Abstract
Several platelet inhibitors were examined in a tissue factor (TF)–initiated model of whole blood coagulation. In vitro coagulation of human blood from normal donors was initiated by 25 pM TF while contact pathway coagulation was suppressed using corn trypsin inhibitor. Products of the reaction were analyzed by immunoassay. Preactivation of platelets with the thrombin receptor activation peptide did not influence significantly the clotting time or thrombin–antithrombin III complex (TAT) formation. Addition of prostaglandin E1 (5 μM) caused a significant delay in clotting (10.0 minutes) versus control (4.3 minutes). The prolonged clotting time is correlated with delays in platelet activation, formation of TAT, and fibrinopeptide A (FPA) release. In blood from subjects receiving acetylsalicylic acid (ASA or aspirin), none of the measured products of coagulation were significantly affected. Similarly, no significant effect was observed when 5 μM dipyridamole (Persantine) was added to the blood. Antagonists of the platelet integrin receptor glycoprotein (gp) IIb/IIIa had intermediate effects on the reaction. A 1- to 2-minute delay in clot time and FPA formation was observed with addition of the antibodies 7E3 and Reopro (abciximab) (10 μg/mL), accompanied by a 40% to 70% reduction in the maximal rate of TAT formation and delay in platelet activation. The cyclic heptapetide, Integrilin (eptifibatide), at 5 μM concentration slightly prolonged clot time and significantly attenuated the maximum rate of TAT formation. The disruption of the gpIIb/IIIa-ligand interaction not only affects platelet aggregation, but also decreases the rate of TF-initiated thrombin generation in whole blood, demonstrating a potent antithrombotic effect superimposed on the antiaggregation characteristics.
Introduction
The importance of the platelet in normal hemostasis and in disease states, such as atherosclerosis and thrombosis, is well established.1-5 A particularly germane example is the platelet-rich thrombus, which forms on fissured or ruptured atherosclerotic plaques.3-5 Although the slow formation of the underlying plaques is a progressive disease that serves to narrow the lumen of the vessel over an extended period of time, it is the sudden and catastrophic superposition of the platelet-rich thrombotic occlusion on these lesions that is central to nearly all ischemic coronary events. General acceptance that the ultimate occlusive event is thrombotic in nature has led to the development of strategies that attempt to ameliorate, inhibit, or reverse this final phase of the coronary disease process. Some of the newest strategies focus on inhibition of platelet functions, which contribute to the formation of the thrombotic occlusion.
The generation of thrombin during the blood clotting process can be divided into 2 semidiscrete intervals, the initiation and propagation phases.6 During the initiation phase nanomolar amounts of thrombin and picomolar amounts of other coagulation enzymes are generated. Clot occurs at the inception of the propagation phase, which is characterized by rapid and near quantitative thrombin generation. By this point activation of platelets and cleavage of factor V and factor VIII are almost complete.7 In normal blood, the thrombin activation process is independent of platelet or factor V activation, but is limited by the generation of factor Xa.6 7
Normal platelet function has been divided into 3 processes: adhesion, aggregation, and activation (including surface phospholipid rearrangements and secretory pathways8-11). These functions bestow on the platelet its antihemorrhagic qualities and are regulated by a variety of mechanisms.12 The most potent platelet agonist is α-thrombin, the central enzyme of coagulation that controls hemorrhage in vivo. Thrombin is generated from the inactive circulating precursor prothrombin via the tissue factor (TF) pathway of coagulation13-16; this serine protease activates platelets via specific cell surface receptors, leading to secretion, aggregation, changes in platelet morphology, and expression of a procoagulant, phospholipid-equivalent, surface.9-11Aggregated thrombin-activated platelets form the basis of the thrombus in normal hemostasis and provide the surface on which the complex-dependent reactions of the TF pathway are localized. Furthermore, thrombin amplifies its own generation by activating circulating plasma procofactors (factor V and factor VIII), critical components of the pathway's proteolytic complex catalysts, which also serve to localize proteolytic activity to the surface of the activated platelet.6,17 Thrombin converts fibrinogen by limited proteolysis to fibrin, which polymerizes throughout the growing thrombus rendering added stability.18-20 The importance of TF-induced thrombin generation on platelets in acute coronary syndromes is strongly suggested by the observation that inflammatory cytokines can up-regulate TF expression in certain cells, which may be found in atherogenic lesions, blood, or the vasculature.21-23Therefore, the interrelationships between platelet functions and thrombin generation are central to an understanding of the thrombotic process in coronary syndromes.
The multifaceted roles of the platelet in hemostasis provide targets for the development of strategies to arrest the occlusive phase of atherosclerotic disease. A number of inhibitors have been described, which are directed at different aspects of platelet function. Prostaglandin E1 (PGE1) is a potent antagonist of platelet activation and functions through the platelet prostaglandin receptor by up-regulating adenylate cyclase production of intracellular cyclic adenosine monophosphate (cAMP).12 The weaker platelet inhibitor aspirin (acetylsalicylic acid or ASA) has been found to be mildly effective at reducing the risk of coronary events or their recurrence.24,25 This effect is presumably due to aspirin's role as irreversible inhibitor of the cyclooxygenase activity of prostaglandin H synthase in the arachidonic acid pathway leading to thromboxane A2 (TXA2) synthesis for release on platelet activation.26,27 Inhibitors of the platelet integrin glycoprotein (gp) IIb/IIIa have been designed, which interfere with the ability of these receptors to bind fibrin(ogen), and thus to form the large platelet-fibrin aggregates, which serve as the foundation of the thrombus.28 Recently, the murine monoclonal antibody 7E3 (and the humanized variant, known as c7E3, Reopro, or abciximab) has proven clinically effective as an adjuvant in the management of high-risk patients undergoing percutaneous transluminal coronary angioplasty (PTCA).29-31 Similarly, a cyclic heptapeptide containing a “KGD” sequence has also been developed as a high-affinity mimic of the fibrinogen “RGD” sequence, which binds to the gpIIb/IIIa receptor (Integrilin, or eptifibatide). This peptidomimetic has proven effective as an adjuvant in PTCA and in treatment of unstable angina.32,33 Although the above agents or their analogs exhibit significant clinical benefits in treating acute coronary syndromes, it is unclear whether their therapeutic properties are localized to the platelet aggregation function alone or extend to thrombin generation within the thrombotic occlusion. The goal of this study was to examine the relationships between therapeutic doses of these reagents and TF-induced coagulation in whole minimally altered human blood. This was accomplished using a laboratory model we have developed, which directly examines various aspects of the coagulation reaction in freshly drawn human blood.7 34
Materials and methods
Materials
Recombinant human TF was provided as a gift by Drs Roger Lundblad and Shu-Len Liu (Hyland Division, Baxter Healthcare, Duarte, CA). 1-Palmitoyl-2-oleoyl phosphatidylserine (PS) and 1-palmitoyl-2-oleoyl phosphatidylcholine (PC) were purchased from either Sigma Chemical (St Louis, MO) or Avanti Polar Lipids (Birmingham, AL).d-Phenylalanyl-l-prolyl-l-arginine chloromethyl ketone (FPRck)35 was obtained as a gift from Hematologic Technologies (Essex Junction, VT), synthesized in house, or purchased from Calbiochem (San Diego, CA). Corn trypsin inhibitor (CTI) was obtained either from Fluka (Ronkonkoma, NY) or prepared by a modification of the procedure of Hojima.34,36PGE1 and dipyridamole (Persantine) were obtained from Sigma Chemical. PGE1 was diluted to 0.564 mM in 95% ethanol and dipyridamole was solubilized at 2 mM in DMSO. Aspirin (100 × 325-mg tablets) was obtained from a local grocery (Hannaford Food and Drug Superstore, Rockland, ME). The antibody 7E3 was kindly provided by Dr Joseph A. Jakubowski (Thrombosis Research Division, Eli Lilly, Indianapolis, IN); stock concentration was 2 mg/mL in 20 mM HEPES, 0.15 M NaCl, pH 7.4 (HBS). Reopro (abciximab) and Integrilin (eptifibatide) were gifts of Dr Uma Sinha, Cor Therapeutics (South San Francisco, CA). Stock concentrations of Reopro and Integrilin were 2 mg/mL and 5 mM, respectively (solutions in HBS). The thrombin receptor activation peptide (TRAP) was provided by Dr Paula Tracy (University of Vermont). Burro antihuman platelet factor 4 (PF4) immunoglobulin G (IgG) antibody was prepared as previously described.7Horseradish peroxidase–labeled goat antihorse IgG antibody was purchased from Southern Biotech (Birmingham, AL). The following analytes were estimated using enzyme-linked immunosorbent assay (ELISA) kits obtained from the manufacturers according to the instructions provided: thrombin-antithrombin III (TAT) (Enzygnost TAT, Behring, Marburg, Germany); fibrinopeptide A (FPA) (Asserachrom FPA, Diagnostica Stago/American Bioproducts, Parsippany, NJ); platelet osteonectin (a gift from Dr Richard Jenny, Hematologic Technologies).
Human donors
All donors were recruited and advised according to a protocol approved by the University of Vermont Human Studies Committee.7 34 Healthy individuals (aged 22-36) were selected so as to exclude donors with a personal or familial history of thrombosis/hemorrhage, or regular aspirin or drug use. All individuals exhibited values in the normal range for the prothrombin time (PT; 11.6-13.8 seconds), activated partial thromboplastin time (aPTT; 27-36 seconds), and platelet counts (172 000-376 000/μL). For the ASA studies, each donor was sampled before ASA treatment (control series—no ASA for > 1 month before experiment) then again after 3 days of 325 mg ASA twice a day (experimental series).
Preparation of TF/lipid reagent
Tissue factor (5 nM) was relipidated into small unilammelar vesicles37 of 25% PS/75%PC (PCPS) (10 μM total lipid) in HBS plus calcium (2 mM) for 30 minutes at 37°C. Concentrated sucrose (60% w/v) was subsequently added to the relipidation mixture to 10% final to stabilize the vesicles for long-term freezer storage (up to 12 months). Aliquots of the reagent (200 μL) were lyophilized and stored at −20°C, which could be rehydrated 60 minutes before each experiment and used with reproducible results.
PT and aPTT assays
Assays were performed in plastic tubes (VWR Scientific, West Chester, PA, no. 60818-270) on 100-μL samples of fresh frozen, citrated human plasma using the manual “tilt-tube” approach described by the manufacturer of the PT or aPTT reagent. When testing the effect of buffer or inhibitor, no more than 10 μL of the additive was mixed with 90 μL plasma, to minimize dilution errors.
Coagulation in whole blood
The protocol used is a modification of Rand and colleagues,7 performed at the Clinical Research Center, Fletcher Allen Health Care (Burlington, VT). For all platelet agents except the aspirin study (see below), studies of clotting in freshly drawn, nonanticoagulated whole blood were performed simultaneously in 2 series (16/series) of capped, polystyrene tubes modified to accept reagents as described.7 One series contained buffer only (control series), the other contained an appropriate amount of the antiplatelet agent under investigation (experimental series). To suppress contact activation, CTI was added to all tubes (10 μL, 5 mg/mL stock); in addition, the first tube of each series of 16 tubes was designated the zero tube and was preloaded with 1 mL 50 mM EDTA in HBS, pH 7.4, and 10 μL 10 mM FPRck (diluted in 0.01 M HCl). As a control for possible TF in the blood resulting from the draw or other sources, all but the last tube in each series (designated “−TF”) were preloaded with relipidated TF.
For studies using platelet agents other than ASA, the platelet agent under investigation was preloaded into the experimental series from a stock prepared at a concentration appropriate to give the desired final concentration in the blood (typically 100- to 400-fold). Care was taken to avoid premixing of the reagents prior to initiation. An equivalent volume of solvent/vehicle (HBS, pH 7.4, or 95% ethanol for the PGE1 or DMSO for dipyridamole studies) was preloaded into each tube of the corresponding control series. Following venipuncture under the protocol approved by the Human Studies Committee at the University of Vermont,7 the initial 2 mL blood was discarded, samples were drawn for blood analyses (complete blood count, PT, aPTT, blood chemistry, etc), and about 40 mL fresh whole human blood was drawn into a repeating pipette or fitted with a plastic barrel syringe (Eppendorf Combi-Tip, Eppendorf, Westbury, NY). Another 1 to 2 mL blood was discarded, and the reaction was initiated by rapid distribution of the remainder (1 mL/tube) to each of the 16 preloaded tubes in each series (control and experimental). The final concentrations of additives in the various experiments are as follows: CTI (50 μg/mL), relipidated TF (3.1 pM, 25 pM, and 200 pM), and, where appropriate, the platelet agonist or antagonist (PGE1, TRAP, dipyridamole, and Integrilin 5 μM; 7E3 and Reopro 10 μg/mL).
For each of the ASA (aspirin) experiments, a similar procedure was used as described above; however, only one series of 16 tubes was prepared as above with no additions other than CTI and TF. Each of the control studies was performed using normal donor whole blood (∼20 mL) drawn prior to administration of ASA; each of the experimental studies was performed on a separate day on blood from the same donor treated according to the ASA regimen described above (see “Human donors”).
For each series in all experiments, periodic quenching of each tube (except the zero tube) was carried out at predefined intervals after initiation using EDTA and FPRck as described above (see discussion of zero tubes). Quenched samples were obtained for each series tracing the reaction progress up to 20 to 30 minutes after initiation. The samples were centrifuged to remove cellular components and clots, and an aliquot (200 μL) of the resulting serum from each tube was filtered to remove cellular contaminants for osteonectin assays (0.2 μm Acrodisc, Gelman Sciences, Ann Arbor, MI). From the remaining serum, both reducing (1% β-mercaptoethanol) and nonreducing sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) samples were prepared (60 μL serum, 190 μL 2% SDS-PAGE sample solution, heated exactly 5 minutes at 98°C), then stored at −20°C for further analysis (PF4). Western blot analysis of PF4 was performed as previously described.7 The remaining serum and cell pellets/clots were aliquotted to screw cap tubes, frozen, then stored at −70°C for immunoblot or immunoassay analysis.
Immunoassays
Commercial ELISAs for FPA, TAT, and platelet α-granule release (osteonectin) were performed according to manufacturers' protocols, with corrections for sample dilution by added quench solution (1.00 mL). Results were analyzed on a Vmax microtiter plate reader (Molecular Devices, Menlo Park, CA) equipped with SOFTMax version 2.0 software and an IBM Personal System 2 Model 30/286 PC. In each assay, a minimum of 5 standard concentrations were run in duplicate, with duplicate sample determinations at each time point. The relationship between concentration of standard and optical density was established by fitting the data to either a 4-parameter or log-logit fit, as described by the manufacturer (Molecular Devices). For each of the analytes (TAT, FPA, and osteonectin), duplicate data describing each time point for a given donor were averaged over all experiments.
Results
Assay conditions developed by Rand and colleagues7allow studies of coagulation directly in whole human blood in vitro under conditions that attempt to model the physiologic coagulation reaction in vivo. A tripartite approach is central to this model, using minimally modified whole blood initiated with limiting (pM) concentrations of relipidated TF (PCPS/TF ratio 2000:1) and CTI to suppress the contact pathway, which does not play a significant role in the normal hemostatic reaction.13-16 Such conditions permit examination of the reaction with clotting at times similar to those observed with in vivo assays like the template bleeding time. The conditions also produce a reaction that is sensitive to factor VIII, factor IX, and factor XI deficiencies known to be associated with hemophilias A, B, and C, and therefore provide a model of hemostasis that is improved over traditional plasma assays such as the PT and aPTT.38 Finally, use of whole human blood as the substrate for the reaction affords direct examination of hemostatic mechanisms on the platelet and cellular surfaces relevant to the reaction in vivo; this aspect is particularly important in allowing study of the effects of pharmaceutical agents (such as antiplatelet drugs) on the coagulation reaction in a safe ex vivo model.
At present, the whole blood model provides simultaneous measurements of clot formation, thrombin generation (via TAT), fibrin formation (via FPA), and platelet activation (via osteonectin release), among others.7 Of particular value have been the determinations of thrombin level over the course of the reaction, which show that thrombin generation proceeds in 3 distinct phases. From initiation of the reaction until the clot is observed, thrombin is produced at very low rates (< 1 nM/min). These low rates of thrombin generation have been ascribed to the limiting concentrations of prothrombinase produced during this initiation phase6 of the reaction, limited by the small amounts of factor Xa being produced. However, at clot time significant platelet and cofactor activation are observed, indicating that the low levels of thrombin generated are sufficient to potentiate the system for an increase in the rate of thrombin generation. This postclot time thrombin generation, propagation phase, is largely driven by factor Xa derived from the intrinsic Xase during this phase. The rate of thrombin generation during the propagation phase routinely exceeds 50 to 100 nM/min. Clotting occurs coincident with the inception of the propagation phase.7 34 Finally, rates of thrombin generation are observed to slow as a result of endogenous regulatory mechanisms in what is referred to as the termination phase of the reaction. Analyses performed across these phases of the reaction provide a means of characterizing the effects of external influences (such as antiplatelet agents) on the reaction as a whole.
Platelet activation and suppression prior to TF: TRAP and PGE1
Under normal conditions, the TF-initiated reaction in whole blood does not appear to be platelet activation limited.39 In keeping with this conclusion the current study found the reaction not to be significantly accelerated by the platelet agonist, TRAP sequence SFLLRN.40 The reaction initiated by 25 pM TF was studied in normal blood in the absence (■, Figure1) and presence (●) of TRAP (Table1), which fully activated the platelets within 1 minute of initiation as illustrated by osteonectin release (Figure 1B). In the absence of the TRAP, clotting occurred at 3.1 minutes (arrow a) and was accelerated slightly by addition of agonist (2.3 minutes, arrow b). However, this observed shortening of the clot time is most likely attributable to increased platelet aggregation leading to an early “call” of the clot formation, because the clotting time is estimated visually and is the most subjective parameter among the evaluated in this model of blood coagulation. This is supported by TAT profiles collected in the absence and presence of activation peptide, which show no significant alteration in thrombin generation (Figure 1A). Thus, TRAP had little effect on the thrombin generation profile, despite significant prior platelet activation. Together with earlier results, demonstrating significant generation of the factor Va and platelet activation prior to clot time,7,34 these data reinforce the conclusion that in the TF-initiated reaction in normal blood (and presumably during physiologic coagulation as well) platelet and factor V activation are not the limiting factors of TF-induced thrombin generation during the initiation phase. Rather, factor Xa appears to be the limiting component of the thrombin-generating prothrombinase complex.6
Whole blood coagulation and TRAP.
Comparison of whole blood coagulation in the absence (▪) and presence (●) of 5μM TRAP. Coagulation was initiated in whole blood by 25 pM relipidated TF as described in “Materials and methods.” Panel A represents thrombin (TAT) generation, panel B platelet activation (osteonectin [ON] release). Arrow “a” indicates clotting times for the control experiment, arrow “b” for the TRAP experiment.
Whole blood coagulation and TRAP.
Comparison of whole blood coagulation in the absence (▪) and presence (●) of 5μM TRAP. Coagulation was initiated in whole blood by 25 pM relipidated TF as described in “Materials and methods.” Panel A represents thrombin (TAT) generation, panel B platelet activation (osteonectin [ON] release). Arrow “a” indicates clotting times for the control experiment, arrow “b” for the TRAP experiment.
Maximum rates of TAT formation, FPA generation, and osteonectin release during TF-initiated (25 pM) whole blood clotting
| Agent . | Subject . | Clotting time (min) . | Maximum rate . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| TAT (nM/min) . | FPA (μM/min) . | Osteonectin (nM/min) . | |||||||
| C . | E . | C . | E . | C . | E . | C . | E . | ||
| TRAP | 2 | 3.1 | 2.3 | 149 | 136 | — | — | 8.2 | 8.0 |
| 5 μM | 6 | 4.7 | 4.2 | 94 | 122 | — | — | 3.9 | 4.0 |
| Average | 3.9 | 3.3 | 122 | 129 | — | — | 6.0 | 6.0 | |
| PGE1 | 1 | 4.2 | 10.7 | 70 | 35 | 6.0 | 5.2 | 9.9 | 7.5 |
| 5 μM | 2 | 4.4 | 9.3 | 54 | 12 | 3.4 | 3.2 | 11.8 | 9.0 |
| Average | 4.3 | 10.0 | 62 | 24 | 4.7 | 4.2 | 10.8 | 8.2 | |
| ASA | 1 | 4.2 | 3.7 | 63 | 53 | 7.6 | 2.7 | 6.5 | 3.9 |
| 2 × 325 mg/d | 2 | 3.1 | 4.1 | 51 | 46 | 4.6 | 2.7 | 5.5 | 2.7 |
| 3 | 5.1 | 3.6 | 105 | 102 | 3.2 | 7.5 | 4.7 | 4.7 | |
| Average | 4.1 | 3.8 | 73 | 67 | 5.1 | 4.3 | 5.6 | 3.8 | |
| Dipyridamole | 4 | 4.2 | 4.5 | 64 | 99 | — | — | 9.7 | 16.8 |
| 5 μM | 5 | 6.0 | 6.1 | 98 | 83 | 9.7 | 10.1 | 14.5 | 21.3 |
| Average | 5.1 | 5.3 | 81 | 91 | 9.7 | 10.1 | 12.1 | 19.0 | |
| 7E3 | 1 | 5.0 | 6.5 | 67 | 42 | 11.4 | 4.6 | 6.5 | 7.1 |
| 10 μg/mL | 2 | 3.1 | 3.7 | 51 | 31 | 4.6 | 5.2 | 7.8 | 10.5 |
| Average | 4.0 | 5.1 | 59 | 36 | 8.0 | 4.9 | 7.1 | 8.8 | |
| Reopro | 1 | 4.0 | 5.9 | 73 | 47 | 8.3 | 4.2 | 13.0 | 8.1 |
| 10 μg/mL | 2 | 4.5 | 6.7 | 90 | 27 | 5.4 | 4.1 | 4.1 | 4.0 |
| Average | 4.2 | 6.3 | 81 | 37 | 6.8 | 4.1 | 8.6 | 6.0 | |
| Integrin | 1 | 4.2 | 4.8 | 76 | 18 | 8.2 | 4.5 | 5.9 | 9.3 |
| 5 μM | 2 | 3.7 | 4.3 | 43 | 26 | 10.5 | 4.0 | 16.8 | 16.5 |
| Average | 3.9 | 4.5 | 59 | 22 | 9.3 | 4.2 | 11.3 | 12.9 | |
| Agent . | Subject . | Clotting time (min) . | Maximum rate . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| TAT (nM/min) . | FPA (μM/min) . | Osteonectin (nM/min) . | |||||||
| C . | E . | C . | E . | C . | E . | C . | E . | ||
| TRAP | 2 | 3.1 | 2.3 | 149 | 136 | — | — | 8.2 | 8.0 |
| 5 μM | 6 | 4.7 | 4.2 | 94 | 122 | — | — | 3.9 | 4.0 |
| Average | 3.9 | 3.3 | 122 | 129 | — | — | 6.0 | 6.0 | |
| PGE1 | 1 | 4.2 | 10.7 | 70 | 35 | 6.0 | 5.2 | 9.9 | 7.5 |
| 5 μM | 2 | 4.4 | 9.3 | 54 | 12 | 3.4 | 3.2 | 11.8 | 9.0 |
| Average | 4.3 | 10.0 | 62 | 24 | 4.7 | 4.2 | 10.8 | 8.2 | |
| ASA | 1 | 4.2 | 3.7 | 63 | 53 | 7.6 | 2.7 | 6.5 | 3.9 |
| 2 × 325 mg/d | 2 | 3.1 | 4.1 | 51 | 46 | 4.6 | 2.7 | 5.5 | 2.7 |
| 3 | 5.1 | 3.6 | 105 | 102 | 3.2 | 7.5 | 4.7 | 4.7 | |
| Average | 4.1 | 3.8 | 73 | 67 | 5.1 | 4.3 | 5.6 | 3.8 | |
| Dipyridamole | 4 | 4.2 | 4.5 | 64 | 99 | — | — | 9.7 | 16.8 |
| 5 μM | 5 | 6.0 | 6.1 | 98 | 83 | 9.7 | 10.1 | 14.5 | 21.3 |
| Average | 5.1 | 5.3 | 81 | 91 | 9.7 | 10.1 | 12.1 | 19.0 | |
| 7E3 | 1 | 5.0 | 6.5 | 67 | 42 | 11.4 | 4.6 | 6.5 | 7.1 |
| 10 μg/mL | 2 | 3.1 | 3.7 | 51 | 31 | 4.6 | 5.2 | 7.8 | 10.5 |
| Average | 4.0 | 5.1 | 59 | 36 | 8.0 | 4.9 | 7.1 | 8.8 | |
| Reopro | 1 | 4.0 | 5.9 | 73 | 47 | 8.3 | 4.2 | 13.0 | 8.1 |
| 10 μg/mL | 2 | 4.5 | 6.7 | 90 | 27 | 5.4 | 4.1 | 4.1 | 4.0 |
| Average | 4.2 | 6.3 | 81 | 37 | 6.8 | 4.1 | 8.6 | 6.0 | |
| Integrin | 1 | 4.2 | 4.8 | 76 | 18 | 8.2 | 4.5 | 5.9 | 9.3 |
| 5 μM | 2 | 3.7 | 4.3 | 43 | 26 | 10.5 | 4.0 | 16.8 | 16.5 |
| Average | 3.9 | 4.5 | 59 | 22 | 9.3 | 4.2 | 11.3 | 12.9 | |
C indicates control; E, experiment.
Like prostaglandin I2, PGE1 is a natural prostanoid, which inhibits intracellular release of stored Ca++ and platelet activation by up-regulation of adenylate cyclase to form cAMP. The clinical usefulness of this reagent is limited by its short half-life in the circulation and an ability to stimulate peripheral vasodilation; however, some preliminary studies have been described with analogs of this reagent for use in controlling pulmonary hypertension.41,42 Figure2 describes results with PGE1(5 μM) added in vitro to whole blood initiated to clot by 25 pM TF. Inhibition of the TF pathway of blood coagulation is observed. The clotting times in the control experiment and in the presence of PGE1 are indicated in each panel of the figure (arrows a and b, respectively). In the presence of PGE1, clot time is prolonged from 4.4 minutes to 9.3 minutes. Thrombin generation, shown as TAT formation over the course of the experiment (Figure 2A), is severely affected by this agent. In the absence of PGE1, thrombin generation is very slow during the initiation phase (■, 0-4 minutes region ending at arrow a), but thereafter occurs in an explosive fashion with a maximum rate near 54 nM/min during the propagation phase of the reaction. This result is similar to those reported for our previous studies of coagulation in blood from normal donors.7 In the presence of PGE1 (●) the initiation phase is prolonged (clotting occurring at arrow b) and the maximum rate of TAT production during the propagation phase is significantly reduced (12 nM/min) (Table 1).
Whole blood coagulation and PGE1.
Comparison of whole blood coagulation in the absence (▪) and presence (●) of 5 μM PGE1. Coagulation was initiated in whole blood by 25 pM relipidated TF as described in “Materials and methods.” Panel A represents thrombin (TAT) generation, panel B fibrin (FPA) formation, and panels C and D platelet activation (osteonectin [ON] and PF4 release, respectively). Arrow “a” indicates clotting times for the control experiment, arrow “b” for the PGE1 experiment. Upper lane in panel D represents control experiment and lower lane represents PGE1experiment.
Whole blood coagulation and PGE1.
Comparison of whole blood coagulation in the absence (▪) and presence (●) of 5 μM PGE1. Coagulation was initiated in whole blood by 25 pM relipidated TF as described in “Materials and methods.” Panel A represents thrombin (TAT) generation, panel B fibrin (FPA) formation, and panels C and D platelet activation (osteonectin [ON] and PF4 release, respectively). Arrow “a” indicates clotting times for the control experiment, arrow “b” for the PGE1 experiment. Upper lane in panel D represents control experiment and lower lane represents PGE1experiment.
Release profiles of FPA for the control and in the presence of PGE1 are compared in Figure 2B. The normal profile (■) exhibits limited FPA formation until just before clot time (arrow a). The burst of FPA slightly precedes the burst of thrombin during the propagation phase (compare to Figure 2A), reaching 30% of the maximum FPA at clot time. FPA levels rapidly go to completion within a 3-minute period, well before peak thrombin levels are generated (< 100 nM TAT). This profile is similar to those described elsewhere,33 34and shows that FPA release is significant at limiting amounts of thrombin. Platelet inhibition by PGE1 (●) produces a delay in the initiation of FPA release, although at clot time FPA release is approximately 35% (arrow b) and proceeds at a rate that is similar to that for the control (3.4 μM/min in control experiment and 3.2 μM/min in the presence of PGE1). Therefore, PGE1 inhibits the onset of explosive fibrin formation, presumably resulting from the reduced levels of thrombin generated during the initiation phase of the reaction in the presence of this inhibitor.
The effect of PGE1 on platelet activation is evident in the platelet osteonectin and PF4 release profiles (Figure 2C and D, respectively). The normal profile of osteonectin release (■) shows that platelet activation begins just before the clot formation and is about 50% complete at clot time (arrow a), consistent with observation of Rand and coworkers.7 However under the influence of PGE1 (●), little or no platelet α-granule release is detectable until after clot time (arrow b), and the profile is delayed overall by 6 minutes (versus control). Similarly, PF4 release in the presence of PGE1 (Figure 2D; lower lane) is delayed by approximately 6 minutes when compared with control experiment (upper lane). A similar effect of 5 μM PGE1 on the clotting time, TAT and FPA formation, and osteonectin release was observed for the blood of another healthy volunteer (Table 1).
In summary, the results suggest that PGE1 at this concentration inhibits the TF-initiated reaction in whole blood in vitro by suppressing formation of the membrane sites required for complex enzyme assembly. Thus, thrombin generation during both the initiation and propagation phases becomes platelet activation dependent and is reduced by PGE1. The results obtained using PGE1 in this model system resemble those obtained in whole blood from thrombocytopenic patients with platelet counts less than 11 000/μL.39
Aspirin (ASA) and dipyridamole (Persantine)
No inhibitory effects on the TF pathway of blood coagulation were discerned in 2 of 3 individuals ingesting aspirin (Table 1). In fact, clotting time in whole blood initiated by 25 pM TF appeared on average to be slightly shorter in the presence of the drug than in control experiment (3.8 and 4.1 minutes, respectively); however, the degree of variability in these experiments precludes conclusions concerning any aspirin accelerating effect. For individual “1” aspirin administration decreased clotting time by 0.5 minute, whereas for individual “3” this decrease reached 1.5 minutes (Figure3). Similarly, thrombin generation (Figure 3A) and fibrin conversion (Figure 3B) profiles for these 2 individuals were shifted to the left in the presence of the drug (●) in comparison with the corresponding cases in the absence of ASA (■), whereas osteonectin release was not significantly influenced by aspirin administration (Figure 3C). For individual “2” the clotting time was prolonged by 1 minute after aspirin administration (3.1 and 4.1 minutes, respectively). The maximum rates of TAT generation, FPA formation, and osteonectin release appeared on average to be slightly slower than normal (Table 1); however, the variability in these assays does not allow any conclusion concerning deceleration of these processes by ASA.
Whole blood coagulation and ASA.
Comparison of whole blood coagulation before (▪) and after (●) administration of ASA, 2 × 325 mg/d for 3 consecutive days. Coagulation was initiated in whole blood by 25 pM relipidated TF as described in “Materials and methods.” Panel A represents thrombin (TAT) generation, panel B fibrin (FPA) formation, and panel C platelet activation (osteonectin [ON] release). Arrow “a” indicates clotting times before ASA administration, arrow “b” after.
Whole blood coagulation and ASA.
Comparison of whole blood coagulation before (▪) and after (●) administration of ASA, 2 × 325 mg/d for 3 consecutive days. Coagulation was initiated in whole blood by 25 pM relipidated TF as described in “Materials and methods.” Panel A represents thrombin (TAT) generation, panel B fibrin (FPA) formation, and panel C platelet activation (osteonectin [ON] release). Arrow “a” indicates clotting times before ASA administration, arrow “b” after.
These studies failed to find a significant role for aspirin at the dose used in attenuating TF-initiated coagulation and underscore the complexity of the physiologic aspirin effect. The data suggest that aspirin's therapeutic effect does not arise primarily from direct modulation of platelet procoagulant function in the TF pathway to thrombin generation because aspirin does not appear to have any measurable effect on platelet activation in this system. This may be due to 2 factors. First, platelet activation in this model system is thrombin mediated as underscored by the observation that addition of nanomolar quantities of hirudin completely inhibit platelet activation. Second, thrombin, a potent platelet agonist, overrides the weak inhibitory effects of aspirin via the TXA2 pathway.
Dipyridamole (Persantine) is a pyrimidopyrimidine derivative that inhibits platelet aggregation, but the mechanism of action of this compound has been controversial.43 It has been established that plasma concentrations of this derivative are 2 to 6 μM after oral administration of pharmaceutical doses. An in vitro addition of 5 μM dipyridamole to whole blood only marginally affected the clotting time, increasing it by 0.1 to 0.3 minute versus control (Table 1). Neither thrombin generation, FPA formation, nor osteonectin release was significantly altered by the presence of this platelet antagonist.
Inhibitors of gpIIb/IIIa
Inhibitors of the gpIIb/IIIa complex on the platelet surface are designed to block the interactions between fibrinogen and platelets, inhibiting the formation of platelet aggregates.28 We examined the effect of the murine antibody 7E3, the chimeric human/murine antibody Reopro (abciximab) and cyclic heptapetide Integrilin (eptifibatide) on blood coagulation induced by 200 pM, 25 pM, and 3.1 pM TF.
In studies of whole blood with TF concentration of 200 pM, the clotting time was 1.25 minutes (Figure 4; arrows a-c). At this initiator concentration no significant effects of gpIIb/IIIa antagonists on the blood coagulation process were observed. Clotting times and maximum rates of TAT formation were similar in the control experiment (■) and in the presence of 10 μg/mL Reopro (●) or 5 μM Integrilin (▴).
Thrombin (TAT) generation in whole blood initiated by 200 pM TF.
Coagulation was initiated by relipidated TF as described in “Materials and methods” in the absence of any adjuvant (▪), in the presence of 5 μM Integrilin (▴) and in the presence of 10 μg/mL Reopro (●). Arrow “a” indicates clotting time for control experiment, arrow “b” for Integrilin experiment, and arrow “c” for Reopro experiment.
Thrombin (TAT) generation in whole blood initiated by 200 pM TF.
Coagulation was initiated by relipidated TF as described in “Materials and methods” in the absence of any adjuvant (▪), in the presence of 5 μM Integrilin (▴) and in the presence of 10 μg/mL Reopro (●). Arrow “a” indicates clotting time for control experiment, arrow “b” for Integrilin experiment, and arrow “c” for Reopro experiment.
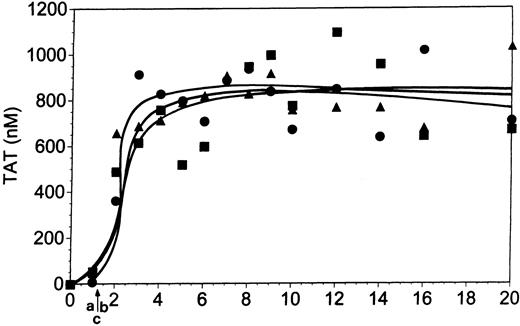
At a TF concentration of 25 pM both forms of antibody (7E3, Figure5; and Reopro, Table 1) at 10 μg/mL modestly delayed clot formation (Figure 5, arrow b). On average, 7E3 prolonged the clotting time from 4 to 5.1 minutes and Reopro from 4.2 to 6.3 minutes (Table 1). This reflects a modest reduction in thrombin generation versus normal in the presence of the inhibitors during the initiation phase of the reaction. With Integrilin (Figure6), clot formation was delayed slightly (by 0.6 minute; arrow b) versus control (arrow a). Consistent with this observation, the initiation phase of thrombin generation (3.9 minutes) was slightly prolonged by this inhibitor (to 4.5 minutes).
Whole blood coagulation and 7E3.
Comparison of whole blood coagulation in the absence (▪) and presence (●) of 7E3 (10 μg/mL). Coagulation was initiated in whole blood by 25 pM relipidated TF as described in “Materials and methods.” Panel A represents thrombin (TAT) generation, panel B fibrin (FPA) formation, and panels C and D platelet activation (osteonectin [ON] and PF4 release, respectively). Arrow “a” indicates clotting times for the control experiment, arrow “b” for the 7E3 experiment. Upper lane in panel D represents control experiment and lower lane represents 7E3 experiment.
Whole blood coagulation and 7E3.
Comparison of whole blood coagulation in the absence (▪) and presence (●) of 7E3 (10 μg/mL). Coagulation was initiated in whole blood by 25 pM relipidated TF as described in “Materials and methods.” Panel A represents thrombin (TAT) generation, panel B fibrin (FPA) formation, and panels C and D platelet activation (osteonectin [ON] and PF4 release, respectively). Arrow “a” indicates clotting times for the control experiment, arrow “b” for the 7E3 experiment. Upper lane in panel D represents control experiment and lower lane represents 7E3 experiment.
Whole blood coagulation and Integrilin.
Comparison of whole blood coagulation in the absence (▪) and presence (●) of 5 μM Integrilin. Coagulation was initiated in whole blood by 25 pM relipidated TF as described in “Materials and methods.” Panel A represents thrombin (TAT) generation, panel B fibrin (FPA) formation, and panel C platelet activation (osteonectin [ON] release). Arrow “a” indicates clotting times for the control experiment, arrow “b” for the Integrilin experiment.
Whole blood coagulation and Integrilin.
Comparison of whole blood coagulation in the absence (▪) and presence (●) of 5 μM Integrilin. Coagulation was initiated in whole blood by 25 pM relipidated TF as described in “Materials and methods.” Panel A represents thrombin (TAT) generation, panel B fibrin (FPA) formation, and panel C platelet activation (osteonectin [ON] release). Arrow “a” indicates clotting times for the control experiment, arrow “b” for the Integrilin experiment.
Thrombin generation during the propagation phase was significantly suppressed by all 3 gpIIb/IIIa inhibitors. With 7E3 (Figure 5A, ●), the maximum thrombin generation rate following clot formation was approximately 60% of that observed in the control experiment (31 nM/min versus 51 nM/min, for the experiment presented in Figure 5A). Reopro decreased the maximum rate of TAT formation on average by 54% (Table 1). Similarly, in the presence of Integrilin this rate was decreased from 59 nM/min to 22 nM/min, a more than 60% reduction (Table 1; Figure 6A).
Both inhibitory antibodies produced delays in FPA release (Figure 5B; 7E3; ●) of approximately 1 to 2 minutes relative to control (■), reflective of the prolonged initiation phase and delay in clot formation. This delay in the FPA profiles is consistent with the influence of these inhibitors on the TF pathway reaction during its initiation phase. Although the FPA release is delayed versus control, the ultimate levels and rates of FPA formation are similar to those observed in the absence of the inhibitors. FPA release in the presence of 5 μM Integrilin (Figure 6B) is also consistent with the depressed thrombin generation although clotting data suggest that this inhibitor has a lesser effect on the initiation phase than the antibodies at this TF concentration. Integrilin did not significantly inhibit the rate, duration, or maximum level of FPA release (●) compared with the control (■).
Platelet osteonectin (Figure 5C for 7E3) and PF4 release (Figure 5D) are delayed versus normal (■) in the presence of inhibitory antibodies (●). With Integrilin, osteonectin release (Figure 6C) is slightly delayed (●) versus normal (■). The maximum rates of osteonectin release are not affected by the presence of these inhibitors (Table 1).
In summary, the antibody inhibitors of the gpIIb/IIIa complex influence thrombin generation, fibrin formation, and platelet activation in both the initiation and propagation phases of the reaction in whole blood induced by 25 pM TF. The largest effect appears to be on the propagation phase of the reaction. Integrilin also has a pronounced effect on the propagation phase, with a more limited effect on the initiation phase of thrombin generation.
Experiments were also performed at a lower (3.1 pM) TF concentration. Under these conditions both agents (Integrilin and Reopro) prolonged the clotting time from 8.2 to 14.3 minutes (Integrilin) and almost 20 minutes (Reopro) and depressed the maximum levels of TAT formed (data not shown).
Discussion
Studies from this laboratory and others7,34,44-46using physiologically relevant systems to model the TF pathway have demonstrated that thrombin generation is not uniform over time but occurs in distinct phases. During an initiation phase, low rates of thrombin generation (< 1 nM/min) are observed as a result of low rates of factor Xa produced primarily by the factor VIIa-TF complex.6,7,34,44-46 Subsequently, enhanced levels of factor Xa are made available by participation of the factor IXa–factor VIIIa complex,38 and a burst of thrombin generation (> 50 nM/min) occurs during a propagation phase of the reaction. Clot formation, the usual end-point of in vitro assays, occurs approximately at the transition between the initiation and propagation phases and coincides with the onset of the thrombin burst.7,34 By this point in the reaction in whole blood, activation of platelets and cleavage of factor V and factor VIII are significant.7
In normal blood, the limiting component in the ultimate activation of prothrombin to thrombin is not platelet or factor V activation, but factor Xa generation.6 7 Thus, as anticipated, preactivation of platelets with TRAP has little effect on thrombin generation.
In contrast, the potent platelet inhibitor PGE1significantly prolongs the initiation phase of thrombin generation, which is reflected in a doubling of the clotting time and delays in the FPA release and thrombin generation. PGE1 also delays platelet degranulation (osteonectin release), with accompanying reduction in the presentation of platelet surface receptors for procoagulant enzyme complex formation thus impairing coagulation complex assembly.10 11 This impaired complex assembly in the presence of PGE1 is reflected by a decreased thrombin generation rate during the propagation phase to less than half that observed normally. Ultimately, platelet activation and fibrin formation, though delayed, proceed to completion despite the reduced prothrombin activation rates. Thus, although thrombin levels are sufficient to fully activate platelets and cleave fibrinogen at maximal rates during the propagation phase, suppression of platelet activation by the potent antagonist PGE1 imparts a substantial platelet activation dependency to the process of thrombin generation.
Aspirin (ASA) at clinically relevant doses did not alter clotting time, thrombin generation, fibrin formation, or platelet activation in blood induced to clot by addition of TF. This result is similar to that noted by Kjalke and associates who reported no effect of aspirin administration on the thrombin generation in a reconstituted system in the presence of platelets when the reaction was initiated by TF.47 Thus, at the doses used, aspirin does not overcome the thrombin activation of the platelets, the agonist principally responsible for platelet activation in the TF-initiated reaction. Similarly, the addition of dipyridamole (Persantine) to whole blood in vitro at pharmacologic concentration43 has no effect on the processes occurring during TF-induced blood coagulation in this model.
The inhibitors of the platelet gpIIb/IIIa receptor exhibit somewhat different behaviors in influencing the TF-initiated reaction. In reactions initiated by 25 pM TF, antibodies 7E3 (the murine form) and Reopro abciximab or c7E3 (the chimeric murine/human form) each provide a modest prolongation of the initiation phase evidenced by slight delays in clotting time and delays in both FPA and platelet osteonectin release profiles. However, both 7E3 and Reopro significantly reduce thrombin generation during the propagation phase of the reaction. Despite reduced thrombin generation the ultimate maximum rates of FPA formation and osteonectin release were observed. Both the fibrin and platelet reactions reached completion. These observations of reduced thrombin generation in the presence of anti-gpIIb/IIIa antibodies are consistent with those described in a defibrinated plasma model.48
The cyclic heptapeptide Integrilin (eptifibatide) slightly delayed thrombin generation at 25 pM TF during the initiation phase, resulting in a slightly prolonged clotting time. However, at pharmacologic concentration the influence of Integrilin was less than that observed for the 7E3 and Reopro antibodies. Integrilin, similarly to antibody inhibitors, reduced the rate of thrombin generation during the propagation phase of the reaction.
At higher TF concentrations (200 pM), neither Reopro nor Integrelin at pharmacologic concentrations affected clot formation, clotting times, or the thrombin generation profiles. At the lower initiator concentration (3.1 pM TF) the efficiencies of both gpIIb/IIIa inhibitors is enhanced. Under these conditions, both Integrilin and Reopro significantly prolonged the clotting time. These significant prolongations of clotting time may explain the bleeding observed with these agents.49-51
Overall, the gpIIb/IIIa inhibitors appear to significantly influence either the quantitative or qualitative expressions of the procoagulant complexes leading to thrombin.
We thank Sara G. Paradis and Joshua D. Dee for the technical assistance. We thank Dr Shu Len Liu and Dr Roger Lundblad for providing us with TF; Dr Joseph A. Jakubowski for providing 7E3; Dr Uma Sinha for providing Reopro and Integrilin; Dr Paula Tracy for providing TRAP; and Dr Richard Jenny for providing osteonectin ELISA kits and FPRck.
Supported in part by RR00109, P01 HL 46703 (K.G.M.), PHS T32 HL07594-12 (K.G.M.) from the National Institutes of Health, and by a TALENT stipend of the Netherlands Organization of Scientific Research (C.v.V.).
Portions of this work were presented at the 39th Annual Meeting of the American Society of Hematology, December 5-9, 1997, San Diego, CA (Blood 90 [suppl 1]:29a, abstract 114).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kenneth G. Mann, Department of Biochemistry, College of Medicine, University of Vermont, Burlington, VT 05405-0068; e-mail: kmann@zoo.uvm.edu.

![Fig. 1. Whole blood coagulation and TRAP. / Comparison of whole blood coagulation in the absence (▪) and presence (●) of 5μM TRAP. Coagulation was initiated in whole blood by 25 pM relipidated TF as described in “Materials and methods.” Panel A represents thrombin (TAT) generation, panel B platelet activation (osteonectin [ON] release). Arrow “a” indicates clotting times for the control experiment, arrow “b” for the TRAP experiment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/8/10.1182_blood.v97.8.2314/5/m_h80810935001.jpeg?Expires=1767734645&Signature=wWEpKXISacnBRcQIlUccGZsu9e6hGTbdWLcDpJzrSTArzrRaZGHW8cdpcoc8twf79bdfOs3zFdiNAiUdFZBR9tRx0a85ZgIyfeN2njO5rTa0pepMiulbpITG5j2yqOFzXAxmpyLsfprZ4P8Rn36JHpcHSiVUpE1y8WBiz~FXag1QFvP8FUyw9I2EUjOWgGGkUIs8x10sva-uuKErrS-tmsq2UNcORgl6jo56QfJHIWKHf2LKDXqnNFcPlf1HwQvwc2oRtuqSaZ2rTKiYxQAC7NLg~aZpTJZljaMGxMbnbLguQMlAScp5CmdbSSkcqQjsWr6Riidq7a2U3zmga1Jz3g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Whole blood coagulation and PGE1. / Comparison of whole blood coagulation in the absence (▪) and presence (●) of 5 μM PGE1. Coagulation was initiated in whole blood by 25 pM relipidated TF as described in “Materials and methods.” Panel A represents thrombin (TAT) generation, panel B fibrin (FPA) formation, and panels C and D platelet activation (osteonectin [ON] and PF4 release, respectively). Arrow “a” indicates clotting times for the control experiment, arrow “b” for the PGE1 experiment. Upper lane in panel D represents control experiment and lower lane represents PGE1experiment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/8/10.1182_blood.v97.8.2314/5/m_h80810935002.jpeg?Expires=1767734645&Signature=s4a59vZioS54Rp8hwCZi92oRwnxFDRWvUyRMXgldb6eBPy06LSFxCXDIYDJzSn7a67lV0spfQdooCSXCdx8JuARvmFQPC-jPmHkHJqOX0EiAHNEFCl0b25XTuqLkdrXIREQDH8Xf9vzQsgMfC4-WhxttlxxpIxUtlqHt6iuLdEELMSsXQF0TNtEXt69RTe6Ve-9Btsi6XAgogFMj2EsX1YygJfULIDQpNmVVUmFmB1gwdTvq3TC3d6JYjdJKKp94uTWC36263QSX1VhF5efEth51uriAbqLs7hMosEz2F-0og6g7gwQW8~fYbP6TcgdPOWQ5J3dz7bDzNnxHiKdxig__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Whole blood coagulation and ASA. / Comparison of whole blood coagulation before (▪) and after (●) administration of ASA, 2 × 325 mg/d for 3 consecutive days. Coagulation was initiated in whole blood by 25 pM relipidated TF as described in “Materials and methods.” Panel A represents thrombin (TAT) generation, panel B fibrin (FPA) formation, and panel C platelet activation (osteonectin [ON] release). Arrow “a” indicates clotting times before ASA administration, arrow “b” after.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/8/10.1182_blood.v97.8.2314/5/m_h80810935003.jpeg?Expires=1767734645&Signature=FAuzJOrRktFSYOl5o~5bO42GVWDNtGTVC4E2iorNrVLRcz0CoQxA6tIVAudJOR2FiX30y7Ns7YwjYhSVULicFdQ5Q6z2omUxooddxdtrpA2R1CKG-YhaFrrQHT9eg5A33jbUuSQjlMMiWIBNeCpg5exTYGvnn-QzVlz4G5I7L9g0EsCumMFJe9UwUzuW-4U4tLuunEYzZW3jiL1DrgfDH~VunsAlVQIJSYhd4qtTxMOX1n7WTt1GRc-2ShE3dm9MLPGhmkBJHiW0lwj0~7J8OSxdbS8WVHdwGc0CKWyAx9fDAp4CHkqNpn0-5be0dT-hehHN5OzV6gb~skSwsOI4og__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Whole blood coagulation and 7E3. / Comparison of whole blood coagulation in the absence (▪) and presence (●) of 7E3 (10 μg/mL). Coagulation was initiated in whole blood by 25 pM relipidated TF as described in “Materials and methods.” Panel A represents thrombin (TAT) generation, panel B fibrin (FPA) formation, and panels C and D platelet activation (osteonectin [ON] and PF4 release, respectively). Arrow “a” indicates clotting times for the control experiment, arrow “b” for the 7E3 experiment. Upper lane in panel D represents control experiment and lower lane represents 7E3 experiment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/8/10.1182_blood.v97.8.2314/5/m_h80810935005.jpeg?Expires=1767734645&Signature=VG7V1nwTU1eeYmWrroX4rVK1t0pAnjcqWYhZNhXKihNriPnCi1VdlYlNDt3XUmKt2OagEvBQcoSHeJkg9UKZ4e~S9oZIRYI4xji1yFuyjDLdX4KmL3ZIgiaW-MaPsUezfInEXkaOaYCkTrA5c8wWjHcZomp3c0UT4Z3-cvt6YmEnY9ShCe97enYOypVxxokzkmYJi0MafXKocMTX8Q95orKMILwybAraLkwsO-BkiauARJZ8h5vj77MZbXyKpEIC28gQ7NPy4uwjBWwAL-VYIJmo3UDn4Jxez5hDLBCUmJMCdm~xyGziBXSW2TSfpcFdycbkeYe5jIytHnOrZ0-bYQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Whole blood coagulation and Integrilin. / Comparison of whole blood coagulation in the absence (▪) and presence (●) of 5 μM Integrilin. Coagulation was initiated in whole blood by 25 pM relipidated TF as described in “Materials and methods.” Panel A represents thrombin (TAT) generation, panel B fibrin (FPA) formation, and panel C platelet activation (osteonectin [ON] release). Arrow “a” indicates clotting times for the control experiment, arrow “b” for the Integrilin experiment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/8/10.1182_blood.v97.8.2314/5/m_h80810935006.jpeg?Expires=1767734645&Signature=Up7fcyUndoTstD8KtUyQldttcJKYzGaMsdMzYvZtOTRBxG3aNtbjUa~h5WU-JVafs6TgyRAB16at2-~UCUgv-pJKAjyMAiGI78hixREhQTFOlCPYHhTEiO58ms3PAHBm95jvZWiTluvB0WwCBHmde9Ob46-ft7SSFCKJr01IG0qubT~0avAtvUq7bDseNx4x~-A9Amjfo3Xv18mnu7dy7jsUs4tp1xynK0imNYjqQHS-OpfDsDgw7Sv74vMv3xWeTtcnDgQP4jLkDNaVlNLY8wMgOgtEO33isCW2N1eMA6aaAJzTaDEMqNpiT5nFCh4DCPmR~E5Ep3Mxa2m7pgpEmg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal