Abstract
The BCR/ABL tyrosine kinase has been implicated in the pathogenesis of chronic myelogenous leukemia (CML) and Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL). STI571 is a novel anticancer agent that selectively inhibits the BCR/ABL tyrosine kinase. The cytotoxic effects of STI571 were studied in combination with antileukemic agents against Ph+leukemia cell lines, KU812, K-562, TCC-S, and TCC-Y. The cells were exposed to STI571 and to other agents simultaneously for 5 or 7 days. Cell growth inhibition was determined by MTT assay. The cytotoxic effects in combinations at the inhibitory concentration of 80% level were evaluated by the isobologram. STI571 produced synergistic effects with recombinant and natural α-interferons in 2 of 3 and 3 of 3 cell lines, respectively. STI571 produced additive effects with hydroxyurea, cytarabine, homoharringtonine, doxorubicin, and etoposide in all 4 cell lines. STI571 with 4-hydroperoxy-cyclophosphamide, methotrexate, or vincristine produced additive, antagonistic, and synergistic effects in 3 of 4 cell lines, respectively. These findings suggest that the simultaneous administration of STI571 with other agents except methotrexate would be advantageous for cytotoxic effects against Ph+ leukemias. Among them, the simultaneous administration of STI571 and α-interferons or vincristine would be highly effective against Ph+ leukemias and these combinations would be worthy of clinical trials. In contrast, the simultaneous administration of STI571 with methotrexate would have little therapeutic efficacy. Although there are gaps between in vitro studies and clinical trials, the present findings provide useful information for the establishment of clinical protocols involving STI571.
Introduction
About 90% of chronic myelogenous leukemia (CML), 20% to 30% of adult acute lymphoblastic leukemia (ALL), and 1% to 2% of acute myeloblastic leukemia (AML) show a reciprocal translocation between chromosome 9 and 22, so-called Philadelphia (Ph) chromosome.1,2 This translocation results in the head-to-tail fusion of the breakpoint cluster region (BCR) gene on chromosome 22 at band q11 with the ABLproto-oncogene located on chromosome 9 at band q34.3 In Ph+ CML and ALL cases, the BCR/ABL chimeric transcripts are expressed; in general the P210 BCR/ABLtranscript is expressed in almost all Ph+ CML cases and in half of Ph+ ALL cases, and the P190 BCR/ABLtranscript is observed in the remaining of Ph+ ALL cases, although only small amounts of the P190 BCR/ABLtranscript have been reported to be expressed in a majority of Ph+ CML.4 The BCR/ABL protein has an antiapoptotic activity and is believed to play a central role in the development of CML and Ph+ ALL.5-7 The leukemia-promoting function of this protein requires its deregulated tyrosine kinase activity, but the precise mechanism of this enzyme to transform cells is still obscure.5-7
The prognosis of CML and Ph+ ALL is poor.8,9CML in the chronic phase can be controlled by anticancer agents such as interferon-α (IFN-α), hydroxyurea, cytarabine, homoharringtonine, and busulfan, but survival is extremely short after the onset of blastic crisis, which usually occurs within a few years after the onset of CML.2,8 Leukemia cells in this phase are extremely resistant to antileukemic agents. Adult Ph+ ALL is also chemoresistant and fewer than 5% of patients may be cured by chemotherapy.9 Allogeneic bone marrow transplantation offers the best chance to cure CML and Ph+ ALL. If transplantation is carried out during the early stage of CML or during the first remission of Ph+ ALL, more than 50% of patients will be cured.8-10 However, transplantation is available for less than 30% of patients.8-10 Clearly, there is an urgent requirement for new anticancer agents and combinations that could improve responses and survival rates for CML and Ph+ ALL.
With increased understanding of the genetic changes that cause malignant transformation, new drugs have been developed that might specifically target the cancer. The BCR/ABL tyrosine kinase could be such a target for the pharmacologic treatment of CML and Ph+ ALL, for example, through the use of BCR/ABL tyrosine kinase inhibitors. Herbimycin A, which effectively reduces intracellular phosphorylation by BCR/ABL tyrosine kinase, preferentially inhibits the growth of Ph+ leukemia cell lines both in vitro and in vivo,11-13 suggesting that herbimycin A and related compounds may be useful for the treatment of Ph+ leukemias. However, the selectivity of herbimycin A for BCR/ABL tyrosine kinase is obscure and clinical application has not yet been performed.
To improve the target specificity of BCR/ABL, many compounds have been synthesized. STI571 is one of 2-phenylaminopyrimidine derivatives, which selectively inhibits the tyrosine kinase activity of ABL and BCR/ABL.14 There was no significant inhibition of the other numerous protein kinases tested with the exception of the platelet-derived growth factor receptor14 and the c-Kit tyrosine kinases. STI571 preferentially inhibited the growth of Ph+ leukemia cell lines in vitro.14-16Injection of Ph+ KU812 and Ph− U937 leukemia cells into mice resulted in the death of all mice due to leukemia. Daily administration of STI571 significantly enhanced the survival of mice inoculated with the Ph+ KU812 cells but barely affected the survival of mice inoculated with Ph− U937 cells.17 A clinical phase I study of STI571showed a mild toxicity but significant activity against patients with CML.18 These preclinical and clinical findings suggest that STI571 may promise a new paradigm for the treatment of Ph+ leukemias. However, both preclinical and clinical studies suggest that the induction of the apoptosis of Ph+leukemia cells by STI571 may be incomplete, and therefore the combination of STI571 with other antileukemic agents would be important.
The agents used for the combination are selected on the basis of single-agent activity, least overlapping toxicity, low cross-resistance, and additive or synergistic interactions. However, experimental findings of STI571 in combination with antileukemic agents are few and controversial.19-22 In the present study, we investigated the in vitro effects of STI571 in combination with commonly used antileukemic agents against 4 Ph+ leukemia cell lines. The dose-response curves for the combinations were obtained using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay23 and the data at the inhibitory concentration of 80% (IC80) level were analyzed by the isobologram method (Steel and Peckham).24 The present findings underline the importance of the design of the combination of STI571 with antileukemic agents for CML and Ph+ ALL.
Materials and methods
Celllines
Experiments were conducted using 4 human Ph+leukemia cell lines: KU812, K-562, and TCC-S from patients with CML myeloblastic crisis, and TCC-Y from a patient with pre-B-cell ALL. KU812 was provided by K. Kishi (Niigata University, Niigata, Japan). K562 was obtained from the Health Science Research Resources Bank (Osaka, Japan). TCC-S and TCC-Y were established at the Tochigi Cancer Center. The doubling times of KU812, K562, TCC-S, and TCC-Y cells under our experimental conditions were 25 ± 2 hours, 23 ± 2 hours, 25 ± 2 hours, and 36 ± 3 hours, respectively. Cells were maintained in 75-cm2 plastic tissue culture flasks containing RPMI1640 medium (Sigma Chemical, St Louis, MO) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Grand Island Biological, Grand Island, NY) and antibiotics in a humidified atmosphere of 95% air/5% CO2 at 37°C.
All 4 Ph+ leukemia cell lines expressed both the P210BCR/ABL transcript and the P190 BCR/ABLtranscript by reverse transcriptase-polymerase chain reaction (RT-PCR) using first and nested primer sets.25 The primer sets for major BCR/ABL transcript detection were: BCR-1S (BCR ex. 13) 5′-ATCCAAGGCTACGGAGAGGC-3′, ABL-1AS (ABL ex.2) 5′-ATGGTACCAGGAGTGTTTCTCC-3′ for the first PCR, and BCR-2S (BCR ex.13) 5′-GGAGCTGCAGATGCTGACCAAC-3′ and ABL-2AS for the nested PCR. The primer sets for minorBCR/ABL transcripts were: BCR-1mS (BCR ex.1) 5′-CAACAGTCCTTCGACAGC-3′ and ABL-1AS for the first PCR, and BCR-2mS (BCR ex.1) 5′-CAGTGCCATAAGCGCACC-3′ and ABL-2AS for the nested PCR. The thermal cycling profile was 95°C for 12 minutes, followed by 35 cycles of 95°C for 30 seconds, 62°C for 45 seconds, and final extension at 72°C for 12 minutes. The nested PCR was performed for 40 cycles with the same parameters.
Drugs
STI571 was kindly provided by Novartis (Basel, Switzerland). Other antileukemic agents used and their sources were: recombinant IFN-α-2b (Nihon Schering, Osaka, Japan), natural IFN-α (Sumitomo, Osaka, Japan), hydroxyurea and homoharringtonine (Sigma Chemical), cytarabine (Nihon Shinyaku, Tokyo, Japan), doxorubicin (Meiji, Tokyo, Japan), etoposide (Nihon Kayaku, Tokyo, Japan), 4-hydroperoxy-cyclophosphamide (the active form of cyclophosphamide) and vincristine (Shionogi, Tokyo, Japan), and methotrexate (Lederle Japan, Tokyo, Japan). All drugs except STI571 were dissolved in RPMI 1640. STI571 was dissolved in dimethyl sulfoxide. The stock solutions of all drugs were prepared at 1 mM (1 000 000 IU/mL for IFN-α) and stored at −80°C. Appropriate drug concentrations were made by dilution with fresh medium immediately before each experiment. The final concentration of dimethyl sulfoxide in the media was less than 0.1%, and it had no effect on the cell growth inhibition in the present study.
Inhibition of cell growth by STI571 alone and by the combination of STI571 and the other agents
The Ph+ leukemia cells were harvested from the medium and resuspended to a final concentration of 6 × 104 cells/mL of fresh medium containing 10% fetal calf serum (FCS) for TCC-S and KU812, 3 × 104 cells/mL for K562, and 2 × 105 cells/mL for TCC-Y cells. Cell suspensions (100 μL) were dispensed into individual wells of a 96-well tissue culture plate with a lid (Falcon, Oxnard, CA). Eight plates were prepared for the testing of each drug combination. Each plate had one 8-well control column containing medium alone and one 8-well control column containing cells but no drugs. Drug solutions of STI571 and other drugs at different concentrations were then added (50 μL) to 8 wells containing cell suspensions. Because daily administration of STI571 has been used in clinical settings, KU812, K562, and TCC-S cells were simultaneously and continuously exposed to the drugs for 5 days. TCC-Y cells, which were very small and had a longer doubling time than other cell lines, were incubated with the drugs for 7 days.
MTTassay
Viable cell growth was determined by MTT reduction assay as described previously.23 For the background control, control (no drug), each drug or drug combination, the 4 intermediate data values among the 8 data values were used for the analysis and the 2 highest and the 2 lowest values were discarded. Hydroxyurea influenced the absorbance at 570 nm and erroneously high MTT values were observed. Thus, each concentration of hydroxyurea was also added to the respective background control for the study of STI571-hydroxyurea combinations. For all cell lines examined, we established a linear relation between the MTT assay value and the cell number within the range of the experiments shown.
Analysis of the evaluation of the cytotoxic effects of STI571 with the other agents
The cytotoxic interactions of STI571 with other agents at the point of IC80 were evaluated by isobologram (Steel and Peckham).24 The IC80 was defined as the concentration of drug that produced 80% cell growth inhibition, that is, 80% reduction of absorbance. We used the IC80 value instead of the more common IC50 value, because IC80 would be more important than IC50 for the evaluation as anticancer agents. Although the drug interaction at IC90 or more would be more important than IC50or IC80, it is difficult to obtain reliable data at the IC90 or greater using MTT assay.
The isobologram of Steel and Peckham
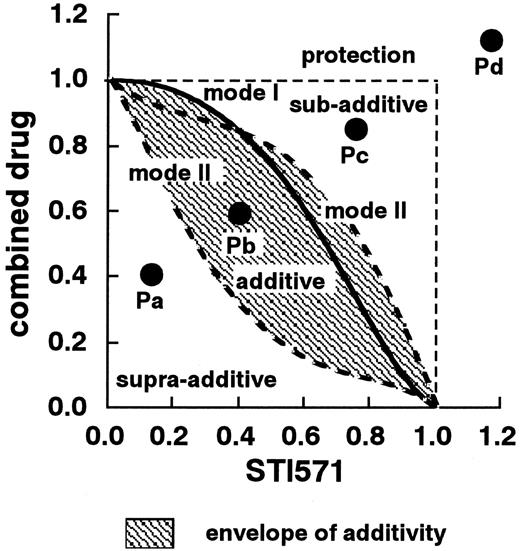
The theoretical basis of the isobologram method and the procedure for making isobolograms have previously been described in detail.24-26 Based on the dose-response curves of STI571 and the other agents, 3 isoeffect curves were constructed (Figure1). If the agents act additively by independent mechanisms, combined data points will lie near the mode I line (hetero-addition). If the agents act additively by similar mechanisms, combined data points will lie near the mode II lines (iso-addition).
Schematic representation of an isobologram (Steel and Peckham).
The concentrations that produced 80% cell growth inhibition were expressed as 1.0 on the ordinate and the abscissa of the isobolograms. Envelope of additivity, surrounded by mode I (solid line) and mode II (broken lines) isobologram lines, was constructed from the dose-response curves of STI571 and the combined drug. When the data points of the drug combination fell within the area surrounded by envelope of additivity (Pb), the combination was regarded as additive. When the data points fell to the left of the envelope (Pa), the drug combination was regarded as having a supra-additive effect (synergism). When the points fell to the right of the envelope but within the square or on the square line (Pc), the combination was regarded as having a subadditive effect. When the data points were outside the square (Pd), the combination was regarded as having a protective effect. Both subadditive and protective interactions were regarded as antagonistic effects.
Schematic representation of an isobologram (Steel and Peckham).
The concentrations that produced 80% cell growth inhibition were expressed as 1.0 on the ordinate and the abscissa of the isobolograms. Envelope of additivity, surrounded by mode I (solid line) and mode II (broken lines) isobologram lines, was constructed from the dose-response curves of STI571 and the combined drug. When the data points of the drug combination fell within the area surrounded by envelope of additivity (Pb), the combination was regarded as additive. When the data points fell to the left of the envelope (Pa), the drug combination was regarded as having a supra-additive effect (synergism). When the points fell to the right of the envelope but within the square or on the square line (Pc), the combination was regarded as having a subadditive effect. When the data points were outside the square (Pd), the combination was regarded as having a protective effect. Both subadditive and protective interactions were regarded as antagonistic effects.
Recent studies suggested that many anticancer agents kill tumor cells by apoptosis. The initiating signals to apoptosis by anticancer agents are still poorly understood. In addition, numerous converging pathways integrate the initiating signals and transmit the results to mitochondria causing activation of caspase cascade. Because it is unknown in advance whether the combined effects of 2 agents will be hetero-additive, iso-additive, or an effect intermediate between these extremes, all possibilities should be considered. Thus, when the data points of the drug combination fell within the area surrounded by 3 lines (envelope of additivity), the combination was regarded as additive. The envelope of additivity should not be considered as a reliable definition of additivity. An expression of the uncertainty is an important concept of the isobologram method of Steel and Peckham.
When the data points fell to the left of the envelope, that is, the combined effect was caused by lower doses of the 2 agents than was predicted, the drug combination was regarded as having a supra-additive effect (synergism). When the points fell to the right of the envelope, that is, the combined effect was caused by higher doses of the 2 agents than was predicted, but within the square or on the line of the square, the combination was regarded as having a subadditive effect; that is, the combination was superior or equal to a single agent but was less than the additive effect. When the data points were outside the square, the combination was regarded as having a protective effect, that is, the combination was inferior in cytotoxic action to a single agent. Both subadditive and protective interactions were regarded as antagonism.
Dataanalysis
When the observed data points in combination mainly fell within the envelope of additivity, the combination was considered as having an additive effect. The mean values of the observed data were compared with those of the predicted maximum values and those of the predicted minimum values for an additive effect.27 If the mean values of the observed data were equal to or smaller than those of the predicted maximum values and equal to or larger than those of the predicted minimum values, the combination was regarded as an additive effect.
When the observed data points in the combinations mainly fell in the area of supra-additivity or in the areas of subadditivity and protection, that is, the mean value of the observed data were smaller than those of the predicted minimum values or larger than those of the predicted maximum values, the combinations were considered to have a synergistic or antagonistic effect, respectively. To determine whether the condition of synergism (or antagonism) truly existed, a statistical analysis was performed. The Wilcoxon signed-rank test was used to compare the observed data with the predicted minimum (or maximum) values for additive effects, which were closest to the observed data (ie, the data on the boundary [mode I or mode II lines] between the additive area and supra-additive area [or subadditive and protective areas]).26 Combinations with significant values (P < .05) were defined as having synergistic effects (or antagonistic effects), and those with insignificant values (P > .05) were defined as having additive to synergistic effects (or additive to antagonistic effects). All statistical analyses were performed using the StatView 4.01 software program (Abacus Concepts, Berkeley, CA).
Results
Cytotoxic effects of STI571 and other agents against Ph+ leukemia cells
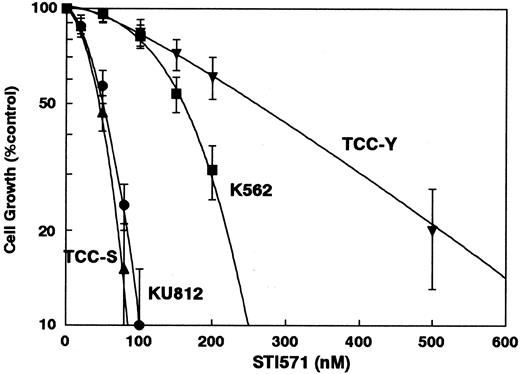
The dose-response curves of STI571 for KU812, K-562, TCC-S, and TCC-Y are shown in Figure 2. The IC80 values of STI571 for these cells were 84 ± 9 nM, 247 ± 30 nM, 72 ± 6 nM, and 510 ± 80 nM, respectively (Figure2). Nine human Ph− leukemia cell lines (HL-60, KG-1, U937, MOLT-3, SALT-3, Raji, HBL, JOK, SKW-3), and 4 solid tumor cell lines (A549, MCF-7, PA-1, WiDr) tested were resistant to STI571 and 10 μM STI571 had almost no effects on the growth inhibition of these cell lines (data not shown). Table 1summarizes the IC80 levels of Ph+ leukemia cell lines to the antileukemic agents used in the present study.
The dose-response curves of STI571 against KU812, K562, TCC-S, and TCC-Y cells.
Cell growth inhibition was measured using the MTT assay after 5 days for KU812, K562, and TCC-S cells or after 7 days for TCC-Y cells and was plotted as a percentage of the control (cells not exposed to drugs). Each point represents the mean ± SEM for at least 3 independent experiments.
The dose-response curves of STI571 against KU812, K562, TCC-S, and TCC-Y cells.
Cell growth inhibition was measured using the MTT assay after 5 days for KU812, K562, and TCC-S cells or after 7 days for TCC-Y cells and was plotted as a percentage of the control (cells not exposed to drugs). Each point represents the mean ± SEM for at least 3 independent experiments.
| Drug . | KU812 . | K562 . | TCC-S . | TCC-Y . |
|---|---|---|---|---|
| ST1571 (nM) | 84 ± 9 | 247 ± 30 | 72 ± 6 | 510 ± 80 |
| Recombinant IFN-α (IU/mL) | 4400 ± 500 | > 10 000 | 1500 ± 300‡ | 4500 ± 600 |
| Natural IFN-α (IU/mL) | 2400 ± 400 | > 10 000 | 1100 ± 200‡ | 2800 ± 450 |
| Hydroxyurea (mM) | 0.75 ± 0.04 | 1.1 ± 0.2 | 0.17 ± 0.02 | 0.52 ± 0.04 |
| Cytarabine (nM) | 8.9 ± 1.1 | 92 ± 17 | 410 ± 56 | 5.1 ± 0.6 |
| Homoharringtonine (nM) | 56 ± 3 | 92 ± 11 | 57 ± 6 | 80 ± 8 |
| Doxorubicin (nM) | 27 ± 3 | 70 ± 6 | 39 ± 6 | 82 ± 11 |
| Etoposide (μM) | 2.0 ± 0.2 | 4.2 ± 0.6 | 2.6 ± 0.4 | 2.1 ± 0.2 |
| Hydroperoxy-cyclophosphamide (μM) | 4.8 ± 0.6 | 8.1 ± 0.6 | 5.3 ± 0.7 | 3 ± 0.5 |
| Methotrexate (nM) | 14 ± 1 | 86 ± 7 | 52 ± 7 | 64 ± 9 |
| Vincristine (nM) | 0.88 ± 0.03 | 7.1 ± 0.9 | 0.75 ± 0.03 | 0.66 ± 0.02 |
| Drug . | KU812 . | K562 . | TCC-S . | TCC-Y . |
|---|---|---|---|---|
| ST1571 (nM) | 84 ± 9 | 247 ± 30 | 72 ± 6 | 510 ± 80 |
| Recombinant IFN-α (IU/mL) | 4400 ± 500 | > 10 000 | 1500 ± 300‡ | 4500 ± 600 |
| Natural IFN-α (IU/mL) | 2400 ± 400 | > 10 000 | 1100 ± 200‡ | 2800 ± 450 |
| Hydroxyurea (mM) | 0.75 ± 0.04 | 1.1 ± 0.2 | 0.17 ± 0.02 | 0.52 ± 0.04 |
| Cytarabine (nM) | 8.9 ± 1.1 | 92 ± 17 | 410 ± 56 | 5.1 ± 0.6 |
| Homoharringtonine (nM) | 56 ± 3 | 92 ± 11 | 57 ± 6 | 80 ± 8 |
| Doxorubicin (nM) | 27 ± 3 | 70 ± 6 | 39 ± 6 | 82 ± 11 |
| Etoposide (μM) | 2.0 ± 0.2 | 4.2 ± 0.6 | 2.6 ± 0.4 | 2.1 ± 0.2 |
| Hydroperoxy-cyclophosphamide (μM) | 4.8 ± 0.6 | 8.1 ± 0.6 | 5.3 ± 0.7 | 3 ± 0.5 |
| Methotrexate (nM) | 14 ± 1 | 86 ± 7 | 52 ± 7 | 64 ± 9 |
| Vincristine (nM) | 0.88 ± 0.03 | 7.1 ± 0.9 | 0.75 ± 0.03 | 0.66 ± 0.02 |
KU812, K562, and TCC-S were exposed to the drugs for 5 days and TCC-Y for 7 days.
Values represent the mean ± SEM of at least 3 independent experiments.
IC40.
Cytotoxic interaction between STI571 and other agents
Figure 3A-D shows the dose-response curves for STI571 in combination with recombinant IFN-α, hydroxyurea, methotrexate, or vincristine in the KU812 cells. Each isobologram was generated based on such dose-response curves.
Dose-response curves.
Dose-response curves for STI571 in combination with (A) recombinant IFN-α (r-α-IFN), (B) hydroxyurea (HU), (C) methotrexate (MTX), and (D) vincristine (VCR) in KU812 cells. Cell growth was measured using the MTT assay after 5 days and was plotted as a percentage of the control (cells not exposed to drugs). The concentrations of STI571 were 0 (○), 10 (●), 20 (▪), 30 (▴), 40 (▾), 50 (♦), 60 (▸), 80 (◂) and 100 (x) nM. The concentrations of the combined drugs are shown on the abscissa. Each point represents the mean value for at least 3 independent experiments; the SEMs were less than 20% and were omitted.
Dose-response curves.
Dose-response curves for STI571 in combination with (A) recombinant IFN-α (r-α-IFN), (B) hydroxyurea (HU), (C) methotrexate (MTX), and (D) vincristine (VCR) in KU812 cells. Cell growth was measured using the MTT assay after 5 days and was plotted as a percentage of the control (cells not exposed to drugs). The concentrations of STI571 were 0 (○), 10 (●), 20 (▪), 30 (▴), 40 (▾), 50 (♦), 60 (▸), 80 (◂) and 100 (x) nM. The concentrations of the combined drugs are shown on the abscissa. Each point represents the mean value for at least 3 independent experiments; the SEMs were less than 20% and were omitted.
Cytotoxic interaction between STI571 and IFNs-α
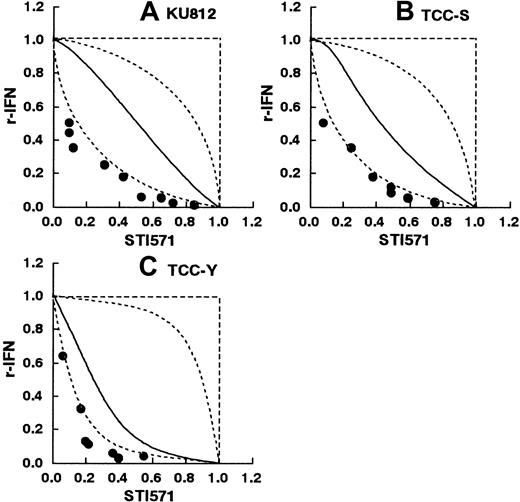
KU812 and TCC-Y cells were sensitive to IFN-α, whereas TCC-S cells were relatively resistant to IFN-α. Thus, the effects of the drug combination at IC40 were used for TCC-S cells. K-562 cells were highly resistant to IFN-α and could not be used for the study. Figure 4A-C shows the isobolograms of STI571 in combination with recombinant IFN-α against KU812, TCC-S, and TCC-Y cells. In the KU812 and TCC-Y cells, all or most of the data points for the combination fell to the left of the envelope of additivity. The mean values of the observed data (KU812: 0.27, and TCC-Y: 0.16) were smaller than those of the predicted minimum additive values (0.40 and 0.32, respectively; Table2). The observed data and the predicted minimum values were compared by Wilcoxon signed-rank test. The observed data in the KU812 and TCC-Y cells were significantly smaller than the predicted minimum values (P < .01 andP < .02, respectively), indicating a synergistic effect of the simultaneous exposure to these 2 agents (Table 2). The observed data (0.29) in the TCC-S cells were not significantly smaller than the predicted minimum values (0.34) (P > .05), indicating additive to synergistic effects of the simultaneous exposure to these 2 agents (Table 2). For the combination of STI571 with natural IFN-α against KU812, TCC-Y, and TCC-S cells, all or most of the data points for the combination fell to the left of the envelope of additivity (isobologram not shown). The observed data in the KU812, TCC-S, and TCC-Y cells were significantly smaller than the predicted minimum values, indicating a synergistic effect of the simultaneous exposure to these 2 agents (Table 2).
STI571 and recombinant IFN-α.
Isobolograms of simultaneous exposure to STI571 and recombinant IFN-α in (A) KU812, (B) TCC-S, and (C) TCC-Y cells. Data are presented as mean values of at least 3 independent experiments. The SEMs were less than 25% and were omitted. In all 3 cell lines, all or most data points of the combinations fell in the area of supra-additivity. The observed data were significantly smaller than the predicted minimum values by Wilcoxon signed-rank test in the KU812 and TCC-S cells (P < .01 and P < .05, respectively), suggesting synergistic effects. The observed data were not significantly smaller with the predicted minimum values in the TCC-Y cells (P > .05), suggesting additive to synergistic effects.
STI571 and recombinant IFN-α.
Isobolograms of simultaneous exposure to STI571 and recombinant IFN-α in (A) KU812, (B) TCC-S, and (C) TCC-Y cells. Data are presented as mean values of at least 3 independent experiments. The SEMs were less than 25% and were omitted. In all 3 cell lines, all or most data points of the combinations fell in the area of supra-additivity. The observed data were significantly smaller than the predicted minimum values by Wilcoxon signed-rank test in the KU812 and TCC-S cells (P < .01 and P < .05, respectively), suggesting synergistic effects. The observed data were not significantly smaller with the predicted minimum values in the TCC-Y cells (P > .05), suggesting additive to synergistic effects.
Mean values of observed data, predicted minimum, and predicted maximum values of ST1571 in combination with other anticancer agents
| Combined drug . | Cell line . | No. of data points . | Observed data* . | Predicted minimum† . | Predicted maximum‡ . | Effect . |
|---|---|---|---|---|---|---|
| Recombinant IFN-α | KU812 | 10 | 0.27 | 0.40 | 0.89 | Synergism (P < .01) |
| K562 | — | ND | ND | ND | ||
| TCC-S | 7 | 0.29 | 0.34 | 0.78 | Additive/synergism (N5)2-153 | |
| TCC-Y | 7 | 0.16 | 0.32 | 0.93 | Synergism (P < .02) | |
| Natural IFN-α | KU812 | 13 | 0.32 | 0.44 | 0.84 | Synergism (P < .01) |
| K562 | — | ND | ND | ND | ||
| TCC-S | 7 | 0.17 | 0.24 | 0.80 | Synergism (P < .05)2-153 | |
| TCC-Y | 6 | 0.18 | 0.31 | 0.92 | Synergism (P< .05) | |
| Hydroxyurea | KU812 | 11 | 0.61 | 0.42 | 0.73 | Additive |
| K562 | 11 | 0.64 | 0.47 | 0.82 | Additive | |
| TCC-S | 8 | 0.66 | 0.50 | 0.74 | Additive | |
| TCC-Y | 7 | 0.82 | 0.74 | 0.85 | Additive | |
| Cytarabine | KU812 | 10 | 0.56 | 0.45 | 0.71 | Additive |
| K562 | 9 | 0.45 | 0.37 | 0.87 | Additive | |
| TCC-S | 12 | 0.51 | 0.36 | 0.91 | Additive | |
| TCC-Y | 7 | 0.54 | 0.52 | 0.63 | Additive | |
| Homoharringtonine | KU812 | 10 | 0.58 | 0.52 | 0.71 | Additive |
| K562 | 12 | 0.68 | 0.48 | 0.74 | Additive | |
| TCC-S | 11 | 0.78 | 0.49 | 0.89 | Additive | |
| TCC-Y | 6 | 0.76 | 0.70 | 0.84 | Additive | |
| Doxorubicin | KU812 | 8 | 0.53 | 0.42 | 0.82 | Additive |
| K562 | 12 | 0.55 | 0.38 | 0.94 | Additive | |
| TCC-S | 11 | 0.51 | 0.34 | 0.77 | Additive | |
| TCC-Y | 7 | 0.51 | 0.49 | 0.60 | Additive | |
| Etoposide | KU812 | 9 | 0.62 | 0.48 | 0.75 | Additive |
| K562 | 9 | 0.67 | 0.33 | 0.86 | Additive | |
| TCC-S | 11 | 0.77 | 0.61 | 0.80 | Additive | |
| TCC-Y | 8 | 0.47 | 0.45 | 0.66 | Additive | |
| 4-Hydroperoxy cyclophosphonamide | KU812 | 10 | 0.53 | 0.44 | 0.70 | Additive |
| K562 | 9 | 0.51 | 0.52 | 0.81 | Additive/synergism (NS) | |
| TCC-S | 10 | 0.67 | 0.55 | 0.80 | Additive | |
| TCC-Y | 5 | 0.50 | 0.46 | 0.68 | Additive | |
| Methotrexate | KU812 | 12 | > 1.10 | 0.31 | 0.52 | Antagonism (P < .01) |
| K562 | 12 | 0.83 | 0.33 | 0.82 | Additive/antagonism (P = NS) | |
| TCC-S | 12 | 0.84 | 0.34 | 0.75 | Antagonism (P< .02) | |
| TCC-Y | 7 | > 1.06 | 0.46 | 0.65 | Antagonism (P < .02) | |
| Vincristine | KU812 | 10 | 0.53 | 0.59 | 0.76 | Synergism (P < .02) |
| K562 | 10 | 0.45 | 0.46 | 0.89 | Additive/synergism (P = NS) | |
| TCC-S | 10 | 0.43 | 0.64 | 0.80 | Synergism (P< .05) | |
| TCC-Y | 6 | 0.42 | 0.49 | 0.67 | Synergism (P < .05) |
| Combined drug . | Cell line . | No. of data points . | Observed data* . | Predicted minimum† . | Predicted maximum‡ . | Effect . |
|---|---|---|---|---|---|---|
| Recombinant IFN-α | KU812 | 10 | 0.27 | 0.40 | 0.89 | Synergism (P < .01) |
| K562 | — | ND | ND | ND | ||
| TCC-S | 7 | 0.29 | 0.34 | 0.78 | Additive/synergism (N5)2-153 | |
| TCC-Y | 7 | 0.16 | 0.32 | 0.93 | Synergism (P < .02) | |
| Natural IFN-α | KU812 | 13 | 0.32 | 0.44 | 0.84 | Synergism (P < .01) |
| K562 | — | ND | ND | ND | ||
| TCC-S | 7 | 0.17 | 0.24 | 0.80 | Synergism (P < .05)2-153 | |
| TCC-Y | 6 | 0.18 | 0.31 | 0.92 | Synergism (P< .05) | |
| Hydroxyurea | KU812 | 11 | 0.61 | 0.42 | 0.73 | Additive |
| K562 | 11 | 0.64 | 0.47 | 0.82 | Additive | |
| TCC-S | 8 | 0.66 | 0.50 | 0.74 | Additive | |
| TCC-Y | 7 | 0.82 | 0.74 | 0.85 | Additive | |
| Cytarabine | KU812 | 10 | 0.56 | 0.45 | 0.71 | Additive |
| K562 | 9 | 0.45 | 0.37 | 0.87 | Additive | |
| TCC-S | 12 | 0.51 | 0.36 | 0.91 | Additive | |
| TCC-Y | 7 | 0.54 | 0.52 | 0.63 | Additive | |
| Homoharringtonine | KU812 | 10 | 0.58 | 0.52 | 0.71 | Additive |
| K562 | 12 | 0.68 | 0.48 | 0.74 | Additive | |
| TCC-S | 11 | 0.78 | 0.49 | 0.89 | Additive | |
| TCC-Y | 6 | 0.76 | 0.70 | 0.84 | Additive | |
| Doxorubicin | KU812 | 8 | 0.53 | 0.42 | 0.82 | Additive |
| K562 | 12 | 0.55 | 0.38 | 0.94 | Additive | |
| TCC-S | 11 | 0.51 | 0.34 | 0.77 | Additive | |
| TCC-Y | 7 | 0.51 | 0.49 | 0.60 | Additive | |
| Etoposide | KU812 | 9 | 0.62 | 0.48 | 0.75 | Additive |
| K562 | 9 | 0.67 | 0.33 | 0.86 | Additive | |
| TCC-S | 11 | 0.77 | 0.61 | 0.80 | Additive | |
| TCC-Y | 8 | 0.47 | 0.45 | 0.66 | Additive | |
| 4-Hydroperoxy cyclophosphonamide | KU812 | 10 | 0.53 | 0.44 | 0.70 | Additive |
| K562 | 9 | 0.51 | 0.52 | 0.81 | Additive/synergism (NS) | |
| TCC-S | 10 | 0.67 | 0.55 | 0.80 | Additive | |
| TCC-Y | 5 | 0.50 | 0.46 | 0.68 | Additive | |
| Methotrexate | KU812 | 12 | > 1.10 | 0.31 | 0.52 | Antagonism (P < .01) |
| K562 | 12 | 0.83 | 0.33 | 0.82 | Additive/antagonism (P = NS) | |
| TCC-S | 12 | 0.84 | 0.34 | 0.75 | Antagonism (P< .02) | |
| TCC-Y | 7 | > 1.06 | 0.46 | 0.65 | Antagonism (P < .02) | |
| Vincristine | KU812 | 10 | 0.53 | 0.59 | 0.76 | Synergism (P < .02) |
| K562 | 10 | 0.45 | 0.46 | 0.89 | Additive/synergism (P = NS) | |
| TCC-S | 10 | 0.43 | 0.64 | 0.80 | Synergism (P< .05) | |
| TCC-Y | 6 | 0.42 | 0.49 | 0.67 | Synergism (P < .05) |
Mean value of observed data.
Mean value of the predicted minimum values for an additive effect.
Mean value of predicted maximum values for an additive effect.
IC40 level.
Cytotoxic interaction between STI571 and hydroxyurea
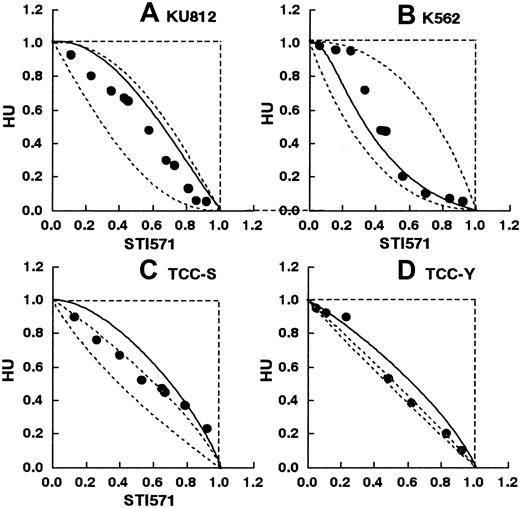
Figure 5A-D shows the isobolograms of this combination in the KU812, K-562, TCC-S, and TCC-Y cells. In the KU812 cells, the combined data points fell within the envelope of additivity (Figure 5A). The mean value of the data (0.61) was larger than that of the predicted minimum values (0.42) and smaller than that of the predicted maximum values for an additive effect (0.73), indicating that the simultaneous exposure to STI571 and hydroxyurea produced an additive effect (Table 2). Similarly, in the K562, TCC-S, and TCC-Y cells, all or most of the data points for the combination fell within the envelope of additivity (Figure 5B-D). The mean values of the observed data were between those of the predicted minimum values and those of the predicted maximum values for an additive effect, indicating an additive effect of the simultaneous exposure to these 2 agents in all 4 cell lines (Table 2).
STI571 and hydroxyurea.
Isobolograms of simultaneous exposure to STI571 and hydroxyurea (HU) in (A) KU812, (B) K-562, (C) TCC-S, and (D) TCC-Y cells. Data are presented as mean values of at least 3 independent experiments; the SEMs were less than 25% and were omitted. In all 4 cell lines, all or most data points fell within the area of the envelope of additivity, suggesting additive effects.
STI571 and hydroxyurea.
Isobolograms of simultaneous exposure to STI571 and hydroxyurea (HU) in (A) KU812, (B) K-562, (C) TCC-S, and (D) TCC-Y cells. Data are presented as mean values of at least 3 independent experiments; the SEMs were less than 25% and were omitted. In all 4 cell lines, all or most data points fell within the area of the envelope of additivity, suggesting additive effects.
Cytotoxic interaction of STI571 in combination with cytarabine, homoharringtonine, doxorubicin, or etoposide
In all 4 cell lines, all or most of the data points for the combination fell within the envelope of additivity (isobologram not shown). The mean values of the data were larger than those of the predicted minimum values and smaller than those of the predicted maximum values for an additive effect, indicating that the simultaneous exposure to STI571 with cytarabine, homoharringtonine, doxorubicin, or etoposide produced additive effects in all 4 cell lines (Table2).
Cytotoxic interaction between STI571 and 4-hydroperoxy-cyclophosphamide
In the KU812, TCC-S, and TCC-Y cells, all or most of the combined data points fell within the envelope of additivity (isobologram not shown). The mean values of the data were between those of the predicted minimum values and those of the predicted maximum values for an additive effect, indicating that the simultaneous exposure to STI571 and 4-hydroperoxy-cyclophosphamide produced additive effects (Table 2). In the K562 cells, the data points for the combination fell within the envelope of additivity and in the area of supra-additivity (isobologram not shown). The mean values of the data were not significantly smaller than those of the predicted minimum values, indicating additive to synergistic effects (Table 2).
Cytotoxic interaction between STI571 and methotrexate
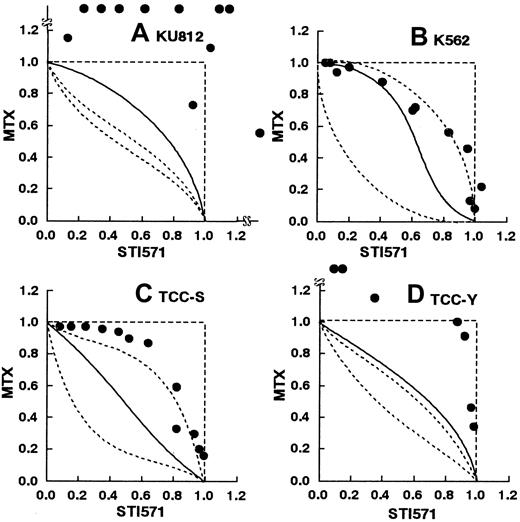
Figure 6A-D shows the isobolograms of this combination. In the KU812, TCC-S, and TCC-Y cells, all or most of the data points for the combination fell in the areas of subadditivity and protection. The mean values of the observed data were larger than those of the predicted maximum additive values. The observed data were significantly higher than the predicted maximum values, indicating antagonistic effects of the simultaneous exposure to these 2 agents (Table 2). In the K562 cells, the data points fell within the envelope of additivity and in the areas of subadditivity and protection. The observed data were not significantly higher than the predicted maximum values, indicating additive to antagonistic effects of the simultaneous exposure to these 2 agents (Table 2).
STI571 and methotrexate.
Isobolograms of simultaneous exposure to STI571 and methotrexate (MTX) in (A) KU812, (B) K-562, (C) TCC-S, and (D) TCC-Y cells. Data are presented as mean values of at least 3 independent experiments. In K562, and TCC-S cells, the SEMs were less than 20% and were omitted. In KU812 and TCC-Y cells, the SEMs were not less than 50%. In KU812, TCC-S, and TCC-Y cells, all or most data points fell in the areas of subadditivity and protection. The observed data were significantly larger than the predicted maximum values (P < .01, .02, and .02, respectively), suggesting antagonistic effects. The observed data were not significantly larger than the predicted maximum values in the K562 cells (P > .05), suggesting additive to antagonistic effects.
STI571 and methotrexate.
Isobolograms of simultaneous exposure to STI571 and methotrexate (MTX) in (A) KU812, (B) K-562, (C) TCC-S, and (D) TCC-Y cells. Data are presented as mean values of at least 3 independent experiments. In K562, and TCC-S cells, the SEMs were less than 20% and were omitted. In KU812 and TCC-Y cells, the SEMs were not less than 50%. In KU812, TCC-S, and TCC-Y cells, all or most data points fell in the areas of subadditivity and protection. The observed data were significantly larger than the predicted maximum values (P < .01, .02, and .02, respectively), suggesting antagonistic effects. The observed data were not significantly larger than the predicted maximum values in the K562 cells (P > .05), suggesting additive to antagonistic effects.
Cytotoxic interaction between STI571 and vincristine
Figure 7A-D shows the isobolograms of this combination. In all 4 cell lines, the data points for the combination fell within the envelope of additivity and in the area of supra-additivity. The mean values of the observed data were smaller than those of the predicted minimum additive values. In the KU812, TCC-S, and TCC-Y cells, the observed data were significantly smaller than the predicted minimum values, indicating synergistic effects of the simultaneous exposure to STI571 and vincristine (Table 2). In the K562 cells, the observed data were not significantly smaller than the predicted maximum value, indicating additive to synergistic effects of the simultaneous exposure to these 2 agents (Table2).
STI571 and vincristine.
Isobolograms of simultaneous exposure to STI571 and vincristine (VCR) in (A) KU812, (B) K-562, (C) TCC-S, and (D) TCC-Y cells. Data are presented as mean values of at least 3 independent experiments; the SEMs were less than 20% and were omitted. In all 4 cell lines, most data points fell in the area of supra-additivity. The observed data were significantly smaller than the predicted minimum values in KU812, TCC-S, and TCC-Y cells (P < .02, .05, and .05, respectively), suggesting synergistic effects. The observed data were not significantly smaller than the predicted minimum values in the K562 cells (P > .05), suggesting additive to synergistic effects.
STI571 and vincristine.
Isobolograms of simultaneous exposure to STI571 and vincristine (VCR) in (A) KU812, (B) K-562, (C) TCC-S, and (D) TCC-Y cells. Data are presented as mean values of at least 3 independent experiments; the SEMs were less than 20% and were omitted. In all 4 cell lines, most data points fell in the area of supra-additivity. The observed data were significantly smaller than the predicted minimum values in KU812, TCC-S, and TCC-Y cells (P < .02, .05, and .05, respectively), suggesting synergistic effects. The observed data were not significantly smaller than the predicted minimum values in the K562 cells (P > .05), suggesting additive to synergistic effects.
We have also made the isobolograms of STI571 in combination with the used agents at the IC50 level (data not shown). Basically, similar effects were observed for all combinations in all 4 cell lines.
Discussion
STI571 is a new antileukemic agent targeting BCR/ABL tyrosine kinase.14 A preliminary clinical study has shown that STI571 has significant activity in patients with chronic phase CML in whom IFN therapy has failed.18 STI571 induced complete hematologic remission in 96% after at least 4 weeks of therapy and cytogenetic remission in 33% of patients within 2 months of therapy. Side effects were mild and no dose-limiting toxicity has been encountered.
The purpose of the present study was to investigate the appropriate combinations of STI571 with antileukemic agents against 4 human Ph+ leukemia cell lines at the cellular level. When the dose-response curves are far from linear, as is usually the case in cancer chemotherapy and was also the case in this study, the nature of an additive response has been controversial.29 We used the Steel and Peckham isobologram method to evaluate the cytotoxic interaction between STI571 and other agents. Although a large number of data points are required, this method can be used to calculate the additive interaction of any combination, irrespective of the shapes of the dose-response curves of the agents and of whether they have independent or overlapping damage.
Currently, the most commonly used agent for CML during the chronic phase is IFN-α.8,10,29,30 IFN-α induced complete hematologic remission in 70% to 80% of patients, whereas a complete cytogenetic response (no detectable Ph+ metaphase) was observed in only 6% to 20% of patients. Patients who had complete cytogenetic response survived much longer than patients with no response or partial response. However, molecular evidence of underlying these differences is rarely, if ever, eliminated. A competitive RT-PCR detected BCR/ABL in most patients with complete cytogenetic response and there was a reverse association between theBCR/ABL/ABL ratio and survival.32 For improving the prognosis of CML, it is important to improve the degree of cytogenetic response and reduce the toxicity by combining IFN-α and other agents.
In the present study, we observed that STI571 produced synergistic or additive to synergistic effects with recombinant and natural IFNs-α in 3 of 3 cell lines. In general, the isobologram of Steel and Peckham is much stricter for the determination of synergism and antagonism than other methods for evaluating the effects of drug combinations, and synergism was rarely observed in the present test system. The present findings suggest that the combination of STI571 and IFN-α may be highly active against IFN-α–responsive Ph+ leukemia at the cellular level. The exact molecular mechanism behind the antileukemic activity of IFN-α remains to be elucidated, and the cytotoxic mechanism of the synergistic interaction between STI571 and IFN-α is still to be clarified. Although it is not clear that the combination of STI571 and IFN-α produces synergistic effects at cytogenetic level, this combination is worthy of preclinical and clinical investigations.
There have been several conflicting experimental findings for the combination of STI571 and IFN-α. Barteneva and coworkers reported that STI571 pretreatment enhanced cytotoxic activity of IFN-α in K562 cells.19 Thiesing and colleagues reported that the combination of STI571 and IFN-α showed an additive effect in a derivative of a human megakaryoblastic leukemia cell line, MO7e, engineered to express BCR/ABL (MO7p210), and K562 cells and colony-forming assay of CML patients,20 whereas Marley and associates reported that the combination of STI571 and IFN-α produced less than additive effects on colony-forming units-granulocye/macrophage from 2 patients with CML.22Marley used lower concentrations of IFN-α (50 and 100 IU/mL) than the concentrations used in this study (100 to 10 000 IU/mL) (Figure 3). The findings of Thiesing and coworkers and Marley and colleagues are different from the present observations. The reason for the differences is unknown. Differences in the experimental conditions and systems such as the cells used, drug concentrations, culture schedules, duration of drug exposure, assay method used to determine viable cells, and differences in the analytical methods for evaluating the effects of the drug combinations might have resulted the different findings.
Hydroxyurea or cytarabine alone or in combination with other agents have also been used for patients with Ph+ and Ph− leukemias.32,33 In present findings, simultaneous exposure to STI571 and hydroxyurea or cytarabine produced additive effects in all 4 Ph+ leukemia cell lines. An additive interaction of the combinations should have therapeutic advantages, when they are less than additive against critical normal tissues or the toxicities of the drugs are different. Because the toxicity profile may vary for STI571 and these agents,18the combinations may reduce doses to a minimum compared with those that would be used if either agent were administered alone. These suggest that the simultaneous administration of STI571 and hydroxyurea or cytarabine might be a preferable therapy against Ph+leukemias.
There have been 2 experimental findings for the combinations of STI571 and hydroxyurea or cytarabine. Thiesing and coworkers reported that the combination of STI571 and hydroxyurea showed less than an additive effect, whereas the combination of STI571 and cytarabine showed synergistic effects against K562 and MO7p210 cells.20Topaly and colleagues reported that the combinations of STI571 and hydroxyurea or cytarabine showed synergistic effects in Ph+BV173 and EM-3 cells using the median effect principle.21The present findings are different from their findings, probably due to different experimental conditions and analytical methods. As described previously, the isobologram of Steel and Peckham is stricter for synergism and antagonism.
Homoharringtonine, an alkaloid extracted from the evergreenCeplalotaxus harringtonia, is a new anticancer agent that is currently incorporated into the combination protocol for CML in chronic phase and AML.8,33 The inhibition of protein synthesis is considered the major cytotoxic mechanism of this agent.34 A recent clinical study showed that homoharringtonine induced complete hematologic remission in 92% and cytogenetic remission in 60% of patients with early CML.35 These findings were significantly better than those observed for IFN-α therapy. In the present study, simultaneous exposure to STI571 and homoharringtonine produced additive effects in all 4 cell lines, suggesting that the combination of STI571 and homoharringtonine has a high therapeutic efficacy as expected.
We further studied the effects of STI571 in combination with doxorubicin, etoposide, 4-hydroperoxy-cyclophosphamide, methotrexate, and vincristine, which are commonly used for the treatment of CML in blastic crisis and Ph+ ALL. Although the clinical activity of STI571 against CML blastic crisis and Ph+ ALL is not yet clear, Ph+ leukemia cell lines, most of which were sensitive to STI571, were established from patients with CML blastic crisis and Ph+ ALL. This suggests that STI571 would have an activity against these Ph+ leukemias.
Simultaneous exposure to STI571 and doxorubicin, etoposide, or 4-hydroperoxy-cyclophosphamide produced additive effects in all 4 or 3 of 4 cell lines. These findings suggest that the simultaneous administration of STI571 with doxorubicin, etoposide, or cyclophosphamide would have the expected activity and would be favorable for the treatment of Ph+ leukemias. 4-Hydroperoxy-cyclophosphamide has been used for purging leukemia cells in vitro.36 STI571 is also considered as an agent for purging Ph+ leukemia cells in vitro. Simultaneous exposure to these 2 agents in vitro appears appropriate for purging Ph+ leukemia cells.
Simultaneous exposure to STI571 and methotrexate produced antagonistic or additive to antagonistic effects in all 4 cell lines. The simultaneous administration of STI571 and methotrexate would be therefore inadequate. The mechanisms of the antagonistic effects of STI571 with methotrexate are unknown. Because methotrexate is a highly cell cycle-specific agent, cell cycle disturbances by STI571 might interfere with the action of methotrexate. Simultaneous exposure to methotrexate and most anticancer agents was also observed to produce antagonistic effects in our previous study (unpublished data).
Simultaneous exposure to STI571 and vincristine produced synergistic or additive to synergistic effects in all 4 cell lines, suggesting that the simultaneous administration of STI571 and vincristine would be highly active against CML in lymphoid crisis and Ph+ ALL. In our previous analysis on the combination of vinca alkaloids with a variety of anticancer agents, no drugs produced synergistic effects with vincristine during simultaneous exposure25 (and unpublished data). The synergism between STI571 and vincristine is therefore exceptional and should draw special attention. Mitotic inhibitors such as vinca alkaloids and taxanes have been shown to induce phosphorylation of bcl-2 family proteins including Bcl-2 and Bcl-x, which appears to be accompanied by the loss of function, and to induce apoptosis.37,38 Recently, Oetzel and colleagues39 reported that STI571 induced apoptosis inBCR/ABL-transfected cell lines by down-regulation of Bcl-x. The synergistic cytotoxicity of STI571 and vincristine may be attributable to their effects on Bcl-x.
These findings may be of importance in the design of STI571-based combination chemotherapy. In the present study, however, we used only simultaneous and continuous exposure to STI571 and other agents for 5 or 7 days. The results obtained on combining STI571 and other agents after 1 or 24 hours of exposure may be different from those obtained after a 5- or 7-day exposure. The order in which STI571 and other agents are administered to cells may also play a role. In addition, there are a number of difficulties in the translation of findings from in vitro to clinical therapy. The pharmacokinetics of STI571 and other agents are significantly different between them. Furthermore, the pharmacokinetic interaction and the toxic effects of the combinations cannot be measured in vitro. In addition, the cell kinetics and cell biochemistry may be quite different. Finally, the administration schedules in the present study were not always similar to those of clinical settings. These differences between in vitro and clinical systems would influence the cytotoxic interaction of STI571 and other agents.
In conclusion, the present study showed that simultaneous exposure to STI571 and IFN-α or vincristine produced mostly synergistic effects, whereas simultaneous exposure to STI571 and methotrexate produced antagonistic effects against Ph+ leukemia cell lines studied. Simultaneous exposure to STI571 and hydroxyurea, cytarabine, homoharringtonine, doxorubicin, etoposide, or 4-hydropeoxy-cyclophosphamide produced mostly additive effects. Although in vitro models are not absolutely predictive of clinical activity, these findings suggest that the simultaneous administration of STI571 with the all agents used in the present study, except methotrexate, may be advantageous for the treatment of CML and Ph+ ALL. Among them, the simultaneous administration of STI571 and IFN-α or vincristine may show high activity against Ph+ leukemias and may be worthy of clinical trials. These findings should provide further insights into and assist in the optimal combination and schedule of STI571 in clinical use.
Supported by a Grant-in-Aid for Cancer Research (11-8) from the Ministry of Health and Welfare, Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Yasuhiko Kano, Division of Medical Oncology, Tochigi Cancer Center, 4-9-13 Yonan, Utsunomiya, Tochigi, 320-0834, Japan; e-mail: ykano@tcc.pref.tochigi.jp.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal