Hematopoietic cells bearing inactivating mutations of Fanconi anemia group C (FANCC) are excessively apoptotic and demonstrate hypersensitivity not only to cross-linking agents but also to interferon γ (IFN-γ) and tumor necrosis factor-α. Seeking essential signaling pathways for this phenotype, this study quantified constitutive and induced RNA-dependent protein kinase (PKR) activation in Fanconi anemia cells of the C complementation group (FA-C). PKR was constitutively phosphorylated and exhibited an increased binding affinity for double-stranded RNA (dsRNA) in FANCC−/− cells. FANCC−/− cells were hypersensitive to both dsRNA and the combination of dsRNA and IFN-γ in that these agents induced a higher fraction of apoptosis in FANCC−/− cells than in normal cells. Overexpression of wild-type PKR-sensitized FANCC−/− cells to apoptosis induced by IFN-γ and dsRNA. Conversely, inhibition of PKR function by enforced expression of a dominant-negative inhibitory mutant of PKR (PKRΔ6) substantially reduced the IFN and dsRNA hypersensitivity of FANCC−/− cells. Two PKR target molecules, IκB-α and IRF-1, were not differentially activated in FANCC−/−cells, but enforced expression of a nonphosphorylatable form of eukaryotic translation initiation factor-2α reversed the PKR-mediated block of messenger RNA translation and partially abrogated the PKR-mediated apoptosis in FANCC−/− cells. Because no evidence was found of a PKR/FANCC complex in normal cells, it was concluded that an essential function of FANCC is to suppress, indirectly, the activity of PKR and that FANCC inactivation results in IFN hypersensitivity, at least in part, because this function of FANCC is abrogated.
Introduction
The hallmark of the rare autosomal recessive disorder Fanconi anemia (FA) is progressive bone marrow failure, deriving, at least in part, from excessive apoptosis in committed progenitor cells.1-4 Hematopoietic progenitor cells from mice nullizygous at the FA group C (FANCC) locus and children with FA of the C complementation group (FA-C) are hypersensitive to the apoptosis-inducing effects of interferon γ (IFN-γ).1,2,5 Although most of the known effects of IFN-γ are thought to be transduced through signal transducer and activator of transcription-1 (STAT1) activation,6,7 and although it seemed reasonable, initially, to expect that STAT1 might be inappropriately activated in FA cells, STAT1 signaling is paradoxically suppressed in FANCC−/− cells because optimal STAT1 activation depends on the presence of a normal FANCC gene product.8Consequently, the excessive apoptotic response to IFN-γ in FANCC−/− cells is STAT1 independent.
If the STAT1 pathway is not directly involved in these aberrant responses of FA-C cells, what signaling pathway is? Any viable candidates should meet the standards of being activatable or inducible by both IFN-γ and tumor necrosis factor (TNF) and should also influence fas activity and activate caspase 3 and 8.2,7 The IFN-inducible, double-stranded RNA (dsRNA)–dependent protein kinase (PKR) meets these criteria.9,10 We carried out studies, reported herein, designed to test the hypothesis that PKR is excessively activated in FANCC−/− cells. We found that FANCC−/−cells are hypersensitive to dsRNA alone and in combination with IFN-γ and that PKR, a known mediator of apoptosis, the expression of which is induced by IFN-γ and the activation of which occurs on exposure to dsRNA and TNF,11 is constitutively activated in FANCC−/− cells. This aberrant activation state is suppressed by expression of the normal FANCC gene. Inhibition of PKR function significantly reduced IFN-γ hypersensitivity of FANCC−/− cells, leading to the conclusion that PKR is an essential mediator of IFN hypersensitivity in FANCC−/−cells.
The capacity of PKR to induce apoptosis can depend on its capacity to activate interferon-responsive factor-1 (IRF-1)12and to inactivate either the eukaryotic translation initiation factor 2α (eIF-2α)13,14 or the transcription factor NF-κB inhibitor (IκB-α),14 15 or both. We also report here that IRF-1 is not excessively activated by PKR in FANCC−/− cells, that dominant negative mutant forms of eIF-2α partially correct the IFN-γ hypersensitivity effect, and that dominant negative mutants of IκB-α do not.
Materials and methods
Cell culture and treatments
Murine embryonic fibroblasts (MEFs) were established from FANCC knockout (FANCC−/−) and wild-type (FANCC+/+) mice1 and maintained in Dulbecco modified Eagle medium (DMEM) (Gibco Life Technologies, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum (FBS). These FANCC−/− and FANCC+/+ MEFs were transformed and immortalized by SV40 large T-antigen.16 Human fibroblasts derived from FA patients with complementation groups A (PD720f), C (PD134f), and G (PD758f) were maintained in DMEM supplemented with 20% FBS. Unless otherwise indicated, cells were stimulated with 10 ng/mL of recombinant murine or human IFN-γ (R & D Systems, Minneapolis, MN) for the indicated time periods. For dsRNA treatment, 5 × 105 MEFs were plated in 60-mm tissue culture dishes and incubated at 37°C in 5% CO2 in air overnight before being transfected with 100 μg/mL of poly(I).poly(C) (Boehringer Mannheim, Indianapolis, IN), using LipofectAMINE (Gibco Life Technologies). The lipo-poly(I).poly(C) complexes were allowed to form for 30 to 45 minutes in serum-free medium before being added to the culture dishes. After incubation at 37°C for 7 hours, the medium was replaced with medium containing 10% serum. Cells were further incubated for 16 to 18 hours, followed by cell viability and flow cytometry analysis.
Construction of retroviral expression vectors and transduction of MEFs
The full-length human PKR (kindly provided by Dr G. N. Barber [Emory University, Atlanta, GA]; GenBank sequence accession number NM002759)17 was amplified by polymerase chain reaction (PCR), using Pfu DNA polymerase (Stratagene, La Jolla, CA) and a primer pair of 5′-CCGGCTCGAGATGGCTGGTGATCTTTCAGCA-3′ and 5′-CGCGGATCCCTAACATGTGTGTCGTTCA-3′. The resulting PCR fragment was subcloned into the XhoI and BamHI sites of vector pCITE-2a (Novagen, Madison, WI). The 1260-base pair (bp) StuI-Bsu36I fragment of amplified PKR sequence in this plasmid was then replaced with the corresponding fragments from the original complementary DNAs (cDNAs) of both wild-type PKR and its dominant negative mutant PKRΔ6.9 Finally, the replaced cDNAs were inserted into the retroviral vector pLXSN18 to create pLXSN-PKR and pLXSN-PKRΔ6, respectively. The cDNAs of wild-type eIF-2α, IκB-α, and their nonphosphorylatable mutants eIF-NP and IκB-M (kindly provided by Dr John W. B. Hershey [University of California, Davis] and Dr R. T. Hay [University of St. Andrews, United Kingdom], respectively) were tagged with the influenza hemagglutinin (HA) epitope (YPYDVPDYA) at the amino-terminus of each protein by PCR. The primers for eIF-2α and eIF-NP were 5′-CCGCTCGAGTACCCATACGATGTTCCTGACTATGCGCCGGGTCTAAGTTGTA-3′ and 5′-CGCGGATCCTAATCTTCAGCTTTGGCTTCCA-3′. For HA-tagged IκB-α and IκB-M, the primers were 5′-CCGCTCGAGTACCCATACGATGTTCCTGACTATGCGTTCCAGGCGGCCGAG-3′ and 5′-CGCGGATCCTCATAACGTCAGACGCTGGCC-3′. The amplified cDNA products were subcloned into the XhoI and BamHI sites of the retroviral vector pLXSH,18 which is different from pLXSN in that the former provides hygromycin resistance, whereas the latter is neomycin resistant. FANCC cDNA19 was subcloned into pLXSN as described previously.2 The pLXSN and pLXSH plasmids (10 μg each) were transfected, by the method of calcium phosphate precipitation, into Ψ2 and the resulting retrovirus was used to infect PA12 cells.20 MEFs were exposed to the retroviral supernatants in the presence of 8 μg/mL polybrene (Sigma Chemical Co, St Louis, MO) and selected in G418 (300 μg/mL) or hygromycin (300 μg/mL). FANCC-transduced cells were exposed to multiple doses (0, 10, 25, 50, 100, and 250 nM) of mitomycin C in cytotoxicity (trypan blue viability) to ensure that the mutant cells transduced with the normal cDNA were fully complemented.
Analysis of cell viability and apoptosis
Cell viability was measured by trypan blue exclusion analysis. To quantify apoptotic cells, we used a polyclonal antibody to the active form of caspase 3 in a flow cytometric assay to detect MEFs in the early stages of apoptosis. Caspase 3 is activated during apoptosis to varying degrees in different cell types, and recent studies from our laboratory3 indicate that the apoptotic response of FANCC−/− cells is caspase 8 and 3 dependent. The active, cleaved form of caspase 3, therefore, provides an instructive and biologically relevant marker of FANCC−/− cells undergoing programmed cell death. We observed that some cells lost adherence to the tissue culture dish after incubation with LipofectAMINE and dsRNA, and many of the apoptotic cells were in the nonadherent fraction. Therefore, assays were performed on pools of adherent and nonadherent cells. Specifically, the cell culture medium containing nonadherent MEF cells was removed and reserved in a separate tube. The adherent cells were then detached by trypsinization. The adherent and suspension cells were then pooled, washed with phosphate-buffered saline and resuspended at a concentration of 1 × 106/mL in staining buffer containing phosphate-buffered saline/2% FCS/0.1% Na Azide. This cell suspension (400 μL) was then added to 400 μL of Cytofix/Cytoperm (Pharmingen, San Diego, CA) and incubated for 20 minutes on ice to fix and permeabilize the cells. The cells were then washed with 2 mL Perm/Wash buffer (Pharmingen) and resuspended in 100 μL Perm/Wash buffer. Purified rabbit immunoglobulin G (IgG; 10 μL; PharMingen) was then added to each sample as a blocking antibody to prevent the nonspecific uptake of fluorochrome-conjugated antibody. After 15 minutes, 20 μL of phycoerythrin-conjugated antiactive Caspase 3 antibody (Pharmingen) was added, and samples were incubated for 30 minutes in the dark at room temperature. Cells were washed with 2 mL Perm/Wash buffer, resuspended in 500 μL of staining buffer, and analyzed by flow cytometry, using a FACSCalibur (Becton Dickinson, San Jose, CA). Camptothecin-treated cells were used as a positive control. Cells were exposed to camptothecin at a concentration of 60 μM for 9 hours at which time caspase 3 activation was optimal.
Immunoprecipitation and immunoblotting
Cells were lysed with Nonidet P-40 (NP-40) lysis buffer (1% NP-40, 20 mM Tris-HCl, pH 8.0, 137 mM NaCl, and 10% glycerol). The lysis buffer was supplemented with 1% aprotinin, 1 μg/mL leupeptin, 1 mM phenylmethylsulfonylfluoride (PMSF), and 2 mM sodium orthovanadate. Cell lysates were cleared by centrifugation at 13 200 rpm for 20 minutes at 4°C, and protein concentrations were determined by the Bradford method,21 using a protein microassay reagent (Bio-Rad, Hercules, CA). For immunoprecipitations with anti-mPKR and anti–eIF-2α antibodies (generous gifts from Dr J. C. Bell of Ottawa Regional Cancer Center Research Laboratories, Ottawa, and Dr B. Datta of University of Nebraska, Lincoln, respectively), whole cell lysates (about 1 mg of total proteins) were precleared with 50 μL of 50% protein A-Sepharose suspension (Pharmacia Biotech, Piscataway, NJ) for 1 hour at 4°C and then incubated with either anti-mPKR or anti–eIF-2α at 4°C for 3 to 5 hours. Immunocomplexes were recovered by incubation with 50 μL of protein A-sepharose beads for 1 to 2 hours at 4°C. For immunoblotting, whole cell lysates or immunocomplexes were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed with the indicated antibodies. To determine whether PKR interacts directly with FA proteins, whole cell lysates (1 mg of total proteins) were incubated with the indicated antibody against the specified FA protein, and the resulting immunocomplexes were analyzed using an anti-PKR antibody.
Reverse transcriptase–polymerase chain reaction
Total RNA was isolated from cells by using Tri Reagent (Molecular Research Center, Cincinnati, OH). First, strand cDNA was reverse transcribed from the indicated RNA by using random hexanucleotide primers (Gibco BRL) and MMLV RNase H−reverse transcriptase (Gibco BRL) as previously described.22 The cDNA was then amplified by polymerase chain reaction (PCR) for 35 cycles (denatured at 94°C for 30 seconds, primer annealed at 53°C for 30 seconds, primer extended at 72°C for 30 seconds). For fas amplification, the primers used were 5′-ACAGACAAAGCCCATTTTTC-3′ (upstream primer) and 5′-TTGCCACTGTTTCAGGATT-3′ (downstream primer), which produced an amplimer with a predicted length of 328 nucleotides.
Electrophoretic mobility shift assay
Nuclear extracts were prepared by the method of Dignam et al.23 A degenerate IRF family consensus oligonucleotide (5′-GAAAAG/CT/CGAAAG/CT/CGAAAG/CT/CG-3′) or a kB sequence (5′-CGGGCCGGGGAATCCCGCTAA-3′) was labeled with γ-32P]-ATP to 2.5 × 104 cpm/ng by using T4 polynucleotide kinase (Boehringer Mannheim). Binding reactions (20 μL) contained 5 μg nuclear extract, 0.2 ng labeled oligo, 1 μg poly (dI-dC), and 10 μg BSA in 10 mM Tris-Cl, pH 7.4, 50 mM NaCl, 1 mM dithiothreitol, 1 mM EDTA, and 10% glycerol. Reactions were incubated at room temperature for 30 minutes, then resolved on a 4% nondenaturing polyacrylamide. For supershift assays, reactions were incubated for a further 45 minutes in the presence of antibodies against IRF-1 (5 μg; Santa Cruz Biotechnology, Santa Cruz, CA), p50 (3μL; Upstate Biotech, New York, NY), or p65 (3 μg; Santa Cruz Biotechnology).
dsRNA binding assay
Whole cell lysates (200 μL) that contained 500 μg of total proteins were mixed with 50 μL of poly(I).poly(C)-agarose (Pharmacia) beads and rocked at 4°C for 60 minutes. The beads were then washed 3 times with 500 μL of NP-40 lysis buffer. Proteins bound to beads were eluted from the beads by heating the samples at 94°C for 5 minutes in 2× Laemmli SDS sample buffer, separated by SDS-PAGE, and subjected to immunoblot analysis by using anti-mPKR.
In vivo 32P and 35S labeling of proteins
Cells were starved for 60 minutes in phosphate-free DMEM that contained 10% dialyzed FBS and were treated with 10 ng/mL of murine recombinant IFN-γ and 100 μg/mL of poly(I).poly(C). Labeling was performed in the same medium by addition of [32P] orthophosphate (150 μCi/mL; DuPont NEN, Boston, MA). After labeling for 3 hours, whole cell lysates were prepared and subjected to immunoprecipitation with antibodies specific for mPKR or eIF-2α. [32P]-phosphate-incorporated immunocomplexes were analyzed by SDS-PAGE, followed by autoradiography. Immunoblot analysis was performed on these samples to determine the quantity of PKR or eIF-2α precipitated by the respective antibody. For 35S labeling, 0.5 × 106 MEFs were seeded in a 60-mm dish and cultured overnight. Cells were rinsed with methionine-cysteine-free DMEM and treated with dsRNA and IFN-γ where indicated. Labeling was again performed in the same medium containing 50 μCi/mL of [35S]methionine-cysteine labeling mix (DuPont NEN) and incubated at 37°C for 60 minutes. Protein synthesis was measured by the incorporation of [35S]methionine and [35S]cysteine into trichloroacetic acid-precipitable proteins.
Results
FANCC mutants are hypersensitive to apoptosis induced by IFN-γ and dsRNA
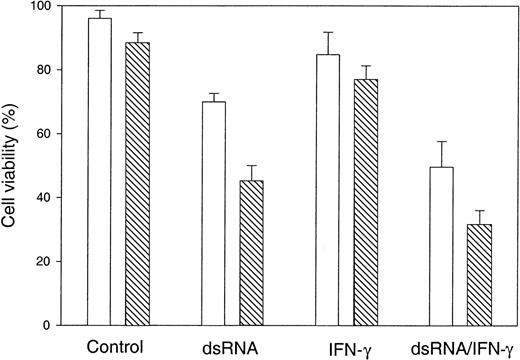
Having previously described hypersensitivity of FANCC−/− cells to IFN-γ-induced apoptosis,1,5 we sought to determine whether the IFN-γ-inducible, dsRNA-dependent PKR plays a role in the FA apoptotic pathway. We initially chose bone marrow cells because such hematopoietic progenitor cells from FANCC knockout mice and from children with FA-C are hypersensitive to IFN-γ and TNF-α-induced apoptosis.1-5 However, we were unable to transfect these bone marrow cells with dsRNA, which is most commonly used as a PKR activator. Recently, MEFs from PKR knockout mice have been successfully used to study dsRNA-dependent PKR-induced apoptosis.10 24Therefore, we employed MEFs derived from normal (FANCC+/+) and FANCC knockout mice (FANCC−/−) to study the involvement of PKR in the FA apoptotic pathway. As shown in Figure1A, a greater fraction of FANCC−/− cells were killed after 24 hours of treatment with dsRNA or IFN-γ in combination with dsRNA when compared to the normal MEFs. Specifically, cell viability in FANCC−/−cells treated with dsRNA alone was reduced to 43% compared to 72% in FANCC+/+ cells (Figure 1A, column dsRNA). Treatment with a combination of dsRNA and IFN-γ caused an increase in cell death of nearly 2-fold in FANCC−/− cells (column dsRNA/IFN-γ).
FANCC−/− MEFs are hypersensitive to apoptosis induced by IFN-γ and dsRNA.
Equivalent numbers of cells were incubated with murine recombinant IFN-γ (10 ng/mL) for 15 hours, followed by transfection with or without 100 μg/mL of poly(I).poly(C) in the presence of LipofectAmine. Mock exposed cells were incubated with LipofectAmine only. After 24 hours, cells were subjected to viability determination by trypan blue exclusion assay. ▧, FANCC−/−; ■, FANCC+/+. Data are the mean ± standard deviations of triplicate determinations.
FANCC−/− MEFs are hypersensitive to apoptosis induced by IFN-γ and dsRNA.
Equivalent numbers of cells were incubated with murine recombinant IFN-γ (10 ng/mL) for 15 hours, followed by transfection with or without 100 μg/mL of poly(I).poly(C) in the presence of LipofectAmine. Mock exposed cells were incubated with LipofectAmine only. After 24 hours, cells were subjected to viability determination by trypan blue exclusion assay. ▧, FANCC−/−; ■, FANCC+/+. Data are the mean ± standard deviations of triplicate determinations.
Caspase 3 activation in single cells, analyzed by flow cytometry, mirrored the cell viability findings. As shown in Table1, IFN-γ (0.78%) or dsRNA (2.1%) alone did not cause a significant increase in caspase 3 activation in normal MEF cells compared to untreated cells (0.46%). The combination of dsRNA and IFN-γ did, however, cause significant induction of caspase 3 (14.5%) in normal MEFs. In FANCC−/− cells, significant caspase 3 activation was induced with IFN-γ alone (9.35%), dsRNA alone (36%), and the combination of both (50.95%).
Flow cytometric analysis of fractional apoptotic responses in murine embryonic fibroblasts treated with interferon γ and double-stranded RNA
| Cells . | % Apoptotic cells . | |||
|---|---|---|---|---|
| Untreated . | IFN-γ . | dsRNA . | IFN-γ/dsRNA . | |
| FANCC+/+ | 0.46 (3937) | 0.78 (4000) | 2.10 (4000) | 14.53 (4000) |
| FANCC−/− | 0.25 (4000) | 9.35 (4000) | 35.98 (4000) | 50.95 (4000) |
| Cells . | % Apoptotic cells . | |||
|---|---|---|---|---|
| Untreated . | IFN-γ . | dsRNA . | IFN-γ/dsRNA . | |
| FANCC+/+ | 0.46 (3937) | 0.78 (4000) | 2.10 (4000) | 14.53 (4000) |
| FANCC−/− | 0.25 (4000) | 9.35 (4000) | 35.98 (4000) | 50.95 (4000) |
Cells were incubated with murine recombinant IFN-γ (10 ng/mL) for 15 hours before transfection with or without 100 μg/mL of poly(I).poly(C) in the presence of LipofectAmine and harvested 24 hours after transfection for flow cytometric analysis as described in “Materials and methods.” The untreated cells were incubated with LipofectAmine only. Treatment of FANCC+/+ murine embryonic fibroblasts with camptothecin was used as a positive control (60 μM for 9 hours) and induced 11.8% positivity (2 916 events). Numbers in parentheses are total events analyzed.
IFN-γ indicates interferon γ; dsRNA indicates double-stranded RNA; FANCC, Fanconi anemia group C.
PKR is constitutively activated in FANCC−/− cells
The above results suggested a possible involvement of PKR in the FA apoptotic pathway. We then asked whether FANCC−/−cells expressed higher levels of PKR. Figure2A shows that IFN-γ but not dsRNA induced PKR expression in both normal and FANCC−/− MEFs and that the levels of PKR proteins were not significantly different between normal and mutant cells. With the reason that there might be differences in the ground-state activation of PKR in these cells, we measured PKR phosphorylation in isogenic FANCC cells. Cells were labeled with [32P]-orthophosphate, and levels of32P-labeled PKR were quantified by using immunoprecipitation. As shown in Figure 2B, PKR activation was undetectable in untreated normal MEFs (top panel, lane 1). Although IFN-γ significantly induced expression of the PKR protein as described in Figure 2A, activated PKR was only slightly increased in these IFN-γ-treated cells (Figure 2B, top panel, lanes 3,7). However, both normal and FANCC−/− MEFs showed greatly elevated levels of activated PKR after treatment with dsRNA (lanes 2,6). This is consistent with previous observations that phosphorylative activation of PKR is dsRNA dependent.11 Significantly, there was approximately 2-fold more phosphorylated PKR precipitated from FANCC−/− cells than normal MEFs treated with dsRNA or dsRNA plus IFN-γ (compare lanes 2 and 4 with 6 and 8). Of equal importance, a significant amount of PKR was constitutively activated in FANCC−/− MEFs (lane 5). Furthermore, the degree to which increased PKR was phosphorylated correlated positively with the fraction of apoptotic cells induced by dsRNA and IFN-γ (compare Table1 with Figure 2B). Immunoblots confirmed that all immunoprecipitates contained similar amounts of PKR proteins (Figure 2B, bottom panel).
PKR is constitutively activated in FANCC−/− cells.
(A) Expression of PKR in MEFs in response to IFN-γ and dsRNA. Whole cell lysates were prepared 48 hours after incubation with IFN-γ (10 ng/mL) and 24 hours after transfection with 100 μg/mL of poly(I).poly(C), separated on a 7.5% SDS-PAGE, and immunoblotted with anti-mPKR antibody. (B) In vivo phosphorylation of PKR in MEFs treated with IFN-γ and dsRNA. FANCC+/− and FANCC−/− MEFs were labeled, 24 hours after incubation with IFN-γ (10 ng/mL) and 6 hours after transfection with 100 μg/mL of poly(I).poly(C), with [32P]orthophosphate (150 μCi/mL) for an additional 3 hours. Cells were lysed, and equal amounts of whole cell lysates (500 μg of total proteins each sample) were immunoprecipitated with antibody specific for mPKR. [32P]phosphate-incorporated PKR immunocomplexes were analyzed by SDS-PAGE, followed by autoradiography (top panel). To ensure additionally that equivalent amounts of the immunocomplexes were measured, immunoblot analysis of equal fractions of the PKR immunoprecipitates was performed by using the mPKR monoclonal antibody (bottom panel). P-mPKR, phosphorylated mouse PKR; HC, IgG heavy chain. (C) dsRNA-agarose affinity chromatography showing significantly more PKR from FANCC−/− cell lysates binding to dsRNA. Equal amounts of whole cell lysates (500 μg of total proteins each sample) were applied to a poly(I).poly(C)-agarose column. The bound proteins were eluted and analyzed by SDS-PAGE and immunoblot, using anti-mPKR antibody.
PKR is constitutively activated in FANCC−/− cells.
(A) Expression of PKR in MEFs in response to IFN-γ and dsRNA. Whole cell lysates were prepared 48 hours after incubation with IFN-γ (10 ng/mL) and 24 hours after transfection with 100 μg/mL of poly(I).poly(C), separated on a 7.5% SDS-PAGE, and immunoblotted with anti-mPKR antibody. (B) In vivo phosphorylation of PKR in MEFs treated with IFN-γ and dsRNA. FANCC+/− and FANCC−/− MEFs were labeled, 24 hours after incubation with IFN-γ (10 ng/mL) and 6 hours after transfection with 100 μg/mL of poly(I).poly(C), with [32P]orthophosphate (150 μCi/mL) for an additional 3 hours. Cells were lysed, and equal amounts of whole cell lysates (500 μg of total proteins each sample) were immunoprecipitated with antibody specific for mPKR. [32P]phosphate-incorporated PKR immunocomplexes were analyzed by SDS-PAGE, followed by autoradiography (top panel). To ensure additionally that equivalent amounts of the immunocomplexes were measured, immunoblot analysis of equal fractions of the PKR immunoprecipitates was performed by using the mPKR monoclonal antibody (bottom panel). P-mPKR, phosphorylated mouse PKR; HC, IgG heavy chain. (C) dsRNA-agarose affinity chromatography showing significantly more PKR from FANCC−/− cell lysates binding to dsRNA. Equal amounts of whole cell lysates (500 μg of total proteins each sample) were applied to a poly(I).poly(C)-agarose column. The bound proteins were eluted and analyzed by SDS-PAGE and immunoblot, using anti-mPKR antibody.
Considering that the higher activation state of PKR might be caused by a higher affinity of PKR for dsRNA in mutant cells, we tested the binding capacity of PKR in isogenic cell lines. RNA affinity binding was performed by using equal amounts of total proteins from treated and untreated normal and FANCC−/− MEFs. Bound PKR was analyzed in eluates by immunoblotting. For reasons that remain to be clarified, the dsRNA binding capacity of PKR was higher in FANCC−/− MEFs compared to the normal cells (Figure 2C).
Overexpression of PKR sensitizes FANCC−/− MEFs to dsRNA- and IFN γ-induced apoptosis
The results described above support the hypothesis that the state of PKR activation may contribute to the IFN-γ hypersensitivity in FA cells. To test this idea, we overexpressed human wild-type PKR (hPKR) and a dominant negative mutant, PKRΔ6 (catalytically inactive9,25), in both normal and FANCC−/−cells and subsequently quantified dsRNA- and IFN-γ-induced apoptosis. After 24 hours of dsRNA transfection, cells were examined for hPKR protein expression by Western blot analysis, for cell viability by trypan blue staining, and for induction of apoptosis by quantification of the fraction of exposed cells containing activated caspase 3. As shown in Figure 3A, endogenous murine PKR (approximately 65 kD) was induced by treatment of IFN-γ but not dsRNA. The expression of hPKR, which migrated at approximately 70 kD on SDS-PAGE, was equivalent between transduced cell lines and was not responsive to IFN-γ, likely reflected the lack of regulatory regions in our constructs. Cell viability assays (Figure 3B) showed that approximately 90% of wild-type PKR-expressing FANCC−/−cells underwent apoptosis after exposure to dsRNA and IFN-γ compared to 50% of normal cells (column PKR wt). Overexpression of PKRΔ6 increased survival of FANCC−/− cells treated with dsRNA and IFN-γ from 30% to more than 50% (compare column VEC with PKR6). Although expression of the human PKR was not induced by IFN-γ and dsRNA treatment (Figure 3A), cell death was substantial in FANCC−/− cells (Figure 3B, column PKRwt). It is possible that overexpression of hPKR increases the amount of total PKR proteins available for activation by dsRNA. Another explanation may pertain to the activation state and binding capacity of the overexpressing hPKR protein in these MEFs. On the latter point, we did observe higher dsRNA-binding capacity by the overexpressed human form of PKR than by the endogenous murine PKR in untreated FANCC−/− cells (data not shown). This may partially explain why overexpression of hPKR alone can induce apoptosis in FANCC−/− MEFs (Table 1, Figure 3B).
Enforced expression of PKR-sensitized FANCC−/− cells to IFN-γ and dsRNA-induced apoptosis.
(A) Retrovirally expressed human PKR proteins in MEFs: control cells carrying vector alone (VEC) or cells expressing wild-type human PKR (PKRwt) or PKRΔ6 were treated with or without IFN-γ (10 ng/mL) or dsRNA (100 μg/mL), lysed, and analyzed by Western blotting by using a polyclonal antibody that reacted with both human PKR (hPKR) and mouse PKR (mPKR) proteins. NS, nonspecific. (B) Cell viability of MEFs carrying vector alone (VEC) or expressing wild-type hPKR (wtPKR) or PKRΔ6, in response to IFN-γ or/and dsRNA treatments; data represent the means (standard deviations of triplicate determinations. ▧, FANCC−/−; ■, FANCC+/−.
Enforced expression of PKR-sensitized FANCC−/− cells to IFN-γ and dsRNA-induced apoptosis.
(A) Retrovirally expressed human PKR proteins in MEFs: control cells carrying vector alone (VEC) or cells expressing wild-type human PKR (PKRwt) or PKRΔ6 were treated with or without IFN-γ (10 ng/mL) or dsRNA (100 μg/mL), lysed, and analyzed by Western blotting by using a polyclonal antibody that reacted with both human PKR (hPKR) and mouse PKR (mPKR) proteins. NS, nonspecific. (B) Cell viability of MEFs carrying vector alone (VEC) or expressing wild-type hPKR (wtPKR) or PKRΔ6, in response to IFN-γ or/and dsRNA treatments; data represent the means (standard deviations of triplicate determinations. ▧, FANCC−/−; ■, FANCC+/−.
To confirm the role of PKR in apoptotic responses of FANCC−/− MEFs, we carried out flow cytometry for caspase 3 activation. Table 2 shows that after exposure either to dsRNA alone or the combination of dsRNA and that IFN-γ caspase 3-positive FANCC−/− cells expressing wild-type PKR were increased 5- to 7-fold over normal MEFs. When compared to cells containing the empty vector, the percentage of apoptosis induced by dsRNA and dsRNA plus IFN-γ in PKR-expressing FANCC−/− cells increased by 12.3- and 8.7-fold, respectively, over the PKR-expressing normal cells. In addition, expression of PKRΔ6 gave more protection to FANCC−/−cells than to normal cells. When compared to cells containing the empty vector, the percentage of apoptosis induced by dsRNA or dsRNA and IFN-γ in PKRΔ6-expressing FANCC−/− cells was reduced by 23.9% and 82.4%, respectively. Less remarkable reductions were seen in PKRΔ6-expressing normal cells (12.1% and 34.5%, respectively). Collectively, these data clearly demonstrate the involvement of PKR in IFN-γ- and dsRNA-induced apoptosis in FANCC−/− MEFs.
Flow cytometric analysis of fractional apoptotic responses in RNA-dependent protein kinase–overexpressing murine embryonic fibroblasts treated with interferon γ and double-stranded RNA
| Cells . | % Apoptotic cells . | ||
|---|---|---|---|
| Untreated . | dsRNA . | IFN-γ/dsRNA . | |
| Vector | |||
| FANCC+/+ | 0.48 (9940) | 0.15 (9950) | 1.15 (9820) |
| FANCC−/− | 0.28 (9906) | 1.84 (9967) | 10.01 (9645) |
| PKR | |||
| FANCC+/+ | 0.14 (9956) | 0.61 (9909) | 1.91 (9821) |
| FANCC−/− | 1.35 (9818) | 4.22 (9675) | 10.86 (9445) |
| PKRΔ6 | |||
| FANCC+/+ | 0.93 (9925) | 0.13 (9930) | 0.75 (9878) |
| FANCC−/− | 3.69 (9621) | 1.40 (9496) | 1.76 (9660) |
| Cells . | % Apoptotic cells . | ||
|---|---|---|---|
| Untreated . | dsRNA . | IFN-γ/dsRNA . | |
| Vector | |||
| FANCC+/+ | 0.48 (9940) | 0.15 (9950) | 1.15 (9820) |
| FANCC−/− | 0.28 (9906) | 1.84 (9967) | 10.01 (9645) |
| PKR | |||
| FANCC+/+ | 0.14 (9956) | 0.61 (9909) | 1.91 (9821) |
| FANCC−/− | 1.35 (9818) | 4.22 (9675) | 10.86 (9445) |
| PKRΔ6 | |||
| FANCC+/+ | 0.93 (9925) | 0.13 (9930) | 0.75 (9878) |
| FANCC−/− | 3.69 (9621) | 1.40 (9496) | 1.76 (9660) |
Murine embryonic fibroblasts carrying vector alone (pLXSH) or expressing (wtPKR) or PKRΔ6 were treated with murine recombinant IFN-γ (10 ng/mL) and/or 100 μg/mL of poly(I).poly(C) as described in Table 1. Numbers in parentheses are total events analyzed.
dsRNA indicates double-stranded RNA; IFN-γ, interferon γ; FANCC, Fanconi anemia group C; wtPKR, wild-type human RNA-dependent protein kinase.
PKR-induced apoptosis in cells treated with a variety of cellular stressors, including dsRNA and IFN-γ occurs, at least in part, through up-regulation of Fas expression.10,26 Indeed, IFN-γ-induced apoptosis involves priming of the Fas pathway in FANCC−/− hematopoietic progenitor cells.1 In addition, Otsuki et al4 reported that apoptosis induced by TNF-α in FANCC−/− hematopoietic progenitor cells involved Fas ligation.4 Therefore, although we have not found that fas expression increases in cells treated with IFN-γ alone,27 we sought to confirm that Fas mRNA levels were unaltered in response to both IFN-γ and dsRNA by reverse transcriptase-PCR, using Fas mRNA-specific primers. We did not observe significant changes in the levels of Fas mRNA in both normal and FANCC−/− MEFs expressing wild-type PKR or PKRΔ6, treated with or without IFN-γ and dsRNA (data not shown).
Li and Youssoufian28 demonstrated that an IFN-responsive gene, MxA, was up-regulated in several FA complementation group mutant cells, including groups A, B, C, and D. By having established the involvement of PKR in IFN-γ and dsRNA-induced apoptosis in FANCC−/− MEFs, we wondered whether other FA group mutant cells were also susceptible to PKR-mediated apoptosis. We treated fibroblasts derived from FA patients of complementation groups A (FANCA−/−), C (FANCC−/−), and G (FANCG−/−) with IFN-γ and dsRNA and determined the sensitivity of these mutant cells in response to the reagents. We found that in contrast to FANCC−/− fibroblasts, which were hypersensitive to IFN-γ and dsRNA treatment, FANCA−/−and FANCG−/− fibroblasts exhibited no more sensitivity than the normal fibroblasts (data not shown).
An eIF-NP mutant inhibits PKR-induced apoptosis in FANCC−/− cells
PKR signaling results in phosphorylation of at least 3 separate substrates that affect apoptotic responses: phosphorylative activation of IRF-112 and phosphorylative inactivation of either translation initiating factor eIF-2α or the transcription factor inhibitor IκB-α.14,29 To test the respective roles of the latter 2 substrates in the apoptotic response of FANCC−/− cells, we expressed wild-type eIF-2α, IκB-α, and their nonphosphorylatable mutants, eIF-NP and IκB-M, as HA-tagged recombinant proteins in both normal and FANCC−/− MEFs. Immunoblot analysis revealed that HA-tagged eIF-2α, eIF-NP, IκB-α, and IκB-M were equally overexpressed in resting normal and FANCC−/− MEFs (Figure4A,B). These cells were then cotransduced with a retroviral wild-type PKR vector to study the interaction of PKR and its 2 substrates in apoptosis in FANCC−/− cells. When normal MEFs were cotransduced with PKR and empty vector alone, treatment with dsRNA or a combination of dsRNA and IFN-γ induced apoptosis (Figure 4C, column VEC). Cotransduction of wild-type eIF-2α neither sensitized nor relieved PKR-induced cytotoxicity (compare column eIF with VEC), but overexpression of the nonphosphorylatable mutants eIF-NP and IκB-M partially abrogated the effects of dsRNA and dsRNA plus IFN-γ (Figure 4C, columns eIF-NP and IκB-M). As has been reported by others,14 30 we also observed partial relief of cytotoxicity by overexpression of wild-type IκB-α (Figure 4C, column IκB). Results differed in studies on FANCC−/−cells. In FANCC−/− cells transduced with empty vector and wild-type eIF-α, treatment with dsRNA or dsRNA plus IFN-γ caused more cell death than in normal MEFs (columns VEC and eIF). Overexpression of eIF-NP in FANCC−/− cells reduced, but not completely, the percentage of apoptotic cells compared to vector alone or wild-type eIF-2α (Figure 4C, compare column eIF-NP with VEC and eIF). However, expression of either wild-type IκB-α or nonphosphorylatable IκB-M in FANCC−/− cells did not relieve cytotoxicity caused by treatment of dsRNA or dsRNA plus IFN-γ (column IκB and IκB-M). We also quantified fractional apoptosis in these cells by flow cytometry. Consistent with the cell viability studies described above, overexpression of eIF-NP, but not that of IκB-M, significantly blocked PKR-mediated apoptosis in FANCC−/− MEFs (data not shown). Immunoblot analysis demonstrated that the levels of retrovirally expressed PKR proteins were similar in all samples (Figure 4D,E, top panels). The bottom panels of Figure 4D and E show the levels of eIF-2α and eIF-NP, and IκB-α and IκB-M, respectively.
Enforced expression of eIF-NP inhibits PKR-induced apoptosis in FANCC−/− MEFs.
(A) Expression of HA-eIF proteins in FANCC+/+ and FANCC−/− cells. Whole cell lysates (80 μg of total proteins) were separated on a 7.5% SDS-PAGE and immunoblotted with the anti-HA antibody (top panel) or the anti–eIF-2α antibody (bottom panel). (B) Expression of HA-IκB proteins in FANCC+/+ and FANCC−/− cells. Total proteins (80 μg) were analyzed as described in (A), using the anti-HA antibody (top panel) or the anti–IκB-α antibody (bottom panel). (C) Cell viability of PKR-expressing MEFs carrying vector alone (VEC), wild-type eIF-2α (eIF), a nonphosphorylatable mutant eIF-NP, wild-type IκB-α (IκB), or a nonphosphorylatable mutant IκB-M; data points were determined by trypan blue exclusion assay as described in Figure 1 and presented as the mean (standard deviations of triplicate determinations. ▧, FANCC−/−; ■, FANCC+/+. (D) Expression of hPKR and eIF proteins in FANCC+/+ and FANCC−/− cells. Total proteins (80 μg) were analyzed by Western blotting with anti-PKR (top panel), anti-HA (middle panel), or anti–eIF-2α antibody (bottom panel). (E) Expression of hPKR and IκB proteins in FANCC+/+ and FANCC−/−cells. Total proteins (80 μg) were analyzed by immunoblotting with anti-PKR (top panel), anti-HA (middle panel), or anti–IκB-α antibody (bottom panel).
Enforced expression of eIF-NP inhibits PKR-induced apoptosis in FANCC−/− MEFs.
(A) Expression of HA-eIF proteins in FANCC+/+ and FANCC−/− cells. Whole cell lysates (80 μg of total proteins) were separated on a 7.5% SDS-PAGE and immunoblotted with the anti-HA antibody (top panel) or the anti–eIF-2α antibody (bottom panel). (B) Expression of HA-IκB proteins in FANCC+/+ and FANCC−/− cells. Total proteins (80 μg) were analyzed as described in (A), using the anti-HA antibody (top panel) or the anti–IκB-α antibody (bottom panel). (C) Cell viability of PKR-expressing MEFs carrying vector alone (VEC), wild-type eIF-2α (eIF), a nonphosphorylatable mutant eIF-NP, wild-type IκB-α (IκB), or a nonphosphorylatable mutant IκB-M; data points were determined by trypan blue exclusion assay as described in Figure 1 and presented as the mean (standard deviations of triplicate determinations. ▧, FANCC−/−; ■, FANCC+/+. (D) Expression of hPKR and eIF proteins in FANCC+/+ and FANCC−/− cells. Total proteins (80 μg) were analyzed by Western blotting with anti-PKR (top panel), anti-HA (middle panel), or anti–eIF-2α antibody (bottom panel). (E) Expression of hPKR and IκB proteins in FANCC+/+ and FANCC−/−cells. Total proteins (80 μg) were analyzed by immunoblotting with anti-PKR (top panel), anti-HA (middle panel), or anti–IκB-α antibody (bottom panel).
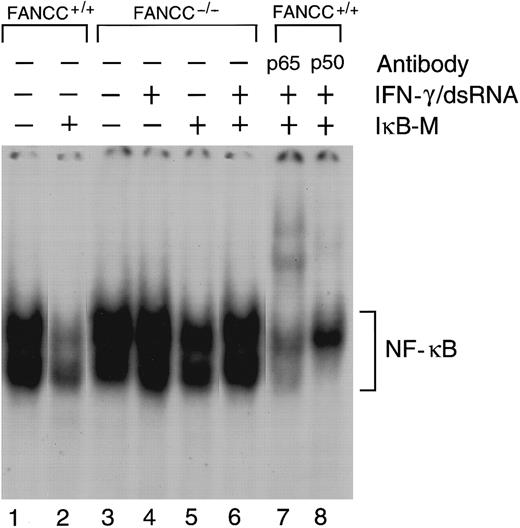
To assure ourselves that PKR-induced apoptosis in FANCC−/− cells does not specifically involve differential NF-κB activation, we performed an electrophoretic mobility shift assay (EMSA) for NF-κB. As shown in Figure5, overexpression of PKR alone induced nuclear NF-κB activity in normal cells (lane 1), and coexpression of IκB-M substantially suppressed this activity (lane 2). Although we observed higher NF-κB DNA binding in PKR-expressing FANCC−/− cells than in normal cells (lane 3 versus lane 1), overexpression of IκB-M did not inhibit NF-κB activity in these mutant cells (compare lane 1 with 2 and lane 3 with 4). Antibody supershift analysis indicated that the NF-κB DNA complexes contained the p50 and p65 subunits (lanes 7,8).
EMSA analysis of NF-κB activation in IκB-M–expressing MEFs.
IκB-M–expressing normal and FANCC−/− MEFs were treated with IFN-γ (10 ng/mL) and dsRNA (100 μg/mL), and EMSA was performed by using 5 μg of nuclear extracts and a kB oligonucleotide sequence as described in “Materials and methods.” The final 2 lanes indicate that binding reactions were incubated with 3 μg of anti-p65 (lane 7) or 3 μL of anti-p50 (lane 8) antibody.
EMSA analysis of NF-κB activation in IκB-M–expressing MEFs.
IκB-M–expressing normal and FANCC−/− MEFs were treated with IFN-γ (10 ng/mL) and dsRNA (100 μg/mL), and EMSA was performed by using 5 μg of nuclear extracts and a kB oligonucleotide sequence as described in “Materials and methods.” The final 2 lanes indicate that binding reactions were incubated with 3 μg of anti-p65 (lane 7) or 3 μL of anti-p50 (lane 8) antibody.
PKR-mediated apoptosis in FANCC−/− MEFs is associated with translational inhibition
To determine whether excessive apoptosis in FANCC−/− cells treated with dsRNA and IFN-γ is induced by excessive PKR-mediated phosphorylation of eIF-2α, we performed in vivo analysis of eIF-2α phosphorylation in MEFs. The eIF proteins were immunoprecipitated from [32P]-orthophosphate-labeled normal and FANCC−/− MEFs that coexpressed PKR and wild-type eIF-2α or eIF-NP. Treatment of PKR-expressing FANCC−/− cells with dsRNA and IFN-γ resulted in significantly more phosphorylation of eIF-2α than in normal cells (Figure 6A, compare lanes 1 versus 2 and 5 versus 6). Expression of eIF-NP dramatically reduced eIF-2α phosphorylation in treated FANCC−/− cells compared to FANCC−/− cells bearing the wild-type eIF-2α or empty vector (Figure 6A, compare lanes 10 with 6 and 8). Phosphorylation of eIF-2α in normal MEFs treated with dsRNA plus IFN-γ (Figure 6A, lane 4) was also inhibited by eIF-NP, albeit to a lesser extent. Immunoblot analysis showed that the amount of eIF-2α immunoprecipitated was not significantly different between samples (Figure 6B).
Expression of the nonphosphorylatable mutant eIF-NP rescued PKR-mediated inhibition of protein synthesis in FANCC−/− cells.
(A) Analysis of phosphorylated eIF-2α levels in PKR-expressing MEFs carrying vector alone (VEC), wild-type eIF-2α (eIF), and a nonphosphorylatable mutant eIF-NP; cells were pretreated with 10 ng/mL of murine recombinant IFN-γ, transfected with 100 μg/mL of poly(I).poly(C), and labeled in a methionine-cysteine-free medium containing 50 μCi/mL of [35S]methionine-cysteine labeling mix for 60 minutes. Whole cell lysates were prepared, and 800 μg of total proteins from each sample were subjected to immunoprecipitation with anti–eIF-2α antibody. The immunocomplexes were analyzed by SDS-PAGE followed by autoradiography. NS, nonspecific. (B) Immunoblot analysis of a fraction of the same immunoprecipitates described in (A) using anti–eIF-2α antibody. HC, IgG heavy chain. (C) Rate of protein synthesis in PKR-expressing MEFs carrying vector alone (VEC), wild-type eIF-2α (eIF), and a nonphosphorylatable mutant eIF-NP. Cells were pretreated with 10 ng/mL of murine recombinant IFN-γ, transfected with 100 μg/mL of poly(I).poly(C), and labeled in a methionine-cysteine-free medium containing 50 μCi/mL of [35S]methionine-cysteine labeling mix for 60 minutes. Protein synthesis was measured by the incorporation of [35S]methionine and [35S]cysteine into trichloroacetic acid-precipitable proteins. The control cells did not carry any expression vectors. ▧, FANCC−/−; ■, FANCC+/+. Data represent means ± standard deviations of 2 independent experiments.
Expression of the nonphosphorylatable mutant eIF-NP rescued PKR-mediated inhibition of protein synthesis in FANCC−/− cells.
(A) Analysis of phosphorylated eIF-2α levels in PKR-expressing MEFs carrying vector alone (VEC), wild-type eIF-2α (eIF), and a nonphosphorylatable mutant eIF-NP; cells were pretreated with 10 ng/mL of murine recombinant IFN-γ, transfected with 100 μg/mL of poly(I).poly(C), and labeled in a methionine-cysteine-free medium containing 50 μCi/mL of [35S]methionine-cysteine labeling mix for 60 minutes. Whole cell lysates were prepared, and 800 μg of total proteins from each sample were subjected to immunoprecipitation with anti–eIF-2α antibody. The immunocomplexes were analyzed by SDS-PAGE followed by autoradiography. NS, nonspecific. (B) Immunoblot analysis of a fraction of the same immunoprecipitates described in (A) using anti–eIF-2α antibody. HC, IgG heavy chain. (C) Rate of protein synthesis in PKR-expressing MEFs carrying vector alone (VEC), wild-type eIF-2α (eIF), and a nonphosphorylatable mutant eIF-NP. Cells were pretreated with 10 ng/mL of murine recombinant IFN-γ, transfected with 100 μg/mL of poly(I).poly(C), and labeled in a methionine-cysteine-free medium containing 50 μCi/mL of [35S]methionine-cysteine labeling mix for 60 minutes. Protein synthesis was measured by the incorporation of [35S]methionine and [35S]cysteine into trichloroacetic acid-precipitable proteins. The control cells did not carry any expression vectors. ▧, FANCC−/−; ■, FANCC+/+. Data represent means ± standard deviations of 2 independent experiments.
We wished to confirm that the excessive phosphorylation of eIF-2α had a functional consequence in FANCC−/− cells. Because phosphorylation of eIF-2α results in translational inhibition,31 we reasoned that global translational activity should be reduced to a greater extent in FANCC−/− cells than in normal cells exposed to IFN-γ and dsRNA. As can be seen in Figure 6C, treatment with dsRNA plus IFN-γ reduced protein synthesis by approximately 60% in FANCC−/− cells compared to approximately 25% in normal MEFs (columns Control and VEC). The percentages of [35S] methionine and [35S] cysteine incorporation in eIF-2α–expressing cells were similar to those in MEFs carrying vector only (column eIF), consistent with the eIF-2α phosphorylation results described above (Figure 6A). Expression of eIF-NP restored protein synthesis to 44% and 50% in PKR/eIF-NP coexpressed FANCC−/− cells treated with and without dsRNA plus IFN-γ, respectively (Figure 6C, column eIF-NP). Normal MEFs coexpressing PKR and eIF-NP also partially rescued the inhibition of protein synthesis.
IRF-1 activation is similar in normal and FANCC−/− cells
PKR has been implicated in regulating the activity of transcription factor IRF-1.9 24 We sought to determine whether constitutive activation of PKR in FANCC−/− cells resulted in up-regulation of IRF-1 activity. EMSA using an IRF consensus oligonucleotide probe showed that treatment of MEFs with IFN-γ and dsRNA greatly increased DNA-binding activity of IRF-1; however, no difference was observed in IRF-1 activity between FANCC−/− and normal cells or between wild-type PKR- and PKRΔ6-expressing FANCC−/− cells (data not shown).
Discussion
Hematopoietic progenitor cells from patients with FA and FANCC knockout mice are hypersensitive to IFN-γ and TNF-α,1-5 agents known to induce apoptotic responses in hematopoietic cells32,33 and may, therefore, play an important role in the pathogenesis of bone marrow failure in patients with FA.1 MEFs from FANCC−/− mice also demonstrate this hypersensitivity, although fibroblasts from fully developed mice do not. The mechanism underlying this hypersensitivity is not clear. We originally hypothesized that constitutive STAT1 activation might account for this phenotype but discovered that this was not the case. Surprisingly, STAT1 activation was suppressed by certain mutations of the FANCC gene, the product of which is normally required for optimal STAT1 activation.8 With the reasoning that the hypersensitive phenotype might reflect constitutive activation of other IFN-responsive factors not directly linked to STAT1 activation, we tested the notion that PKR plays such a role. PKR, an IFN-induced protein, activated by dsRNA or by single-stranded RNAs that possess substantial secondary structure, plays an important role in the antiviral effects of the interferons and in control of cell survival.11 Other features of PKR function, especially its inducibility by TNF-α and certain chemicals,10,24 34factors that are also excessively toxic to FA cells, made PKR a particularly attractive molecule to evaluate functionally in FA cells.
We report here that cultured embryonic fibroblasts derived from FANCC−/− mice5 are also hypersensitive to dsRNA and that PKR is a key mediator of the high level of apoptosis induced by IFN-γ and dsRNA in FANCC−/− cells. We argue that these results reflect, in large part, the distinctly abnormal high-level constitutive activation of PKR in FANCC−/−cells (Figure 2B). Furthermore, treatments with IFN-γ and dsRNA induced a significantly higher level of activated PKR in FANCC−/− cells than in normal MEFs. With the use of a dominant negative mutant of human PKR (PKR6), we showed that excessive apoptosis in FANCC−/− MEFs treated with IFN-γ and dsRNA requires PKR activation. Our results also confirm recent studies demonstrating that PKR is a general transducer of the apoptotic response.10,13,26,35 36 In addition, our studies showed that priming with IFN-γ greatly sensitized FANCC−/− MEFs to apoptosis following treatment with dsRNA (Figure 1).
It is known that IFN primes or enhances dsRNA-mediated cytotoxicity in NIH3T3 cells,13 and that IFN-γ treatment of MEFs leads to a posttranslational modification of PKR (by phosphorylation).24 37 One possibility for the hypersensitive response in FA cells is that normal FANCC modulates induced expression of PKR, and, therefore, IFN-γ more effectively induces expression of PKR in FA cells. In this circumstance, PKR would be more substantially activated by treatment with dsRNA because there is simply more substrate. However, our results indicate that this is not the case. PKR expression was equally induced in both normal and FANCC−/− MEFs in response to IFN-γ stimulation (Figure2A), indicating that FANCC does not govern expression of PKR and that the constitutive activation (Figure 2B) and high affinity (Figure 2C) of PKR, rather than its overproduction, is unique to the FANCC−/− cells. We argue that this represents a pathogenically important phenotype linked to the FANCC mutation.
The capacity of PKR to influence cell survival involves inactivation of at least 2 factors critical to the survival of normal cells, eIF-2α and IκB-α.13-15 38 To identify which of these PKR substrates is necessary to effect hypersensitivity of FA-C cells to IFN-γ and dsRNA, we coexpressed PKR and nonphosphorylatable forms of eIF-2α (eIF-NP) or IκB-α (IκB-M). We found that excess apoptosis in FANCC−/− cells correlated only with increased phosphorylation of eIF-2α (Figures 4,6). Collectively, our results indicate that hypersensitivity of FANCC−/− cells to IFN-γ involves translational inhibition through aberrant activation of the dsRNA-dependent protein kinase PKR. It is important to note that, whereas overexpression of the dominant negative mutant IκB-M failed to block PKR-mediated apoptosis in FANCC−/− MEFs, probably as a result of its inability to inhibit NF-κB activation (Figure 5), we cannot exclude an additional role of high-level activated NF-κB on PKR-induced apoptosis in these mutant cells. Further experiments are required to determine the functionality of the overexpressing IκB-M protein in FANCC−/− cells, namely, its capacity to compete with endogenous IκB-α as the substrate for PKR.
That the dominant negative eIF-NP construct was less capable of completely abrogating induced apoptosis in FANCC−/− cells than was the dominant negative mutant of PKR, suggests that additional PKR substrates may be involved. One such candidate is the transcription factor IRF-1. IRF-1, a known effector of programmed cell death in hematopoietic cells,39 is constitutively expressed in FA cells, including MEFs, from FANCC knockout mice40 and in bone marrow cells from children with inactivating mutations of FANCC.1 FANCC−/− cells are known to be hypersensitive to IFN-γ and TNF-α1-5, and expression of both PKR and IRF-1 is induced by both IFN-γ and TNF-α.24,36 In fact, there is evidence that IRF-1 cannot function unless a phosphorylation signal is provided, potentially by PKR.12 Studies by Der et al10 have also shown that PKR is required for DNA-binding activity of IRF-1 in MEFs treated with stress-related agents, including TNF-α and dsRNA. Therefore, constitutive activation of PKR combined with known constitutive expression of IRF-1 in FANCC−/− cells would be expected to have serious apoptotic consequences. We found no differences in constitutive or induced nuclear IRF-1 DNA-binding activity between FANCC−/− and normal cells in EMSAs.
The mechanism by which FANCC protein suppresses PKR activation in normal cells is not clear. We did not find that the FANCC protein associates with PKR in normal cells. However, FANCC exists in a complex with at least 2 other FA proteins,41 and the same is true of PKR that is known to complex with a variety of other proteins, including itself.42,43 Consequently, further studies are warranted because one of the other FANCC-binding proteins might associate with one of the PKR-associated proteins, thus suppressing PKR activation. It is clear that phosphorylation of eIF-2α is involved in the FANCC phenotype, but it is also apparent that other priming mechanisms may be involved. Recent studies implicating certain MAP kinase pathways in PKR responses44 suggest that p38 MAPK and JNK might also be involved in some cell types. PKR-induced apoptosis occurs at least in part through up-regulation of fas expression,10,26 a pathway we and others have shown to be involved in IFN-γ and TNF-α responses of FANCC−/−hematopoietic progenitor cells.1 4 However, we did not observe significant changes in the levels of fas mRNA in both PKR-expressing normal and FANCC−/− MEFs treated with or without IFN-γ and dsRNA.
Although the signaling pathways responsible for constitutive activation of PKR and PKR-dependent apoptosis in FANCC−/− cells are not yet clear, our study does provide unambiguous evidence of a pivotal role of PKR in the characteristic hypersensitivity of FANCC−/− cells to IFN. We speculate that appropriately targeted inhibitors of PKR activity might prevent bone marrow failure in children with this disease.
We thank Drs B. Magun and G. N. Barber for providing the human PKR clones, Dr A. D. Miller for the retroviral vector pLXSN, Dr J. W. B. Hershey for the eIF-2α clones, Dr R. T. Hay for the IκB-α clones, Dr J. C. Bell for anti-mPKR, Dr B. Batta for anti–eIF-2α antibodies, and Tara Koretsky for valuable technical assistance. We also thank Dr Markus Grompe for the FANCC mutant cell lines, knockout mice, and for helpful discussions.
Supported by grant HL48546 from the National Institutes of Health and a Department of Veterans Affairs Merit Review Grant to G.C.B.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Grover C. Bagby, Oregon Cancer Center, Department of Medicine (Division of Hematology and Medical Oncology) and Department of Molecular and Medical Genetics, Oregon Health Sciences University, Portland, OR 97201; e-mail: grover@ohsu.edu.

![Fig. 2. PKR is constitutively activated in FANCC−/− cells. / (A) Expression of PKR in MEFs in response to IFN-γ and dsRNA. Whole cell lysates were prepared 48 hours after incubation with IFN-γ (10 ng/mL) and 24 hours after transfection with 100 μg/mL of poly(I).poly(C), separated on a 7.5% SDS-PAGE, and immunoblotted with anti-mPKR antibody. (B) In vivo phosphorylation of PKR in MEFs treated with IFN-γ and dsRNA. FANCC+/− and FANCC−/− MEFs were labeled, 24 hours after incubation with IFN-γ (10 ng/mL) and 6 hours after transfection with 100 μg/mL of poly(I).poly(C), with [32P]orthophosphate (150 μCi/mL) for an additional 3 hours. Cells were lysed, and equal amounts of whole cell lysates (500 μg of total proteins each sample) were immunoprecipitated with antibody specific for mPKR. [32P]phosphate-incorporated PKR immunocomplexes were analyzed by SDS-PAGE, followed by autoradiography (top panel). To ensure additionally that equivalent amounts of the immunocomplexes were measured, immunoblot analysis of equal fractions of the PKR immunoprecipitates was performed by using the mPKR monoclonal antibody (bottom panel). P-mPKR, phosphorylated mouse PKR; HC, IgG heavy chain. (C) dsRNA-agarose affinity chromatography showing significantly more PKR from FANCC−/− cell lysates binding to dsRNA. Equal amounts of whole cell lysates (500 μg of total proteins each sample) were applied to a poly(I).poly(C)-agarose column. The bound proteins were eluted and analyzed by SDS-PAGE and immunoblot, using anti-mPKR antibody.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/6/10.1182_blood.v97.6.1644/6/m_h80610772002.jpeg?Expires=1765883297&Signature=bhuw2fIMncvqv9P-JdGIG3ZrZGlLmQH72w7e2CGnFA4tEDULd5T3K1eV8Jhlt63gw4P3Y-jOn6gM~YhPdsLv6q34OvG-jhuwtw9niZ8ntSEih6JEt4sgfU3Q0G2-C9Ff-Un30WHoEr51QmuDML7x8~dPwsBUghS1YI9QCIWtv6ZF4yXuQGbK6ZcO~j3exjH3ZajkkgLLBO5Z0mRgrn3ZrYeQjk8HVMRKy2bcZphH-wd8rYLMACvDZ2kvYYlsbFINeWfvPCHmsaVrstr7OHDlNncGj-a0PqE5UrSxMC~80UQAqVaH3g6r0L21k4iagzfMZpLoFb-gH8TYZKzvwOtoow__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 6. Expression of the nonphosphorylatable mutant eIF-NP rescued PKR-mediated inhibition of protein synthesis in FANCC−/− cells. / (A) Analysis of phosphorylated eIF-2α levels in PKR-expressing MEFs carrying vector alone (VEC), wild-type eIF-2α (eIF), and a nonphosphorylatable mutant eIF-NP; cells were pretreated with 10 ng/mL of murine recombinant IFN-γ, transfected with 100 μg/mL of poly(I).poly(C), and labeled in a methionine-cysteine-free medium containing 50 μCi/mL of [35S]methionine-cysteine labeling mix for 60 minutes. Whole cell lysates were prepared, and 800 μg of total proteins from each sample were subjected to immunoprecipitation with anti–eIF-2α antibody. The immunocomplexes were analyzed by SDS-PAGE followed by autoradiography. NS, nonspecific. (B) Immunoblot analysis of a fraction of the same immunoprecipitates described in (A) using anti–eIF-2α antibody. HC, IgG heavy chain. (C) Rate of protein synthesis in PKR-expressing MEFs carrying vector alone (VEC), wild-type eIF-2α (eIF), and a nonphosphorylatable mutant eIF-NP. Cells were pretreated with 10 ng/mL of murine recombinant IFN-γ, transfected with 100 μg/mL of poly(I).poly(C), and labeled in a methionine-cysteine-free medium containing 50 μCi/mL of [35S]methionine-cysteine labeling mix for 60 minutes. Protein synthesis was measured by the incorporation of [35S]methionine and [35S]cysteine into trichloroacetic acid-precipitable proteins. The control cells did not carry any expression vectors. ▧, FANCC−/−; ■, FANCC+/+. Data represent means ± standard deviations of 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/6/10.1182_blood.v97.6.1644/6/m_h80610772006.jpeg?Expires=1765883297&Signature=3lzkimhoIPjvmxXdTDy3lIMiXDIhILHU8MQrt5ugudSp7Uh3kM9fsEjaAc-ZPaj8ZYexs2HrfKMOxgthWd8yuCiLY49WGd0Qe0xt-ji2Mv36LFzOSztrHyhgIUBoD61B9hYLWAhMDvk9emNU0qhV5wdYbVtnDCmycPysNgQzVVqoJU2jMk7aDHSQZfQ8G62AQthaE0a8w2m07zdMQBhshDsWhZRZTmgZSHoa1cqi6tp2Vq18BX13gERdLy5YXR38aiCTNXdkrpRjh0zdiigOXs3mKTNJPNRPI5UO4Tm22aisRfFBXmruOb3wYim95TuFB0DpNKfV2bbuZAVCkNvOkw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal