PTLD is thought to represent a spectrum of EBV-driven lymphoid proliferations ranging in histologic appearance from a reactive polymorphic expansion of EBV-infected lymphocytes to monoclonal B-cell lymphomas.7Studies of patients who developed PTLD have implicated several risk factors, including T-cell depletion and the degree of immunosuppression; however, the pathogenesis of PTLD is not completely understood.8 9 The lack of a reproducible large-animal model of PTLD has limited progress in understanding the pathogenesis of and in developing therapy for this clinically important disease.
Materials and methods
Animals
The original herd of National Institutes of Health (NIH) miniature swine inbred at the MHC locus has been described in detail.10,11 Animals from this herd, bred and maintained at our current facility, are referred to as MGH MHC inbred miniature swine. Transplant donors (4-6 months old) and recipients (8-12 weeks old) were selected from our herd of MGH MHC inbred miniature swine. Donor and recipient animals were selected as pig allelic antigen positive or negative (PAA+ or PAA−), respectively, to facilitate chimerism detection.12 All experiments were performed in accordance with NIH guidelines for the care and use of laboratory animals.
Thymicirradiation
Thymic irradiation (700-1000 cGy) was administered on day −2 as previously described.13
Recipient T-cell depletion
Cyclosporin A treatment
Cyclosporine A (CyA; Neoral; Novartis, East Hanover, NJ) was administered orally at approximately 15 to 30 mg/kg per day in divided doses from day −1 to day 30 with or without tapering to day 60. Serum CyA levels were monitored daily and dosage of CyA was adjusted to attempt to maintain a level of 400 to 800 mg/dL over the first 30 days.
Peripheral blood stem cell collection
A stem cell mobilizing regimen was administered to donors of peripheral blood stem cells (PBSCs). This regimen consisted of daily treatments with porcine stem cell factor (pSCF; 100 μg/kg) in combination with porcine interleukin 3 (pIL-3; 100 μg/kg), both from Biotransplant (Boston, MA), administered subcutaneously. Collection of PBSCs was achieved by leukapheresis (Cobe Spectra Apheresis System, Lakewood, CO) beginning on day 5 of cytokine therapy and continuing daily until sufficient numbers of cells were collected. PBSCs, either fresh or frozen and thawed, were adjusted to a concentration of 2.0 × 108/mL, and the appropriate volume was infused via catheter over 15 to 20 minutes. PBSCs were administered beginning on day 0, and on 2 to 3 successive days, for a total of 100 to 200 × 108/kg.
Antibodies and flow cytometry
Antibodies used for flow cytometry were as follows: CD1 76-7-4 Balb/c IgG2aK16; CD2 MSA-4; CD3 898H2-6-15 C3H/HEJ IgG2aK15; CD5 BB6-9G12 IgG117; CD16 G7; class II DR 40D; CD21 BB6-11C9; anti-IgM 5C9; anti-κ light-chain K139 3E1; pig monocyte/granulocyte-specific SWC3a 74-22-15 (Balb/c, IgG1K)16; and PAA 1038H-10-9 (B10.PD1, IgMK).12 Flow cytometry was performed using a Becton Dickinson FACScan (San Jose, CA). Staining of whole blood and lymph node cell suspensions was performed as previously described.13 Data were analyzed using Winlist list mode analysis software (Verity Software House, Topsham, ME).
Detection of cytoplasmic immunoglobulin by flow cytometry was done using Fix & Perm cell permeabilization kit (Caltag Laboratories, Burlingame, CA) according to the manufacturer's instructions. Cytoplasmic staining for immunoglobulin was done using fluorescein isothiocyanate (FITC) goat antiswine IgM (Kirkegaard and Perry, Gaithersburg, MD) and FITC K139 3E1 mouse antiswine light-chain mAb.
Histology
Lymph node tissues were obtained by sequential biopsies and at autopsy. Representative sections were fixed in formalin and snap frozen. Formalin-fixed tissue sections were stained with hematoxylin and eosin.
Polymerase chain reaction to identify novel porcine gammaherpesviruses
Sample preparation.
Genomic DNA from the lymph nodes of miniature swine that were observed to have PTLD was extracted using the Qiamp Blood Kit (Qiagen, Santa Clara, CA). Then, 100 ng of the DNA pool was added to each 50-μL polymerase chain reaction (PCR) containing the following reagents: 25 mM KCl, 10 mM Tris-HCl (pH 8.3), 3.5 mM MgCl2 (Stratagene, La Jolla, CA), 0.2 mM dNTP, and 2.5 U Amplitaq Gold (PerkinElmer, Norwalk, CT) along with 20 pM of each primer. The following primer pair designed to amplify the glycoprotein B (gpB) gene of known gammaherpesviruses yielded a 627-bp product: QLIVF4 5′-CAR ITI CAR TWT GCM TAY GAC-3′; FREYNR4 5′-GTA RTA RTT RTA YTC YCT RAA-3′; (R = A + G, Y = C + T, M = A + C, W = A + T, I = Inosine).
The PCRs were cycled 9 minutes at 95°C followed by 30 cycles of 94°C for 30 seconds, 45°C for 1 minute, and 72°C for 1 minute. The program concluded with a 5-minute incubation at 72°C. The PCR products were purified using Microspin G-50 columns (Amersham Pharmacia Biotech, NJ), ligated into the pCRII-TOPO vector (Invitrogen, Carlsbad, CA), and used to transform competent TOP10F′Escherichia coli. Colonies were grown up and plasmid DNA extracted using the Wizard miniprep kit (Promega, Madison, WI). The plasmids were sent to Lark Technologies (Houston, TX) for automated sequencing.
PCR to detect relative level of porcine gammaherpesvirus DNA in miniature swine lymph node
Genomic DNA from peripheral blood mononuclear cells (PBMCs) was extracted using the Qiamp Blood Kit (Qiagen, Valencia, CA) or from tissue samples using the Puregene DNA Isolation Kit (Gentra Systems, Minneapolis, MN) following the manufacturers' instructions. Sequence information from the PCR product derived from degenerate herpesvirus primers was used to design specific primers to the porcine gammaherpesvirus (pGHV) gpB gene: TEF-16 (forward primer) 5′-CAC AAG CGT CAT GAG CAT G-3′; TER-13 (reverse primer) 5′-TAA GCC TCT TCT CGT CCC TG-3′.
The final PCR mixture consisted of 25 mM KCl, 10mM Tris-HCl, pH 8.3, 1.5 mM MgCl2 (Stratagene), 0.4 mM dNTP, and 2.5 U Amplitaq Gold (PerkinElmer), 15 pmol of each primer and 50 ng genomic DNA. Cycling conditions were as follows: 1 cycle of 9 minutes at 95°C followed by 25 cycles of 96°C for 10 seconds, 59°C for 30 seconds, and 72°C for 30 seconds. The program concluded with a 5-minute incubation at 72°C.
Plasmid DNA containing the fragment of the pGHV gpB gene was quantitated on an agarose gel. Known amounts of the plasmid were added with 50 ng pGHV-negative pig DNA to a PCR tube containing the PCR reagents as detailed above. Thus, the standards were amplified simultaneously with the test samples.
Results
Lymphoproliferation in miniature swine
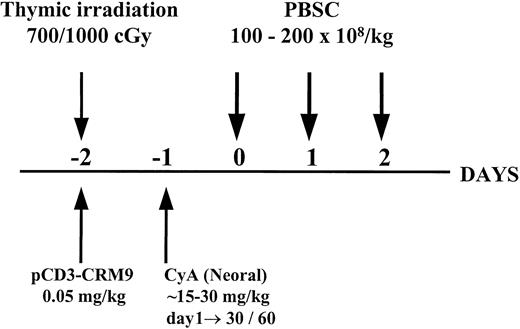
Twenty-one miniature swine were conditioned with thymic irradiation and in vivo T-cell depletion on day −2, followed by a short course (either 30 days or 60 days with tapering from day 30) of CyA starting on day −1. Eight animals were treated with the 30-day CyA protocol and 13 were treated with the 60-day CyA protocol, although not all animals completed their CyA course (see below). Conditioned recipients received PBSC transplants on days 0 to 2 with a high dose (1-2 × 1010/kg) of donor PBSC mobilized for 5 to 7 days with pIL-3 and pSCF as shown in Figure 1. This regimen was successful in establishing long-term mixed chimerism and donor-specific tolerance across both minor and major histocompatibility barriers in miniature swine, as previously reported.1 However, as summarized in Table1, a high percentage (43%, 9 of 21) of animals treated with this protocol developed a lymphoproliferative disorder, which was transient in some animals (3 of 9) and fatal in others (6 of 9).
Schematic representation of the nonmyelosuppressive preparative regimen for hematopoietic cell transplantation.
Schematic representation of the nonmyelosuppressive preparative regimen for hematopoietic cell transplantation.
Summary of animals developing PTLD
| Animal no. . | CyA treatment (d) . | Time to PTLD onset (d) . | Clinical outcome . | Histology of PTLD lymph node (LN) . | pGHV (LN) . |
|---|---|---|---|---|---|
| 12 757 | 30 | 34 | PTLD completely resolved | ND | ND |
| 13 100 | 30 | 35 | PTLD resolved/SAC due to GvHD d 73 | ND | ND |
| 13 318 | 30 | 32 | Died due to complications of PTLD d 32 | Polymorphous | +++ |
| 13 271* | 24 | 21 | Died due to complications of PTLD d 24 | Polymorphous | +++ |
| 13 432* | 36† | 34 | Died due to complications of PTLD d 39 | Polymorphous | +++ |
| 13 433* | 37 | 34 | Died due to complications of PTLD d 37 | Polymorphous | +++ |
| 13 629* | 48† | 48 | PTLD resolved/SAC due to GvHD d 69 | Polymorphous | +++ |
| 13 813* | 28 | 26 | Died due to complications of PTLD d 28 | Polymorphous | +++ |
| 13 801* | 45† | 45 | Died due to complications of PTLD d 48 | Polymorphous | +++ |
| Animal no. . | CyA treatment (d) . | Time to PTLD onset (d) . | Clinical outcome . | Histology of PTLD lymph node (LN) . | pGHV (LN) . |
|---|---|---|---|---|---|
| 12 757 | 30 | 34 | PTLD completely resolved | ND | ND |
| 13 100 | 30 | 35 | PTLD resolved/SAC due to GvHD d 73 | ND | ND |
| 13 318 | 30 | 32 | Died due to complications of PTLD d 32 | Polymorphous | +++ |
| 13 271* | 24 | 21 | Died due to complications of PTLD d 24 | Polymorphous | +++ |
| 13 432* | 36† | 34 | Died due to complications of PTLD d 39 | Polymorphous | +++ |
| 13 433* | 37 | 34 | Died due to complications of PTLD d 37 | Polymorphous | +++ |
| 13 629* | 48† | 48 | PTLD resolved/SAC due to GvHD d 69 | Polymorphous | +++ |
| 13 813* | 28 | 26 | Died due to complications of PTLD d 28 | Polymorphous | +++ |
| 13 801* | 45† | 45 | Died due to complications of PTLD d 48 | Polymorphous | +++ |
Eight of 21 animals were treated with the 30-day CyA protocol. Two developed transient PTLD and one died of PTLD complications. Thirteen of 21 animals were treated with the 60-day CyA protocol (none of the 6 developing PTLD completed their 60-day CyA course).
+++ indicates greater than 102 copies pGHV/cell; ND, not determined; SAC, sacrificed.
Animals included in 60-day CyA protocol.
CyA stopped early in attempt to resolve PTLD.
Transient lymphoproliferation was observed in 2 animals receiving PBSC transplants, one MHC-matched (no. 12757) and one MHC-mismatched (no. 13100), with a 30-day course of CyA. The peripheral blood white blood cell (WBC) count rapidly rose in these animals reaching a maximum on days 34 and 35 (Table 1). The rise in WBCs was coincident with a drastic decline in the degree of lymphocyte chimerism in the peripheral blood, as previously reported.1 Immediately following cessation of CyA on day 30, both animals developed a high fever (106°F) with loss of appetite and lethargy, and a slight decline in platelet count and blood hemoglobin level (data not shown). Blood cultures were consistently negative. The blood counts returned to normal, and both animals appeared clinically well with no evidence of PTLD by day 40. The animal that received MHC-matched PBSC remained healthy, with stable chimerism for over 550 days, whereas the animal that received MHC-mismatched PBSC developed signs of skin and intestinal graft-versus-host disease (GVHD) beginning on day 43 and was killed on day 73 due to prolonged, persistent diarrhea associated with chronic GVHD.1 A third animal (no. 13 318) treated with the 30-day CyA protocol died of complications due to PTLD on day 32 (Table 1).
In an attempt to avoid GVHD in an additional series of animals receiving transplants across an MHC barrier, we extended CyA administration to 60 days, with tapering from day 30 to 60. Six of 13 (46%) animals treated with this extended CyA protocol developed PTLD (Table 1). Clinical signs of PTLD in miniature swine included lethargy, anorexia, rapid rise in WBC and palpable lymph nodes on physical examination. Five of these animals died of respiratory failure in 24 to 48 days (median, 35 days). Autopsy examination revealed massive enlargement of tonsils and lymph nodes throughout the body with involvement of the gastrointestinal tract and spleen. The enlarged pulmonary hilar lymph nodes and palatine tonsils resulted in airway obstruction and respiratory failure in these animals. Histologic examination of lymph node tissue showed typical polymorphous changes of PTLD with a mixture of immunoblasts, plasmacytoid cells, and plasma cells (Table 1). The sixth animal (no. 13 629) was killed because of complications of GVHD, which developed after CyA was stopped in an effort to halt the progression of PTLD (see below).
Although PTLD was more apparent when we began treating animals using the 60-day CyA protocol, the prevalence of PTLD was similar among animals treated with either CyA protocol. Of 8 animals intentionally treated with the 30-day CyA protocol, 2 (no. 12 757 and no. 13 100) developed transient PTLD that resolved following CyA cessation, and one (no. 13 318) died of complications due to PTLD on day 32 (Table 1). Because 2 of the animals in the 60-day CyA protocol (no. 13 271 and no. 13 813) developed PTLD and died prior to day 30 (Table 1), the prevalence of PTLD among the 10 animals given CyA for no more than 30 days (5 of 10) was comparable to the prevalence of PTLD among the 11 animals receiving a course of CyA beyond 30 days (4 of 11).
Immunophenotyping of peripheral blood and lymph node cells
Flow cytometry and immunohistochemistry were performed to determine the immunophenotype of the cells proliferating in the peripheral blood and lymph nodes of animals with PTLD (Table2). Donor cells can be distinguished from the host by flow cytometry using a mAb that recognizes a PAA present on all swine leukocytes.12 Eight of 9 cases of PTLD were of host origin. In all cases examined, the abnormal cells were class II+, CD3−, CD2dim, and CD16− (Table 2). These cells were negative for the available swine peripheral B-cell marker, CD21 (data not shown). Flow cytometric analysis of immunoglobulin μ heavy-chain and κ light-chain staining was performed on lymph node cell suspensions from 7 of the 9 PTLD animals. Five of 7 examined were positive for surface immunoglobulin. Cytoplasmic staining was performed on the 2 samples that were negative for surface immunoglobulin. Both were positive for cytoplasmic μ and one of the 2 was also positive for cytoplasmic κ light chain. Based on this analysis, all 7 cases examined were determined to be of B-cell origin (Table 2). The variable pattern of κ light-chain staining of involved lymph node cells in 6 of the 7 cases examined suggested that the lesions were most likely either polyclonal or oligoclonal (data not shown). In one case (no. 13 271), more than 95% of the lymph node consisted of PTLD cells and they were all surface and cytoplasmic κ light-chain negative (Table 2), suggesting a possible monoclonal outgrowth. This was a very aggressive case with rapid onset at 21 days (Table 1). Interestingly, this is the only case so far in which we have been successful in growing out a continuous cell line in culture.
Immunophenotyping of PTLD by flow cytometry
| Animal no. . | % CD3-CD16-lymphocytes . | Immunophenotype . | ||||||
|---|---|---|---|---|---|---|---|---|
| Origin . | CD2 . | CD3 . | CD16 . | Class II . | IgM . | Igκ . | ||
| 12 757 | 50 (PBMC) | Host | Dim | — | ND | ND | ND | ND |
| 13 100 | 57 (PBMC) | Host | Dim | — | — | ND | ND | ND |
| 13 318 | > 70 (LN) | Host | Dim | — | — | + | + | + |
| 13 271 | > 95 (LN) | Host | Dim | — | — | + | + | — |
| 13 432 | > 70 (LN) | Host | Dim | — | — | + | + | — |
| 13 433 | 47 (LN) | Host | Dim | — | — | + | + | + |
| 13 629 | 40 (LN) | Donor | Dim | — | — | + | Dim | + |
| 13 813 | > 95 (LN) | Host | Dim | — | — | + | + | + |
| 13 801 | 85 (LN) | Host | Dim | — | — | + | + | + |
| Animal no. . | % CD3-CD16-lymphocytes . | Immunophenotype . | ||||||
|---|---|---|---|---|---|---|---|---|
| Origin . | CD2 . | CD3 . | CD16 . | Class II . | IgM . | Igκ . | ||
| 12 757 | 50 (PBMC) | Host | Dim | — | ND | ND | ND | ND |
| 13 100 | 57 (PBMC) | Host | Dim | — | — | ND | ND | ND |
| 13 318 | > 70 (LN) | Host | Dim | — | — | + | + | + |
| 13 271 | > 95 (LN) | Host | Dim | — | — | + | + | — |
| 13 432 | > 70 (LN) | Host | Dim | — | — | + | + | — |
| 13 433 | 47 (LN) | Host | Dim | — | — | + | + | + |
| 13 629 | 40 (LN) | Donor | Dim | — | — | + | Dim | + |
| 13 813 | > 95 (LN) | Host | Dim | — | — | + | + | + |
| 13 801 | 85 (LN) | Host | Dim | — | — | + | + | + |
Analysis was done on PBMC or lymph node (LN) on day of autopsy or maximal lymphoproliferation as indicated in Table 1.
Dim indicates diminished; ND, not determined.
Surface negative—confirmed positive by cytoplasmic staining.
Resolution of PTLD following CyA cessation
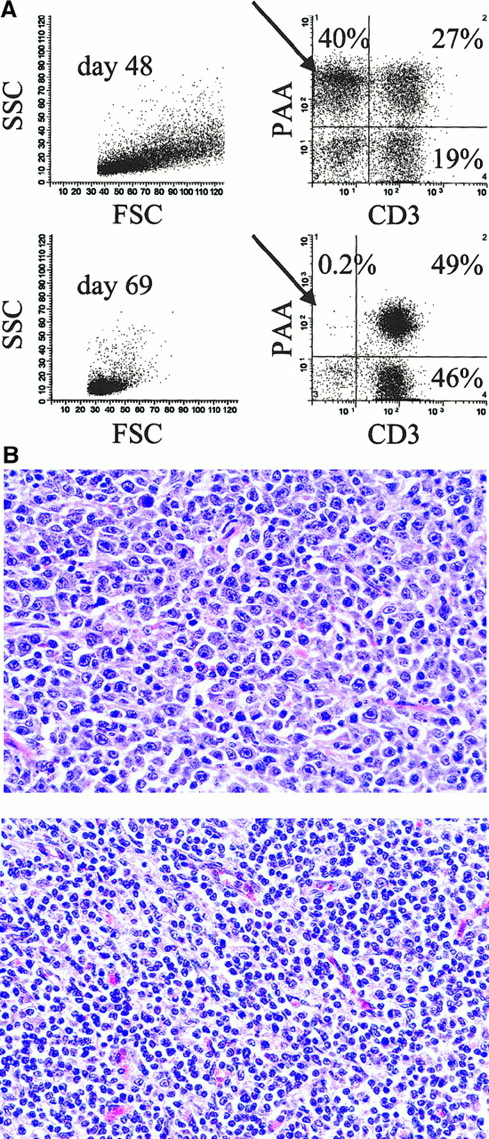
One of the 6 animals that developed PTLD on the 60-day CyA protocol survived the disease (no. 13 629, Table 1). In an attempt to limit progression of PTLD, CyA was stopped in this animal at 48 days when clinical signs of PTLD first appeared. Clinical symptoms including fever, lethargy, anorexia, high WBC count, and palpable lymph nodes on physical examination all completely resolved in animal no. 13 629 by day 57. Figure 2A shows cell surface staining and FACS analysis of lymph node biopsy samples taken from this animal at the time of PTLD before CyA cessation (day 48) and after resolution of PTLD (day 69). At day 48, approximately 40% of the lymph node suspension consisted of CD3− donor-type (PAA+) cells, which were almost completely absent by day 69. Figure 2B shows the histology of the same lymph node biopsy samples. Typical polymorphous PTLD cells with a mixture of immunoblasts, plasmacytoid cells, and plasma cells were seen throughout the lymph node sample taken on day 48, but were not present in the day 69 sample. Immunohistochemistry revealed only CD3+ cells remaining in the lymph node on day 69 with no distinct B-cell follicles (data not shown).
Resolution of PTLD after CyA cessation in animal no. 13 629.
(A) Two-color staining and flow cytometry (CD3 versus PAA) of lymph node cells during PTLD (day 48) and following complete resolution of PTLD (day 69). PAA is a marker of donor-type cells (see “Results” and “Materials and methods”). Forward scatter (FSC), side scatter (SSC), pig allelic antigen (PAA). (B) Hematoxylin and eosin staining of mesenteric lymph node tissue taken on day 48 (top) and day 69 (bottom). Typical polymorphous PTLD with a mixture of immunoblasts, plasmacytoid cells, and plasma cells can be seen in the day 48 but not the day 69 sample (original magnification × 500).
Resolution of PTLD after CyA cessation in animal no. 13 629.
(A) Two-color staining and flow cytometry (CD3 versus PAA) of lymph node cells during PTLD (day 48) and following complete resolution of PTLD (day 69). PAA is a marker of donor-type cells (see “Results” and “Materials and methods”). Forward scatter (FSC), side scatter (SSC), pig allelic antigen (PAA). (B) Hematoxylin and eosin staining of mesenteric lymph node tissue taken on day 48 (top) and day 69 (bottom). Typical polymorphous PTLD with a mixture of immunoblasts, plasmacytoid cells, and plasma cells can be seen in the day 48 but not the day 69 sample (original magnification × 500).
Identification of a pGHV associated with PTLD
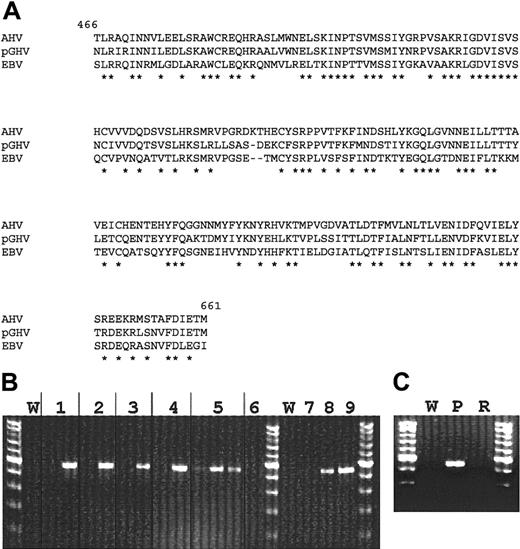
Degenerate PCR primers were designed based on sequences of known gammaherpesviruses to amplify the highly conserved gpBenvelope gene. DNA extracted from autopsy lymph node tissue of miniature swine that died of PTLD was amplified in a PCR by using the designed oligonucleotides. The resulting 627-bp PCR product was cloned and sequenced. The fragment was identified as a previously unknown gpB DNA sequence related to gammaherpesviruses. Amino acid sequence comparison revealed a high similarity (71% homology) to the gpB sequence of the wildebeest virus Alcelaphine herpesvirus (AHV) and also significant similarity to EBV and other gammaherpesviruses (Figure3A and Table3).
Molecular analyses of pGHV sequences.
(A) Alignment of pGHV gpB amino acid sequence with those of AHV (GenBank accession no. T03107, amino acids 466-661) and EBV (GenBank accession no. P03188, amino acids 467-660). Amino acid identity is represented by an asterisk. (B) PCR amplification of pGHV sequences of DNA derived from lymph node samples. Lane pairs 1 to 5 represent animals 13 271, 13 432, 13 433, 13 813, and 13 801, respectively. The first lane of each pair represents pretreatment samples and the second a sample taken during PTLD. The third lane for animal no. 13 801 is a PBMC sample taken during PTLD. Lane 6 is a lymph node sample from animal no. 13 793 that was transplanted with the same donor cells at the same time as no. 13 801 but did not develop PTLD. A lymph node from this control animal was taken during the time of PTLD for animal no. 13 801. Lanes 7, 8, and 9 represent control amplifications of 2 × 103, 1 × 104, and 5 × 104 copies of PGHV gpB DNA, respectively. W represents water control amplifications. (C) Presence of pGHV sequences in DNA from lymph nodes during PTLD (P) and after resolution of PTLD (R). The tests indicate significantly elevated levels (4-5 log increase) of pGHV gpB sequences in all samples taken in animals exhibiting symptoms of PTLD.
Molecular analyses of pGHV sequences.
(A) Alignment of pGHV gpB amino acid sequence with those of AHV (GenBank accession no. T03107, amino acids 466-661) and EBV (GenBank accession no. P03188, amino acids 467-660). Amino acid identity is represented by an asterisk. (B) PCR amplification of pGHV sequences of DNA derived from lymph node samples. Lane pairs 1 to 5 represent animals 13 271, 13 432, 13 433, 13 813, and 13 801, respectively. The first lane of each pair represents pretreatment samples and the second a sample taken during PTLD. The third lane for animal no. 13 801 is a PBMC sample taken during PTLD. Lane 6 is a lymph node sample from animal no. 13 793 that was transplanted with the same donor cells at the same time as no. 13 801 but did not develop PTLD. A lymph node from this control animal was taken during the time of PTLD for animal no. 13 801. Lanes 7, 8, and 9 represent control amplifications of 2 × 103, 1 × 104, and 5 × 104 copies of PGHV gpB DNA, respectively. W represents water control amplifications. (C) Presence of pGHV sequences in DNA from lymph nodes during PTLD (P) and after resolution of PTLD (R). The tests indicate significantly elevated levels (4-5 log increase) of pGHV gpB sequences in all samples taken in animals exhibiting symptoms of PTLD.
Comparison of gpB amino acid sequence homology among different gammaherpesviruses
| Gammaherpesvirus . | Genus . | % Identity to pGHV (amino acid sequence) . |
|---|---|---|
| Alcelaphine herpesvirus-1 | Rhadinovirus | 71 |
| Equine herpesvirus-2 | Unclassified | 60 |
| Rhesus rhadinovirus H26-95 | Rhadinovirus | 59 |
| Ateline herpesvirus-3 | Rhadinovirus | 58 |
| Human herpesvirus-8 (Kaposi sarcoma-associated) | Rhadinovirus | 57 |
| Human herpesvirus-3 (Epstein-Barr virus) | Lymphocriptovirus | 53 |
| Bovine herpesvirus-4 | Unclassified | 50 |
| Gammaherpesvirus . | Genus . | % Identity to pGHV (amino acid sequence) . |
|---|---|---|
| Alcelaphine herpesvirus-1 | Rhadinovirus | 71 |
| Equine herpesvirus-2 | Unclassified | 60 |
| Rhesus rhadinovirus H26-95 | Rhadinovirus | 59 |
| Ateline herpesvirus-3 | Rhadinovirus | 58 |
| Human herpesvirus-8 (Kaposi sarcoma-associated) | Rhadinovirus | 57 |
| Human herpesvirus-3 (Epstein-Barr virus) | Lymphocriptovirus | 53 |
| Bovine herpesvirus-4 | Unclassified | 50 |
Comparisons between the porcine gammaherpesvirus and other known gammaherpesviruses were made using the NCBI's Blast 2 sequences program (http://www.ncbi.nlm.nih.gov/gorf/bl2.html) using the blastp option. The following sequences and their accession numbers were obtained from the Entrez-Protein database: Alcelaphine herpesvirus-1,AAC58059.1; equine herpesvirus-2, AAC13795.1; rhesus rhadinovirus H26-95, AAC58686.1; Ateline herpesvirus-3, AAC95532.1; human herpesvirus-8, AAC57085.1; human herpesvirus-4, NP_039909.1; bovine herpesvirus-4, CAA78761.1.
Using pGHV gpB sequence data, specific PCR primers were designed to detect pGHV in lymph node and peripheral blood samples. Figure 3B shows the results of PCR analysis of lymph node DNA from miniature swine that developed PTLD. All samples showed a significant increase in the level of pGHV (Figure 3B). Semiquantitative PCR indicated that the level of pGHV went from less than 1 copy in 103 cells prior to PTLD to approximately 10 to 100 copies per cell during PTLD (data not shown). Samples were not available for animal no. 13 100 or no. 12 757. A high copy number of pGHV could also be detected in DNA derived from PBMCs at the time of PTLD progression, as seen for animal no. 13 801. Figure 3C shows PCR results for a lymph node sample from animal no. 13 629 before and after PTLD resolution. After complete resolution of PTLD in animal no. 13 629, no pGHV sequences could be amplified in the DNA derived from lymph node samples.
Discussion
We have identified a large-animal model of PTLD in miniature swine conditioned for hematopoietic stem cell transplantation. Here we describe the PTLD occurring in our partially inbred, MHC-defined herd of miniature swine and its close association with pGHV. Other animal models in which herpesvirus infection causes similar disease include human severe combined immunodeficiency models of EBV-induced PTLD,18 murine gammaherpesvirus 68 (MHV-68)–induced lymphoproliferation in mice,19 and simian herpesvirus–induced lymphomas in immunosuppressed primates.20 The availability of this reproducible miniature swine model of PTLD provides a unique opportunity to test approaches for diagnosis, treatment, and prevention of this disease.
The PTLD observed in miniature swine closely resembles that observed in humans undergoing transplantation (Table4). The incidence of PTLD observed in our transplant model, however, is much higher than that reported in human allograft recipients.8,9 In human transplant recipients, PTLD is generally associated with the intensity of immune suppression, particularly the use of antilymphocyte preparations, with primary EBV infection, and with coinfection by cytomegalovirus.6 The high incidence in the miniature swine model may be related to the absence of WBI or other myelosuppressive treatments in this protocol. We have not observed PTLD or lymphoma in animals treated under any other transplantation protocol used for miniature swine, including a similar preparative regimen for PBSC transplantation that differs only in the addition of 300 cGy WBI.13 Myelosuppressive host conditioning including WBI results in a reduction of the host B-cell pool, which may contribute to a reduction in the prevalence of host-type PTLD. We observed only one case of donor-type PTLD among 21 animals treated with this regimen, and this prevalence is within the range of what has been reported for patients having bone marrow transplants.9 Of note, host-type PTLD is more often seen in those receiving organ transplants (Table 4).8 This is primarily due to the fact that the lymphoid system of organ transplant recipients remains of host type, whereas that of bone marrow transplants is generally converted to donor type. Because it is among these cells that PTLD arises, it is understandable that organ transplant recipients generally have PTLD of host type and bone marrow transplant recipients have PTLD of donor type. In our model, where we strive to achieve mixed chimerism rather than complete bone marrow chimerism, we have seen PTLD of both host and donor types. Host PTLD predominates, presumably because recipient B-cell precursors are more prevalent at the time of origin of the disease than are the corresponding donor elements.
Comparison of PTLD in humans and miniature swine
| . | Humans . | Miniature swine . |
|---|---|---|
| Mean time to onset | Variable, may be < 6 mo | ∼34 d |
| Increased risk with increased immunosuppression | Yes | Likely, but not definitively demonstrated |
| Sites involved | Lymph nodes, GI tract, tonsils and adenoids, allograft, others | Lymph nodes, tonsils |
| Response to decreased immunosuppression | Sometimes | Sometimes |
| Outcome | Fatal in approximately 50% | Fatal in 6 of 9 |
| Incidence | < 9% | > 40% |
| Histology | Polymorphous or monomorphous | Polymorphous |
| Lineage | B cell | B cell |
| Origin | Mainly host (solid organ) mainly donor (BMT) | Host, 8 of 9 |
| Virus present | EBV, most cases | pGHV, high copy number |
| . | Humans . | Miniature swine . |
|---|---|---|
| Mean time to onset | Variable, may be < 6 mo | ∼34 d |
| Increased risk with increased immunosuppression | Yes | Likely, but not definitively demonstrated |
| Sites involved | Lymph nodes, GI tract, tonsils and adenoids, allograft, others | Lymph nodes, tonsils |
| Response to decreased immunosuppression | Sometimes | Sometimes |
| Outcome | Fatal in approximately 50% | Fatal in 6 of 9 |
| Incidence | < 9% | > 40% |
| Histology | Polymorphous or monomorphous | Polymorphous |
| Lineage | B cell | B cell |
| Origin | Mainly host (solid organ) mainly donor (BMT) | Host, 8 of 9 |
| Virus present | EBV, most cases | pGHV, high copy number |
GI indicates gastrointestinal; BMT, bone marrow transplantation.
Human neoplasms in immunosuppressed patients have been associated with gammaherpesviruses such as EBV (Burkitt lymphoma) and human herpesvirus 8 (Kaposi sarcoma, primary effusion lymphoma21). In this report, we identify a pGHV associated with lymphoproliferative disease in swine. The pGHV was found to be most similar to the wildebeest virus AHV with 71% homology at the amino acid level. AHV is known to cause wildebeest-associated malignant catarrhal fever,22 a fatal lymphoproliferative disease found in cattle. The pGHVs have only recently been identified23,24 and we are currently addressing whether the herein described pGHV is identical to either of the 2 other pGHVs described. The similarity of pGHV to a virus (AHV) known to infect ungulates other than its natural host may have important implications for xenotransplantation25 given that swine are currently favored as potential organ donors for xenotransplantation.26 However, pGHV transmission does not appear to occur in utero and can be prevented at birth by using clean-catch procedures (C. Patience, manuscript in preparation). Therefore, and because swine to be used for xenotransplantation will be screened extensively for all human pathogens, pGHV should pose a manageable risk.
The observation of transient lymphoproliferation in 2 of the initial animals (no. 12 757 and no. 13 100) receiving PBSC transplantation with a 30-day course of CyA was thought to be a hematopoietic graft-versus-host (GVH) or host-versus-graft (HVG) reaction that became evident only after CyA was stopped at day 30.1 Despite a marked increase in peripheral lymphocyte count, the absolute number ofdonor-type lymphocytes in the peripheral blood in both animals remained constant suggesting that an expansion ofhost-type lymphocytes had occurred. If this phenomenon were due to an HVG reaction, an expansion of host T cells at this time would be predicted. However, phenotypic analysis of the peripheral blood by surface staining and FACS analysis on the day of maximal WBC in these animals revealed predominantly very large lymphocytes, the majority of which were host-type non-T cells (PAA−, CD3−, CD2dim) (Table 2). The fact that these cells were CD2dim is consistent with a non–T-cell phenotype, because CD2 expression has been documented on swine B cells and natural killer cells.27 28 In retrospect, a more plausible explanation for these observations was that host-type B-cell lymphoproliferation occurred and completely resolved after CyA cessation. PTLD became apparent as the cause of host-type lymphoproliferation in animals no. 12 757 and no. 13 100 after several additional animals died of respiratory complications due to upper airway obstruction from enlarged lymphoid tissue following similar hematopoietic manifestations.
The elimination of WBI has greatly reduced the acute toxicity associated with preparative regimens for hematopoietic cell transplantation in miniature swine, which could, in turn, increase the potential clinical applicability of this approach.1,13 The frequent occurrence of PTLD among animals treated with this regimen, however, could have opposite implications. We are therefore currently working to identify the risk factors associated with PTLD. A similar lymphoproliferative disorder has recently been described in NIH miniature swine receiving liver allografts with a 12-day course of FK506.29 The degree of immunosuppression, viral exposure status of donor and recipient, and allogeneic transplantation could all play a role in development of lymphoproliferative disease in these animals. The availability of this genetically defined large-animal model of PTLD will allow us to study the role of the many potential contributing factors in the pathogenesis of PTLD. In this regard, we are planning to investigate the role of CyA, T-cell depletion, allogeneic stem cell transplantation, and the pretransplant pGHV and porcine cytomegalovirus exposure status of donor and recipient animals. The availability of a large-animal model of PTLD should facilitate the development of diagnostic and therapeutic approaches for prevention or treatment of PTLD in the clinical setting.
The authors would like to thank Drs Kai Sonntag and Yong-guang Yang for critical review of the manuscript; Drs David M. Neville, Jr and Joshua Scharff (NIH-NIMH, Bethesda, MD) for collaborating to provide the pCD3-CRM9 used in these studies; Jason Bailey for excellent technical assistance; and Lisa Bernardo for assistance in preparation of the manuscript. We also wish to acknowledge the following companies for generous gifts of their products—Novartis Pharmaceuticals (Neoral) and Abbot Laboratories (Ancef).
Supported by National Institutes of Health grant 1R01 HL63430-01 and by a grant from The Cure for Lymphoma Foundation. C.A.H. is a Cure for Lymphoma Foundation Fellow and a recipient of the MGH Claflin Distinguished Scholar Award.
C.A.H. and Y.F. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
David H. Sachs, Transplantation Biology Research Center, Massachusetts General Hospital, MGH-East, Bldg 149-9019, 13th St, Boston, MA 02129; e-mail: sachs@helix.mgh.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal