Various studies have demonstrated the aberrant expression of normal testicular proteins in neoplastic cells. These proteins collectively form the new class of tumor antigens called cancer-testis (CT) antigens. Their selective normal tissue expression makes them ideal antigens for immune targeting of the malignant disease. In this study, the expression of a spermatozoa protein, Sp17, in multiple myeloma was investigated. It was found that Sp17 is detectable in tumor cells from 12 of 47 (26%) myeloma patients. Reverse transcription polymerase chain reaction (RT-PCR) and Western blot analysis detected Sp17 transcripts and proteins, respectively. Northern blot analysis and RT-PCR demonstrated that Sp17 transcripts were detected only in normal testis, supporting its tissue specificity. Since a high proportion of normal individuals develop antibodies against Sp17 following vasectomy, Sp17 is likely to be a highly immunogenic protein in vivo. Sp17 is therefore a novel member of the CT antigen family and should be an ideal target for immunotherapy of multiple myeloma.
Introduction
Therapeutic approaches for multiple myeloma remain a challenge. Although it is possible to induce disease remission in 50% to 60% of patients using aggressive combination chemotherapy, only a small proportion of patients have long-term disease-free survival.1 Most patients die of disease relapse. Present therapeutic approaches aim to reduce the relapse rate by using maintenance chemotherapy or immunotherapy. Because immunotherapy is more specific and less toxic, it is an ideal approach, but at present there is a general lack of suitable candidate antigens. The myeloma idiotypic protein is clone-specific and has been previously used.2-6 However, the clinical results have been disappointing, most likely owing to the weak immunogenicity of the idiotype proteins and low effector-to-target ratio generated by the vaccines. Therefore, isolation and identification of other novel tumor antigens in myeloma will contribute to the ultimate development of a polyvalent vaccine that elicits consistent and strong immune responses and generates a high effector-to-target ratio in vivo.
In this study, we demonstrated that sperm protein 17 (Sp17) is a potential tumor antigen in myeloma. Both the transcripts encoding Sp17 and the protein are expressed in human myeloma cell lines and cells from fresh myeloma bone marrow specimens.
Study design
Materials
We studied 2 myeloma cell lines (ARP-1 and ARK-B, gifts from J. Epstein, University of Arkansas for Medical Sciences, Little Rock, AK) and bone marrow from 13 normal donors and 47 myeloma patients. The degree of bone marrow myeloma involvement ranged from 10% to 80% (in 6 patients, only BB4 antibody–enriched myeloma cells were used). All clinical materials were obtained with patients' consent and with approval from the local ethics committee. Rabbit polyclonal anti–human Sp17 antibody was kindly provided by Michael O'Rand (The University of North Carolina at Chapel Hill, NC).
Reverse transcription-polymerase chain reaction
Total RNA was extracted from cells by means of the Tri-reagent (Sigma, St Louis, MO). All RNA specimens were first treated with DNAse I (Ambion, Austin, TX) to remove genomic DNA contamination. First strand cDNA was synthesized from 1 μg of total RNA by means of random hexamer primer. The polymerase chain reaction (PCR) primers were as follows: for 5′Sp17 PCR, 5′-GGC AGT TCT TAC CAA GAA GAT-3′; for 3′Sp17 PCR, 5′-GGA GGT AAA ACC AGT GTC CTC-3′ They amplify a complementary DNA (cDNA) of approximately 500 base pairs (bp). PCR was performed by means of 35 amplification cycles at an annealing temperature of 55°C. Positive control amplification contained a plasmid with the Sp17 cDNA and a negative control of the PCR reaction mixture except for substitution of cDNA by water. RNA integrity in each sample was checked by amplification of a GAPDH gene segment. Successful removal of genomic DNA contamination was confirmed in each sample by amplification of the RNA without prior reverse-transcription (RT) reaction. PCR products were visualized on an ethidium bromide agarose gel for a DNA band of the expected size. All results were confirmed in 2 independent RT-PCRs.
Northern blot analysis
We electrophoresed 15 μg of total RNA from each normal tissue (Invitrogen, Leeks, The Netherlands) on a 1.2% agarose/formaldehyde gel and transferred it onto a nitrocellulose membrane. Subsequent hybridization to a 32P-labeled probe (derived from a plasmid containing the full-length Sp17 cDNA) and washing were performed under high-stringency conditions. Hybridization was performed at 60°C overnight, and final washes of the membranes were performed at 60°C with 0.1 × SSC in 0.1% sodium dodecyl sulfate (SDS) solution.
Western blot analysis
Lysates from tumor cells and cells from normal donor marrow were fractionated in a 12% SDS-polyacrylamide gel and transferred onto a nitrocellulose membrane. Sp17 protein was detected by rabbit polyclonal anti-Sp17 antibody followed by an alkaline phosphatase-conjugated goat anti–rabbit immunoglobulin G antibody. Antibody binding was visualized by reaction with the Western blue stabilized substrate (Promega).
Flow cytometric analysis
This was carried out on myeloma cell lines by means of a FACScan. Rabbit polyclonal anti-Sp17 antibody was used, and 2-stage indirect staining was carried out.
Results and discussion
In addition to idiotypes, T-lymphocyte targets on myeloma cells that may be suitable molecules for immunotherapy include MUC-1,7 mutant ras oncogene protein,8 and the new class of tumor antigens, known as CT (cancer-testis) antigens.9 The CT antigens include members of the MAGE family, BAGE, GAGE, andNY-ESO-1. They are normal testicular antigens expressed aberrantly in tumor cells. Their restricted normal tissue expression makes them ideal molecules for immune targeting. CT antigens are expressed in some myeloma cells.10,11Anti–MAGE-A3 cytotoxic lymphocyte clones raised from normal healthy donors could lyse myeloma cells in an HLA-A1– and HLA-A2–specific manner.11
There are a number of criteria that a protein should fulfill to be an ideal tumor antigen for immunotherapy. In addition to being expressed by tumor cells, the antigen must be immunogenic in vivo and show limited normal tissue expression that would make the T-cell targeting safe. To address these points, we have investigated in this study the expression of Sp17, a novel CT antigen that may be suitable for immunotherapy of myeloma. Sp17 is a protein of apparent molecular weight of 24.5 kd that is involved in acrosome reactions in spermatozoa. It has, in the last few years, been the target of investigation as an immunocontraceptive. Sp17 was chosen for this study because of its potential tissue specificity. In addition, it is likely to be a highly immunogenic protein in vivo on the basis of previous works showing a high incidence of auto-antibodies against Sp17 in vasectomized normal healthy males.12 Both B- and T-cell responses against Sp17 have also been defined in mice.13
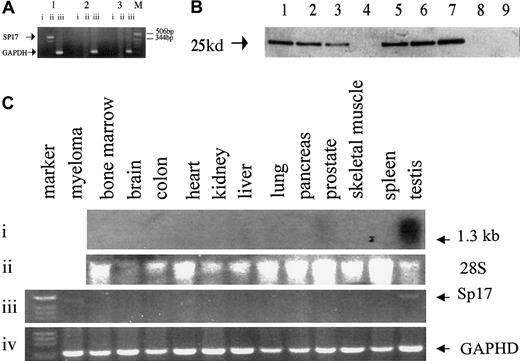
Using a pair of sequence-specific primers, we first demonstrated the presence of Sp17 messenger RNA (mRNA) in 2 out of 2 myeloma cell lines and 7 of 41 (17%) total RNA samples from fresh unfractionated myeloma bone marrow (Figure 1A). In another 6 bone marrow specimens obtained from myeloma patients, myeloma cells were purified with the use of the BB4 antibodies (directed at syndecan-1 expressed on myeloma cells); Sp17 transcripts were detected in 5 of these 6 specimens. In contrast, Sp17 mRNA was not detected in the bone marrow from any of the 13 normal healthy donors (Fisher exact test, P = .05). To confirm that the Sp17 mRNA resulted in the production of the Sp17 protein, we also used the 6 BB4 antibody–purified fresh myeloma cells and ARK myeloma cell lines for Western blot analysis. Sp17 protein was detected in all of the 5 BB4-enriched specimens that were also positive by RT-PCR and in the ARK cell line but not detected in either of the 2 normal bone marrows (Figure 1B). Our results therefore suggest that Sp17 is expressed, at both the mRNA and the protein level, in myeloma cells but not in normal bone marrow. It is likely that the prevalence of Sp17 expression in myeloma is underestimated by RT-PCR of total RNA derived from unfractionated bone marrow because of the relative low mRNA copy number within the myeloma cells that are actively synthesizing high levels of idiotypic protein. The detection may, however, be increased by enrichment of the tumor cell population.
Analysis showing that Sp17 is expressed in myeloma cells and only in normal testis.
(A) PCR analysis using a pair of sequence-specific primers for Sp17 produced a positive signal of around 500 bp. Lane 1 shows Sp17-positive myeloma bone marrow; lane 2, Sp17-negative myeloma bone marrow; lane 3, normal bone marrow; M, molecular marker. Lane i shows PCR of DNAse I-treated RNA; lane ii, PCR of RNA that had undergone RT; lane iii, control amplification for GAPDH gene segment. (B) Western blot analysis showing the expression of Sp17 protein in myeloma cells and not normal bone marrow cells. Lanes 1 through 6 show lysates from fresh myeloma cells; lane 7, lysate from ARK-B myeloma cell line; lanes 8 and 9, lysates from normal bone marrow. (C) Northern blot and RT-PCR analysis of RNA from a panel of normal tissue. Panel i shows a strong signal, of approximately 1.3 kb, in normal testis and a weak signal in normal prostate detected in Northern blot analysis; panel ii, 28S ribosomal RNA; panel iii, RT-PCR showing Sp17 transcripts in only normal testis and a myeloma tumor cell RNA; panel iv, control amplification for GAPDH gene segment.
Analysis showing that Sp17 is expressed in myeloma cells and only in normal testis.
(A) PCR analysis using a pair of sequence-specific primers for Sp17 produced a positive signal of around 500 bp. Lane 1 shows Sp17-positive myeloma bone marrow; lane 2, Sp17-negative myeloma bone marrow; lane 3, normal bone marrow; M, molecular marker. Lane i shows PCR of DNAse I-treated RNA; lane ii, PCR of RNA that had undergone RT; lane iii, control amplification for GAPDH gene segment. (B) Western blot analysis showing the expression of Sp17 protein in myeloma cells and not normal bone marrow cells. Lanes 1 through 6 show lysates from fresh myeloma cells; lane 7, lysate from ARK-B myeloma cell line; lanes 8 and 9, lysates from normal bone marrow. (C) Northern blot and RT-PCR analysis of RNA from a panel of normal tissue. Panel i shows a strong signal, of approximately 1.3 kb, in normal testis and a weak signal in normal prostate detected in Northern blot analysis; panel ii, 28S ribosomal RNA; panel iii, RT-PCR showing Sp17 transcripts in only normal testis and a myeloma tumor cell RNA; panel iv, control amplification for GAPDH gene segment.
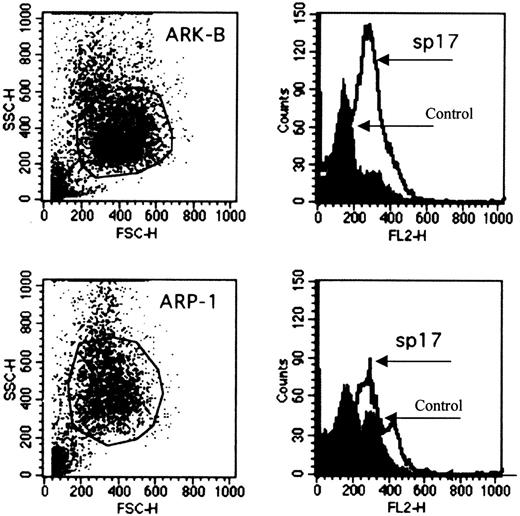
Since tissue specificity is a vital consideration in the choice of an antigen for immunotherapy, we proceeded to determine the expression of Sp17 transcripts in a panel of normal tissue RNA. Positive signals of approximately 1.3 kilobases (kb) were detected strongly only in normal testis and weakly in normal prostate (Figure 1C). By RT-PCR, we also excluded low copy number expression in these normal tissues (Figure 1C) and showed that the transcripts were detected only in normal testis, confirming the restricted normal tissue distribution of the Sp17 transcripts. Although the PCR was not designed primarily to give accurate mRNA quantitation, the reproducibly weaker signal from Sp17-positive myeloma cells when compared with those obtained with normal testicular PCR products suggests a lower level of Sp17 expression in myeloma cells (Figure 1C). Sp17 may therefore be a suitable molecule for T-cell targeting in myeloma. Sp17 is also expressed on the 2 myeloma cell lines as a surface protein (Figure2) and may therefore be a suitable surface antigen for antibody targeting.
Expression of Sp17 protein on the surface of 2 myeloma cell lines.
The expression of Sp17 protein (ARP-1, upper panel; ARK-B, lower panel) is demonstrated by flow cytometry (2-stage cell-staining technique). Rabbit Sp17 polyclonal antiserum was used to stain the cells. Phosphate-buffered saline was used as a control.
Expression of Sp17 protein on the surface of 2 myeloma cell lines.
The expression of Sp17 protein (ARP-1, upper panel; ARK-B, lower panel) is demonstrated by flow cytometry (2-stage cell-staining technique). Rabbit Sp17 polyclonal antiserum was used to stain the cells. Phosphate-buffered saline was used as a control.
In conclusion, our findings support the suitability of Sp17 as an immunologic target in myeloma and indicate that Sp17 is a new member of the class of CT antigens. Work is ongoing to use Sp17 as a vaccine for multiple myeloma.
Supported by grant RO1 CA88434-01 from the National Institutes of Health/National Cancer Institute, Bethesda, MD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Seah H. Lim, Center for Immunology and Microbial Disease, Albany Medical College, 47 New Scotland Ave, MC 151, Albany, NY 12208; e-mail: seah.lim@mail.amc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal