Cell cycle checkpoints ensure orderly progression of events during cell division. A microtubule damage (MTD)-induced checkpoint has been described in G1 phase of the cell cycle (G1MTC) for which little is known. The present study shows that the G1MTC is intact in activated T lymphocytes from mice with the p21waf-1 gene deleted. However, p21waf-1 gene deletion does affect the ratio of cells that arrest at the G1MTC and the spindle checkpoint after MTD. The G1MTC arrests T lymphocytes in G1 prior to cdc2 up-regulation and prior to G1arrest by p21waf-1. Once cells have progressed past the G1MTC, they are committed to chromosome replication and metaphase progression, even with extreme MTD. The G1MTC is also present in a human myeloid cell line deficient in p21waf-1gene expression. The p21-independent G1MTC may be important in cellular responses to MTD such as those induced by drugs used to treat cancer.
Introduction
All proliferating animal cells must accurately duplicate and transmit the total of their genome during each cell division. This remarkable fidelity of chromosome replication and segregation depends on cell cycle checkpoints,1 especially in highly proliferative cells. Loss of mitotic checkpoint function has been linked to the origin and progression of human malignancy.2 The microtubule/mitotic spindle assembly checkpoint (SAC) is essential to normal growth and development in mice.3 Signaling molecules of the SAC have garnered intense recent interest because mutations in these mitotic checkpoint genes have been demonstrated in human cancers.2 Moreover, the tumor suppressor, p53, which is mutated in more than half of all human tumors, along with one of its downstream effectors, p21waf-1 (p21), are required for proper cell cycle arrest after spindle disruption.4-7
We recently described a human growth-factor dependent hematopoietic cell line that is defective in levels of p21 expression (AS21), especially in response to microtubule damage (MTD).7 These cells display a similar, albeit less penetrant, defect in mitotic checkpoints compared with human cells completely lacking p21. In those studies, p21 was implicated in loss of both G1 and M phase MTD checkpoints, observations further substantiated in human colorectal cell lines with p21 gene deletion. We proposed that p21 was important for proper SAC responses and involved in a new interphase MTD checkpoint (G1MTC).
We have now investigated by intracellular flow analysis the requirement for p21 in the G1MTC using proliferating murine T lymphocytes with the p21 gene deleted and AS21 cells. We find that p21 gene is not required for the G1MTC arrest, but it is involved in regulating G1 progression past the G1MTC commitment point. This point is now defined as the point at which cdc2 is up-regulated in G1 phase and the cell becomes refractory to G1 arrest by MTD and becomes committed to completion of DNA replication and mitotic initiation, even in the presence of extreme MTD.
Study design
Cells, antibodies, and treatments
The MO7e cells containing antisense p21 (AS21) or empty vector (LXSN) were generated and maintained as described.7,8Asynchronously growing cells were treated with 15 μg/mL nocodazole or taxol (Sigma Chemical, St Louis, MO), or with control diluent for 24 hours, then analyzed by multivariate flow cytometric cell cycle analysis. For flow cytometry, fluorescein-isothiocyanate (FITC)-conjugated antihuman/mouse cdc2 rabbit polyclonal IgG1 or rabbit FITC-IgG1 isotype control antibodies were used to determine specific and nonspecific cdc2 immunofluorescence intensity, respectively. Activated splenic T lymphocytes were obtained9 from p21−/− and p21+/+ mice.10 11
Multivariate flow cytometric cell cycle analysis
Flow cytometric analysis was performed on cells treated with nocodazole, taxol, or diluent by first fixation and simultaneous permeabilization using Cytofix/Cytoperm reagent (Pharmingen, San Diego, CA) according to the manufacturer's instructions. After washing to remove the fixative, intracellular staining was done by incubating the cells for 1 hour with 10 μg/mL FITC-conjugated antibody to cdc2 or with 10 μg/mL FITC-conjugated isotype control antibody. After washing 3 times, the intracellularly labeled cells were counterstained with propidium iodide (Sigma) for 30 minutes to quantitate DNA. Cellular fluorescence intensity was measured with a FACscan flow cytometer (Becton-Dickinson, San Jose, CA). Laser light scatter was used to gate out dead cells. Cell cycle proportions were calculated using the Modfit computer program (Verity Software House, Topsham, ME). Density scatter diagrams were constructed and analyzed using the WinMDI program (J. Trotter, The Scripps Research Institute, La Jolla, CA,http://facs.scripps.edu/). Other details including multicolor compensation have been described.12 Statistical comparisons used the Student t test. Experiments were performed at least 3 times.
Results and discussion
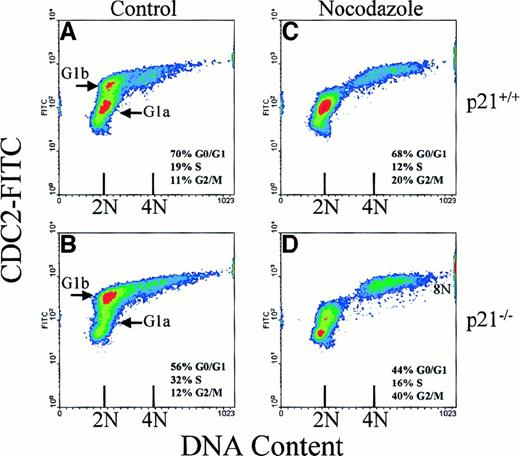
Figure 1 shows cdc2 expression in activated murine T lymphocytes as a function of DNA content. The cdc2 content is up-regulated in G1 phase. There are at least 2 separate populations of G1 cells with respect to cdc2 levels, G1a and G1b. Cells enter S and G2/M phases with very little further increase in cdc2 expression. After mitosis, cdc2 is degraded and the daughter cells return to G1 phase with little or no cdc2. A 3-fold shift in the relative proportions of G1a and G1b cells is observed in the cells with the p21 gene deleted. This is consistent with the proposed role of p21 in G1 phase progression and “threshold” events as reported.11,13After treatment with the MT depolymerizing agent, nocodazole, the G1b population could not be observed in wild-type orp21 knockout mouse cells (Figure 1C,D). However, the relative proportion of G2/M phase cells was higher in thep21 knockout cells after treatment compared to wild-type cells. There was also an increase in the number of 8N cells in thep21 knockout cultures after nocodazole treatment compared to wild-type cells.4-7 Treatment with taxol resulted in a similar response (not shown). We interpret this as indicating that G1a cells are arresting after MTD at a point in G1 phase before cdc2 expression is up-regulated. Once cells have progressed in the cell cycle past this point, and cdc2 expression is turned on, subsequent MTD no longer arrests the cells in G1, but they progress through S and G2 phases to arrest at the SAC in mitosis. Thus, the point of cdc2 up-regulation defines the point of passage of the G1MTC when cells become committed to DNA replication, even in the presence of MTD. The absence of p21 does not appear to influence G1 arrest by the G1MTC. However, the temporal effect of p21deletion on the proportions of cells at G1a versus G1b leads to fewer cells that arrest at the G1MTC after MTD, and leads to the increased proportion ofp21 knockout cells arrested in mitosis as reported.4-7
Expression of cdc2 and cell cycle arrest of T lymphocytes after MTD.
Scatter density diagrams of bivariate (cdc2/DNA) cell cycle analysis of activated T lymphocytes from wild-type mice (p21+/+; A,C) and p21 knockout mice (p21−/−; B,D) are shown. After activation, cells were treated for 24 hours with 15 μg/mL nocodazole (C,D) or control diluent (A,B). 2N (G0/G1) and 4N (G2/M) DNA content is indicated along with the percentages of cells in different cell cycle phases. Data are representative of at least 2 replicate experiments from 6 sets of mutant mice and their wild-type littermate controls.
Expression of cdc2 and cell cycle arrest of T lymphocytes after MTD.
Scatter density diagrams of bivariate (cdc2/DNA) cell cycle analysis of activated T lymphocytes from wild-type mice (p21+/+; A,C) and p21 knockout mice (p21−/−; B,D) are shown. After activation, cells were treated for 24 hours with 15 μg/mL nocodazole (C,D) or control diluent (A,B). 2N (G0/G1) and 4N (G2/M) DNA content is indicated along with the percentages of cells in different cell cycle phases. Data are representative of at least 2 replicate experiments from 6 sets of mutant mice and their wild-type littermate controls.
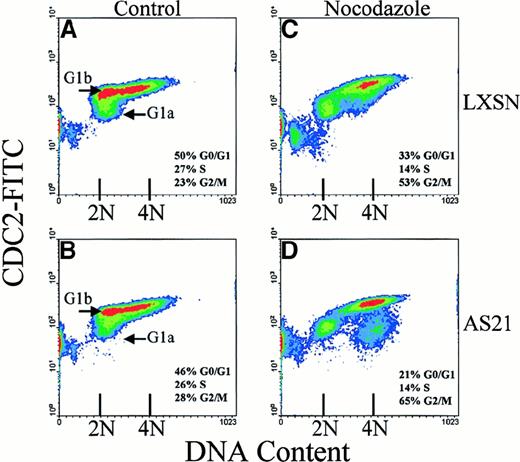
An identical experiment was performed on p21-deficient human myeloid AS21 cells,7 to determine if the human G1MTC arrest is also independent of p21 (Figure2). A similar pattern of cdc2 expression occurred during cell cycle progression in these cells as observed in murine T lymphocytes. The relative proportions of G1a and G1b cells are shifted just as in the mouse cells (Figure2A,B) and after treatment with nocodazole or taxol (not shown), the G1b population disappears with a commensurate shift in the G1 and G2/M arrested proportions (Figure 2C,D).
Expression of cdc2 and cell cycle arrest of human myeloid cell line, MO7e, after MTD.
Scatter density diagrams of bivariate cell cycle analysis of vector control cells (LXSN; A,C) and p21 antisense (AS21; B,D) are shown. Day 3 cell cultures were treated for 24 hours with 15 μg/mL nocodazole (C,D) or control diluent (A,B). The 2N (G0/G1) and 4N (G2/M) DNA content is indicated along with the percentages of cells in different cell cycle phases. Data are representative of at least 6 separate experiments done in triplicate.
Expression of cdc2 and cell cycle arrest of human myeloid cell line, MO7e, after MTD.
Scatter density diagrams of bivariate cell cycle analysis of vector control cells (LXSN; A,C) and p21 antisense (AS21; B,D) are shown. Day 3 cell cultures were treated for 24 hours with 15 μg/mL nocodazole (C,D) or control diluent (A,B). The 2N (G0/G1) and 4N (G2/M) DNA content is indicated along with the percentages of cells in different cell cycle phases. Data are representative of at least 6 separate experiments done in triplicate.
An interesting finding is the appearance of a population of 4N human cells with low (Figure 2C) or negative (Figure 2D) cdc2 content that is not observed in murine cells. This population could represent p21-deficient cells that have prematurely exited mitosis without cytokinesis and thus have escaped cell cycle arrest induced by SAC activation, suggesting a difference between human and murine responses to MTD. Embryonic cells from p21−/− mice have been reported to have an intact SAC response, but do fail to prevent re-replication of DNA4 events consistent with our analysis. On the other hand, loss of p53 in a human tumor cell line is reported to be without effect on nocodazole arrest.6Rodent cells are known to have “leaky” or missing cell cycle checkpoints compared to human cells. Also, somatic cells may have different or additional checkpoints compared to embryonic cells. Therefore, cell type and species differences in response to MTD are possible. These issues are currently under investigation.
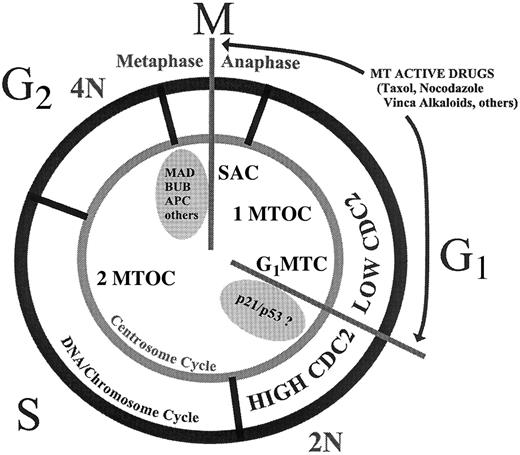
Questions yet to be answered are: What is the purpose of the G1MTC and what is the nature of the MTD sensing mechanism? The sensing apparatus of the SAC is believed to reside at the kinetochore, and tension on 2 juxtaposed MT attachment points is believed to be the key mechanochemical process that senses correct chromosome-spindle alignment and sends a “go” signal for anaphase initiation and chromosome congression.14 Because the centrosome is the MT organizing center of interphase cells, and because this organelle must duplicate and separate in G1 phase ultimately to become the poles of the mitotic spindle, we speculate that the G1MTC defines a cell cycle checkpoint ensuring proper centriole duplication and separation. Figure3 illustrates the “location” of the G1MTC in relation to the SAC, the centrosome cycle, and the DNA cycle. The relationship between the G1MTC and the restriction point, or other cell cycle commitment points or checkpoints, remains to be determined. However, the existence of an interphase MTD checkpoint has significant implications in treatment strategies of cancer by drugs that exert selective toxicity on cancer cells by interfering with MT dynamics.
Two MTD-induced cell cycle checkpoints.
This illustration shows the proposed relative location of the 2 known cell cycle arrest points in cells with MTD. SAC indicates spindle assembly checkpoint; G1MTC, G1 phase microtubule checkpoint. The centrosome cycle (1 or 2 MTOC indicates microtubule organizing centers) is shown to be coordinated with the DNA/chromosome cycle (2N or 4N DNA content). The arrests the cell cycle in G1 phase between the shift from a low cdc2-expressing state to a high cdc2-expressing state. Some of the proteins implicated in each MT checkpoint are listed (see reference 15 for a recent review).
Two MTD-induced cell cycle checkpoints.
This illustration shows the proposed relative location of the 2 known cell cycle arrest points in cells with MTD. SAC indicates spindle assembly checkpoint; G1MTC, G1 phase microtubule checkpoint. The centrosome cycle (1 or 2 MTOC indicates microtubule organizing centers) is shown to be coordinated with the DNA/chromosome cycle (2N or 4N DNA content). The arrests the cell cycle in G1 phase between the shift from a low cdc2-expressing state to a high cdc2-expressing state. Some of the proteins implicated in each MT checkpoint are listed (see reference 15 for a recent review).
The authors wish to thank Patricia Mantel for help in editing the manuscript.
Supported by Public Health Service grants RO1 HL 56416 and RO1 DK 53674 to H.E.B.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Charlie R. Mantel, Walther Oncology Center, 1044 West Walnut St, Indianapolis, IN 46202-5121; e-mail:cmantel@iupui.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal