Abstract
Translocations involving fibroblast growth factor receptor 3 (fgfr3) have been identified in about 25% of patients with myeloma. To directly examine the oncogenic potential offgfr3, murine bone marrow (BM) cells were transduced with retroviral vectors containing either wild-type fgfr3 or an activated mutant form of the receptor, fgfr3-TD. Mice transplanted with FGFR3-TD–expressing BM developed a marked leukocytosis and lethal hematopoietic cell infiltration of multiple tissues within 6 weeks of transplantation. Secondary and tertiary recipients of spleen or BM from primary fgfr3-TD mice also developed tumors within 6 to 8 weeks. Analysis of the circulating tumor cells revealed a pre-B-cell phenotype in most mice, although immature T-lymphoid or mature myeloid populations also predominated in some animals. Enhanced lymphoid but not myeloid colony formation was observed in the early posttransplantation period and only interleukin 7 and FGF-responsive pre-B-cell lines could be established from tumors. Cell expansions in primary recipients appeared polyclonal, whereas tumors in later passages exhibited either clonal B- or T-cell receptor gene rearrangements. Mice transplanted with wild-type FGFR3-expressing BM developed delayed pro-B-cell lymphoma/leukemias approximately 1 year after transplantation. These studies confirm that FGFR3 is transforming and can produce lymphoid malignancies in mice.
Introduction
The recent description of ubiquitous yet heterogeneous chromosome translocations into the immunoglobulin heavy (IgH) chain switch region in multiple myeloma (MM) has provided an opportunity to dissect the molecular basis of this malignancy.1-3 Some of the more common translocation partners identified include cyclin D1,fgfr3/mmset,2,4-6c-maf,7 and mum1/irf4.8It is thought that these translocations into the IgH switch region represent an early event in the subsequent development of MM.1 9
The translocation of fibroblast growth factor receptor 3 (fgfr3), a receptor tyrosine kinase, into the IgH locus in MM has been identified in approximately 25% of patients and cell lines studied to date.2,5 This receptor is important in the negative regulation of bone formation in mammals.10,11Studies of fgfr3 knockout mice have shown that mice deficient in this gene develop long bone overgrowth and other skeletal abnormalities.10 11 In humans, it has been noted that activating mutations in fgfr3 result in chondrodysplasias (varying severity of dwarfism depending on the placement of the mutation within the gene) and craniosynostosis (premature fusion of the skull bones).
Interestingly, the translocated fgfr3 gene in myeloma is sometimes noted to contain such activating mutations.2,4 One such mutation occurs within the second kinase domain of fgfr3, a lysine to glutamic acid residue substitution (K650E; single-letter amino acid codes).12When this mutation arises within the germline it results in the development of thanatophoric dysplasia type II (TDII), a neonatal lethal form of dwarfism. The TDII mutation occurs within the activation domain of the receptor and is thought to relieve the inhibitory conformation of the kinase enabling constitutive activation of the receptor.13 Indeed, it has been demonstrated that the TDII form of FGFR3 (FGFR3-TD) is capable of autophosphorylating at a level 100-fold higher than wild-type FGFR3.13 However, neither wild-type nor mutant FGFR3 has been previously shown to have transforming activity in vitro or in vivo.
Previous work by our laboratory has demonstrated that overexpression of FGFR3 in myeloma cells leads to enhanced interleukin 6 (IL-6)–dependent proliferation and myeloma cell survival in the absence of IL-6.14 Furthermore, expression of both the R248C and K650E (constitutively active forms of FGFR3) in BaF3, a pro-B-cell line, demonstrated that constitutively active FGFR3 could lead to ligand-independent proliferation.15 To further investigate the potential in vivo effects of overexpression of wild-type FGFR3 and FGFR3-TD in hematopoietic cells, we performed bone marrow (BM) transplants in mice. We postulated that FGFR3 expression would cause expansion of clonal cells within the BM and lead to development of tumors or perhaps myeloma formation. The experiments reported here demonstrate that mice receiving transplants with FGFR3-expressing BM cells develop hematologic malignancy most commonly of pre-B-cell phenotype.
Materials and methods
Construction of FGFR3 retroviruses
Two pcDNA3 plasmids containing full-length human complementary DNAs (cDNAs) of the wild-type or TDII mutant form of fgfr3were obtained from Daniel J. Donoghue (San Diego, CA).13The fgfr3 was released by restriction cutting withHindIII and SpeI, which also removed the 3′ untranslated region. The recessed 3′ termini of the cDNA fragments were filled and the fragments ligated into aHpaI/SnaBI-digested MINV retrovirus,16 containing the neomycin resistance gene as the selectable marker. The resulting vectors were termed MFINV-WT (wild-type fgfr3) and MFINV-TD (TDII K650 mutantfgfr3). The parental MINV vector (neor only) was used as a negative vector control.
Retroviral packaging lines
Twenty micrograms of the constructed retroviral vectors and the parental MINV vector were electroporated into 1.5 × 106PA317 amphotropic packaging cells.17 After 48 hours the supernatant was collected, passed through a 0.45 μm filter, and overlaid on subconfluent monolayers of GP+E 86 ecotropic packaging cells18 in the presence of 8 μg/mL polybrene (Sigma, St Louis, MO). On days 3 and 4 of culture this process was repeated. Transfected GP+E 86 cells were selected in 1.2 mg/mL G418 (Life Technologies, Gaithersburg, MD) for 14 days. The resulting packaging lines were GP+E 86/MINV (empty vector), GP+E 86/WT (wild-typeFGFR3), and GP+E 86/TD (TDII mutant FGFR3).
Transduction of BM precursors and transplantation
Eight- to 10-week-old female BALB/c mice (Charles River Canada, Montreal, QB) were used as BM donors or transplant recipients. The transplantation of BM cells was performed as previously described.19,20 In brief, BM was flushed from the hind limbs of donor mice that had been injected 4 days previously with 150 mg/kg 5-flurorouracil (Roche, Laval, QB) in phosphate-buffered saline (PBS). Following erythrocyte lysis, nucleated cells were cultured in 100-mm dishes at a density of 5 × 105 cells/mL in prestimulation medium (Iscoves modified Dulbecco medium [IMDM]; Life Technologies), 10% fetal calf serum (FCS; Life Technologies), 1% penicillin/streptomycin (Life Technologies), 2 mM glutamine (Life Technologies), 50 μM 2-mercaptoethanol (2-ME) supplemented with 10% conditioned medium from X630-rIL3 cells,21 10% conditioned medium from Sp2/mIL6 cells,22 and 2% to 5% conditioned medium from Sp2/soluble Flt3 ligand (sFLt3L) for 2 days. BM cells were then cocultured with 40% to 50% confluent GP+E-86/MINV, GP+E-86/WT, or GP+E-86/TD that had previously been γ-irradiated with 20 Gy (from a 137Cs source; Gammacell 3000 Elan, Nordion International, Kanata, ON), in the presence of 8 μg/mL polybrene. After 48 hours, nonadherent cells were collected and cultured in prestimulation medium in the presence or absence of 1.25 mg/mL G418 for 24 hours. Transduced cells were harvested, washed, resuspended in PBS, and injected into the tail vein of recipient mice that had been administered 7 Gy γ-irradiation. Each recipient mouse received between 0.5 × 106 and 2 × 106transduced cells. For serial transplantation, 106 BM or 2 to 5 × 106 spleen cells or thymocytes from primary recipients were injected intravenously into 4-Gy γ-irradiated hosts.
Hematologic evaluations and tissue processing
Peripheral blood (PB) of transplant recipients was sampled at weekly intervals by tail vein bleeding. Wright-Giemsa–stained PB smears were scored by light microscopy for differential counting. Mice were killed by cervical dislocation when moribund. Tissue samples were preserved in 10% neutral-buffered formalin before embedding in paraffin for sectioning and staining with hematoxylin and eosin. Single-cell suspensions of hematopoietic tissues (BM, spleen, thymus, and lymph nodes) were cultured in vitro or processed for isolation of genomic DNA and proteins.
Colony assay and cell line establishment
Pre-B-cell and myeloid colony-forming unit assays were performed on both in vitro cultured, retrovirus-engineered BM cells and on BM cells harvested from transplant recipient mice. Pre-B and myeloid colonies were cultured in Methocult M3630 or Methocult M3434 methylcellulose medium, respectively (Stemcell Technologies, Vancouver, BC) at 1 × 104 cells/mL per dish. In attempts to establish cell lines, retroviral-transduced BM cells or tumor-derived cells from mice were also cultured in IMDM, 10% FCS, 50 μM 2-ME, 2 mM l-glutamine, and 1% penicillin/streptomycin plus 10 ng/mL murine stem cell factor (mSCF), 10% mIL-3–conditioned medium, 2% mIL-6–conditioned medium, 2% murine granulocyte-macrophage colony-stimulating factor (mGM-CSF)–conditioned medium with or without 2 ng/mL recombinant mIL-7 (Pharmingen, Mississauga, ON). Cytokines were individually withdrawn to determine the dependence of cultured cells on growth factors.
Western blot analysis
Tissue samples were minced and incubated in RIPA buffer (50 mM Hepes pH 7.23, 150 mM NaCl, 50 μM ZnCl2, 50 μM NaF, 50 μM orthophosphate, 2 mM EDTA, 1% Nonidet P40, 0.1% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS]) containing 2 mM phenylmethylsulfonyl fluoride and 2 mM sodium orthovanadate while being pressed through a 70-μM nylon cell strainer (Becton Dickinson, San Jose, CA). Lysates were fractionated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose (Costar, Acton, MA). Membranes were probed with antiphosphotyrosine, anti-FGFR3, anti-bcl-xS/L, or anti-ERK1 (all from Santa Cruz Biotechnology, Santa Cruz, CA) and goat antirabbit IgG horseradish peroxidase (HRP; Pharmingen) was used as a secondary antibody. Blots were developed by enhanced chemiluminescence (ECL; Amersham, Arlington Heights, IL).
Flow cytometric analysis
Reagents used included antimouse B220-PE, CD43-FITC, CD3e-FITC, CD4-biotin, CD4-FITC, CD8a-PE, CD11b-FITC, CD16/CD32 (FcγIII/II receptor, Fc blocker) (all the preceding from Pharmingen); antimouse CD117-FITC (c-kit), CD25-biotin, streptavidin-PE-Cy5 (all 3 from Cedarlane, Hornby, ON); and antimouse IgM-FITC (Southern Biotechnology Associates, Birmingham, AL). Single-cell suspensions from tissues were prepared, washed in a solution of PBS, 0.1% NaN3, and 1.0% FCS and resuspended. Cells were analyzed by flow cytometry on a FACScan (Becton Dickinson) using CellQuest software.
Nucleic acid analysis
Southern blot analysis was carried out according to standard procedures.23 Probes included: a 1.4-kbBamHI-NotI fragment of the neo gene (MINV);16 a 0.5-kb EcoRI-XbaI fragment of a murine T-cell receptor (TCR) (chain gene cDNA (pCOWβ.R) containing Cβ2 constant region sequences;24 a 2.0-kb BamHI-EcoRI fragment of the murine IgH chain locus (pSv2-gpt-BamC5′)containing the J3 and J4 joining gene segments (provided by Dr Tak Mak, Amgen Institute, Toronto, ON). VDJH rearrangements were assessed by using 5′ VALL degenerate VH-specific primer and the 3′ JH4 primer.25 Polymerase chain reaction (PCR) products were separated by gel electrophoresis, transferred to Hybond N nylon membrane (Amersham), and hybridized with a JH4 internal probe.25 Mutation analysis of FGFR3 was conducted using single-strand conformation polymorphism (SSCP) analysis as previously described.2
Results
Lethal hematopoietic tumors develop in mice transplanted with FGFR3-expressing BM
The BM from donor mice was transduced with either MFINV-WT (wild-type fgfr3), MFINV-TD (TDII K650 mutantfgfr3), or parental MINV (neor only) retroviruses to assess the potential effect of ectopic FGFR3 expression on hematopoiesis. The transduced BM cells from donor mice were injected intravenously into lethally irradiated recipient BALB/c mice. Compared to control mice, mice receiving BM expressing wild-type FGFR3 displayed only a transient leukocytosis. However, those mice receiving FGFR3-TD–expressing BM developed a leukemic blood picture with marked leukocytosis, polychromasia, and thrombocytopenia within 3 to 4 weeks after transplantation (Figure 1A). High white blood cell (WBC) counts of FGFR3-TD mice coincided with systemic illness and organ infiltration with immature hematopoietic cells. The morphology of the blood cells of FGFR3-TD mice suggested that there was an increase in the number of immature lymphoid cells (Figure2).
Leukocyte (WBC) counts and survival of primary marrow transplant recipients.
(A) Mean WBC values for each treatment group are shown over time. Only mice receiving MFINV-TD–expressing BM displayed extremely high WBC counts initially (TD). At 1 year, mice receiving MFINV-WT transplants began to develop leukocytosis (WT). Control mice receiving empty vector–transduced BM are also shown (MINV). (B) MFINV-TD (TD) mice rapidly became moribund and required sacrifice by 6 weeks after transplantation. MFINV-WT (WT) mice became sick and died or required sacrifice at approximately 1 year after transplantation. All control (MINV) vector group mice remained alive. ○, TD, n = 5; ▪, WT, n = 4; ▵, MINV, n = 3.
Leukocyte (WBC) counts and survival of primary marrow transplant recipients.
(A) Mean WBC values for each treatment group are shown over time. Only mice receiving MFINV-TD–expressing BM displayed extremely high WBC counts initially (TD). At 1 year, mice receiving MFINV-WT transplants began to develop leukocytosis (WT). Control mice receiving empty vector–transduced BM are also shown (MINV). (B) MFINV-TD (TD) mice rapidly became moribund and required sacrifice by 6 weeks after transplantation. MFINV-WT (WT) mice became sick and died or required sacrifice at approximately 1 year after transplantation. All control (MINV) vector group mice remained alive. ○, TD, n = 5; ▪, WT, n = 4; ▵, MINV, n = 3.
Hematopoietic cell infiltration of organs and PB.
(A) PB of MFINV-TD secondary recipient (pre-B cell by FACS) (original magnification × 40). (B) Peripheral blood of MFINV-TD secondary recipient demonstrates an acute lymphoblastic leukemia confirmed to be of pre-B-cell phenotype by FACS (original magnification × 100).
Hematopoietic cell infiltration of organs and PB.
(A) PB of MFINV-TD secondary recipient (pre-B cell by FACS) (original magnification × 40). (B) Peripheral blood of MFINV-TD secondary recipient demonstrates an acute lymphoblastic leukemia confirmed to be of pre-B-cell phenotype by FACS (original magnification × 100).
Among 9 primary recipients of FGFR3-TD–expressing BM, 4 mice died between weeks 4.5 and 6 and the remaining 5 mice had to be killed at 6 weeks after transplantation due to morbidity. Postmortem analysis of FGFR3-TD mice revealed splenomegaly. Most of the FGFR3-TD mice also had enlarged thymuses and lymph nodes and exhibited fragile bones. Tumors arising in FGFR3-TD mice were then serially passaged by transferring either spleen or BM cells from the primary tumor-bearing mice to a second and subsequently third or fourth generation sublethally irradiated recipient mouse. All mice receiving serial transplants also became moribund within 6 to 8 weeks of transplantation. Necropsy of these mice revealed hematopoietic tumors infiltrating major organs.
In contrast, mice overexpressing FGFR3-WT were initially well but began to develop profound leukocytosis and became moribund around 1 year of age (Figure 1B). Of 4 FGFR3-WT mice alive at late reconstitution times, 2 looked in poor health and died spontaneously at 9 and 12 months, respectively. The remaining 2 animals became sick at about 12 months and were killed. PB films demonstrated very elevated WBC counts with a morphology resembling that of acute lymphoblastic leukemia in humans. Histologic examination of major organs at autopsy demonstrated infiltration of tissue with lymphoid cells. Three control mice transplanted with BM cells expressing parental MINV and left to live did not develop neoplasms up to 18 months after transplantation.
FGFR3-induced tumors are multilineage in nature but pre-B-cell leukemia/lymphomas predominate
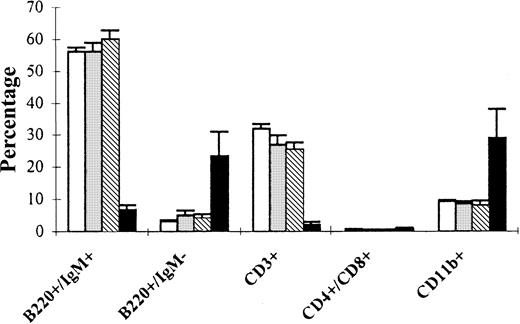
Flow cytometry on the autopsied spleens of primary FGFR3-TD mice 6 weeks after transplantation revealed a marked increase in overall numbers of both B220+IgM− pre-B cells and CD11b+ myeloid cells (Figure3). Analysis of tumors arising in secondary and tertiary FGFR3-TD recipients demonstrated a predominant pre-B (B220+/IgM−/CD43+/CD25+/c-kit−) cell population (Figure 4). In total, 9 of 13 secondary recipients of tumor cells from FGFR3-TD mice had a distinguishable pre-B cell population that infiltrated the BM, spleen, and thymus; in some cases, the malignant pre-B cells also invaded liver, kidney, and lung. In contrast, 3 of the secondary recipient mice displayed predominantly T-cell abnormalities (CD4+, CD8+, CD3−) (Figure5) and one secondary recipient mouse had more than 90% B220−CD11b+ mature-appearing neutrophils within the PB and BM (not shown). One of the secondary recipients of FGFR3-TD–expressing cells displayed abnormalities in all 3 cell lineages: B cells, T cells, and myeloid cells (not shown). In wild-type FGFR3 mice, tumors developing 1 year after transplantation were exclusively pro-B cell (B220+, c-kit+) in nature both in primary and secondary recipients (not shown).
Phenotype of spleen cells from primary FGFR3 transplant recipients.
Single-cell suspensions of spleen were prepared from primary BM transplant recipients 6 weeks after transplantation. Cell populations analyzed by flow cytometry are shown for normal littermate controls not receiving transplants (normal, ■), mice receiving empty vector-transplants (MINV, ░), and mice transplanted with MFINV-WT– (WT, ▧) or MFINV-TD– (TD, ▪) expressing BM. MFINV-TD mice displayed a decrease in both normal B (B220+/IgM+) and T (CD3+) lymphocytes, whereas expansions of immature B (B220+/IgM−) lymphocytes and of CD11b+ cells were evident. Mice receiving MFINV-WT BM displayed the same splenic cell phenotype at 6 weeks after transplantation as controls.
Phenotype of spleen cells from primary FGFR3 transplant recipients.
Single-cell suspensions of spleen were prepared from primary BM transplant recipients 6 weeks after transplantation. Cell populations analyzed by flow cytometry are shown for normal littermate controls not receiving transplants (normal, ■), mice receiving empty vector-transplants (MINV, ░), and mice transplanted with MFINV-WT– (WT, ▧) or MFINV-TD– (TD, ▪) expressing BM. MFINV-TD mice displayed a decrease in both normal B (B220+/IgM+) and T (CD3+) lymphocytes, whereas expansions of immature B (B220+/IgM−) lymphocytes and of CD11b+ cells were evident. Mice receiving MFINV-WT BM displayed the same splenic cell phenotype at 6 weeks after transplantation as controls.
Abnormal pre-B-cell lineage expansions in secondary FGFR3-TD overexpressing transplant recipients.
Single-cell suspensions of BM, spleen, or PB cells from mice 6 weeks after secondary transplantation were prepared and analyzed by flow cytometry. Shown in panel A are malignant cells from an MFINV-TD recipient mouse that displayed a pre-B-cell phenotype (B220+, IgM−, CD43+/−, CD25+) in spleen. Flow cytometric analysis of spleen cells from an MFINV-WT recipient mouse, shown in panel B, demonstrated a normal pattern in this early posttransplantation period.
Abnormal pre-B-cell lineage expansions in secondary FGFR3-TD overexpressing transplant recipients.
Single-cell suspensions of BM, spleen, or PB cells from mice 6 weeks after secondary transplantation were prepared and analyzed by flow cytometry. Shown in panel A are malignant cells from an MFINV-TD recipient mouse that displayed a pre-B-cell phenotype (B220+, IgM−, CD43+/−, CD25+) in spleen. Flow cytometric analysis of spleen cells from an MFINV-WT recipient mouse, shown in panel B, demonstrated a normal pattern in this early posttransplantation period.
Abnormal T- and B-cell lineage expansions in secondary FGFR3-TD overexpressing transplant recipients.
Malignant cells (staining as pre-B) from a primary recipient (TD5) were transplanted into secondary recipients (TD5.1 and TD5.2). Of note, malignant populations that arose in these mice had different phenotypes. Spleen cells from the TD5.1 secondary recipient mouse exhibited an abnormal T-cell phenotype (CD3−, CD4+, CD8+), whereas in mouse TD5.2 the pre-B cell population continued to predominate. Analysis of the same cell populations from an empty vector MINV recipient control mouse are shown to demonstrate a normal pattern.
Abnormal T- and B-cell lineage expansions in secondary FGFR3-TD overexpressing transplant recipients.
Malignant cells (staining as pre-B) from a primary recipient (TD5) were transplanted into secondary recipients (TD5.1 and TD5.2). Of note, malignant populations that arose in these mice had different phenotypes. Spleen cells from the TD5.1 secondary recipient mouse exhibited an abnormal T-cell phenotype (CD3−, CD4+, CD8+), whereas in mouse TD5.2 the pre-B cell population continued to predominate. Analysis of the same cell populations from an empty vector MINV recipient control mouse are shown to demonstrate a normal pattern.
FGFR3 expression promotes pre-B-cell expansion
To further investigate the progenitor cell type targeted by the transforming capacity of FGFR3-TD, primary murine BM cells were transduced and divided for use in either transplantation models or for long-term in vitro suspension culture. In long-term culture, pre-B-cell colony frequency was increased after 4 weeks in comparison to controls and a predominant B220+ cell phenotype was evident by FACS analysis at week 7 (Figure 6A,B). In contrast no increase in myeloid colony formation was observed (not shown). A c-kit+B220− colony arising from FGFR3-TD–expressing cells was plucked and recultured in suspension. After 3 months in culture, a predominant B220+ B-cell population was again evident (Figure 6C). Finally, in mice receiving transplants, serial analysis of PB demonstrated the emergence of a pre-B-cell population only 2 weeks after transplantation (Figure 6D). The frequency of pre-B-cell colonies in the BM of FGFR3-TD–transplanted mice was increased at all tested time points above control levels (not shown).
FGFR3-TD targets an early B-cell progenitor.
Serial analysis of FGFR3-TD–expressing BM cultured in vitro revealed an excess of pre-B cells by week 7 (A) and an excess of early B-cell colonies by week 4 to 5 (B) when compared to FGFR3-WT and MINV vector only controls. ○, TD; ▪, WT; ▵, MINV. (C) A single plucked colony from an FGFR3-TD–transduced BM culture differentiated into a predominantly B-cell population after 3 months in culture. (D) Circulating pre-B cells were increased in the blood of mice receiving transplants as early as 2 weeks after transplantation. ■, MINV; ░, WT; ▪, TD.
FGFR3-TD targets an early B-cell progenitor.
Serial analysis of FGFR3-TD–expressing BM cultured in vitro revealed an excess of pre-B cells by week 7 (A) and an excess of early B-cell colonies by week 4 to 5 (B) when compared to FGFR3-WT and MINV vector only controls. ○, TD; ▪, WT; ▵, MINV. (C) A single plucked colony from an FGFR3-TD–transduced BM culture differentiated into a predominantly B-cell population after 3 months in culture. (D) Circulating pre-B cells were increased in the blood of mice receiving transplants as early as 2 weeks after transplantation. ■, MINV; ░, WT; ▪, TD.
The ability to establish permanent cell lines from the FGFR3-TD tumors was then investigated. Single-cell suspensions of tumor-infiltrated spleen or BM were cultured in varying combinations of cytokines in attempts to generate cell lines of multiple lineage. This method enabled the consistent establishment of only B220+/IgM−/c-kit− IL-7– and FGF-responsive cell lines. Thus the available evidence suggests that FGFR3 predominantly targets an early B-cell progenitor population.
Signaling pathways in FGFR3-induced tumors
The FGFR3 protein was overexpressed in the tumor samples from FGFR3-TD and wild-type FGFR3 mice when compared to controls (Figure 7). Because transformation in FGFR3-WT–expressing mice was delayed, this suggested that cooperating oncogenes were involved or alternatively that the FGFR3-WT transgene had undergone activating mutation(s). Analysis of FGFR3-WT by SSCP failed to demonstrate any acquired mutations. As expected from previous studies, both STAT3 phosphorylation and bcl-xL expression were increased in FGFR3-expressing tumors (Figure 7). Of interest, global tyrosine phosphorylation patterns also varied between controls, wild-type FGFR3, and FGFR3-TD–induced tumors (Figure8).
FGFR3 is overexpressed and STAT3 and bcl-xLup-regulated in tumors originating after BM transplantation.
Western blot analysis for FGFR3 expression was performed on tissues derived from mice that developed tumors (WT1.1.1.1, WT2.1.1, TD5.2, TD5.1, TD2.2.1) and from controls (MINV5.1, WT6). The 2 expected isoforms of FGFR3 at 125 and 135 kd were observed in all tumor-bearing mice. Concomitantly, phosphorylated STAT3 and bcl-xL levels are increased coincident with elevations in FGFR3. The ERKgene is used as a housekeeping control on the lower panel.
FGFR3 is overexpressed and STAT3 and bcl-xLup-regulated in tumors originating after BM transplantation.
Western blot analysis for FGFR3 expression was performed on tissues derived from mice that developed tumors (WT1.1.1.1, WT2.1.1, TD5.2, TD5.1, TD2.2.1) and from controls (MINV5.1, WT6). The 2 expected isoforms of FGFR3 at 125 and 135 kd were observed in all tumor-bearing mice. Concomitantly, phosphorylated STAT3 and bcl-xL levels are increased coincident with elevations in FGFR3. The ERKgene is used as a housekeeping control on the lower panel.
Variation in global tyrosine phosphorylation patterns in FGFR3-TD and FGFR3-WT mice.
Global phosphotyrosine patterns were compared from mice that developed tumors (WT1.1.1.1, WT2.1.1, TD5.2, TD5.1, TD2.2.1) and from controls (MINV5.1, WT6). Distinct variation in banding patterns was observed.
Variation in global tyrosine phosphorylation patterns in FGFR3-TD and FGFR3-WT mice.
Global phosphotyrosine patterns were compared from mice that developed tumors (WT1.1.1.1, WT2.1.1, TD5.2, TD5.1, TD2.2.1) and from controls (MINV5.1, WT6). Distinct variation in banding patterns was observed.
FGFR3-induced tumors can be serially passaged and exhibit clonal DNA rearrangements
Malignant cells of both T- and B-cell phenotype from the BM or spleen of secondary FGFR3-TD recipient mice could be passaged through third or fourth generation sublethally irradiated syngeneic recipients with hematopoietic tumors consistently developing. Primary tumors arising in wild-type FGFR3 mice could also be serially passaged.
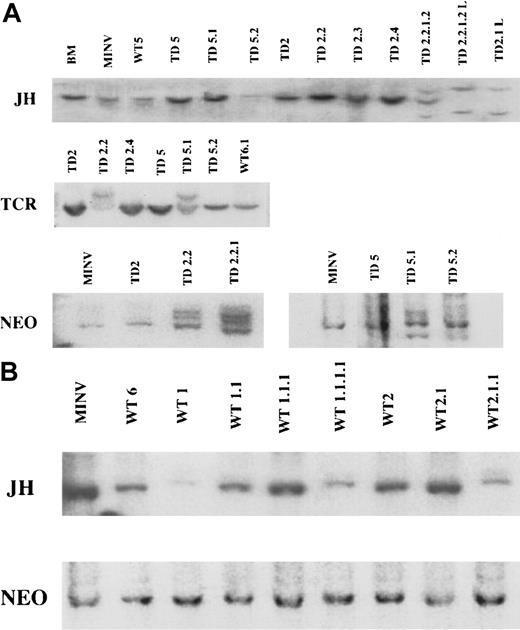
To examine clonality of the tumors, the status of the IgH and TCRβ loci were investigated by Southern blots using JHand TCR Cβ2 constant region probes, respectively. Southern blot analysis demonstrated rearranged JH bands only in late generation B-cell tumors, whereas primary and secondary recipients only showed a germline configuration. JH was also rearranged in B220+ cell lines derived from both secondary and later recipient mice (Figure9A). PCR analysis using degenerate primers for the IgH locus and subsequent Southern blotting with a JH4 probe demonstrated that some FGFR3-TD secondary recipient mice had more intense IgH bands than primary recipients suggesting progressive clonal expansion of B-cell populations (data not shown). Taken together, these data demonstrated that initial cell expansions were polyclonal with clonal cell populations gradually emerging. Hybridization of the TCR Cβ2 probe indicated the presence of uniquely rearranged TCRβ bands only in mice that showed abnormal T-lineage cells by flow cytometry, whereas germline TCR configuration was detected in mice with B-cell tumors (Figure 9A). All tumors arising in wild-type mice receiving FGFR3 transplants exhibited germline JH configuration (Figure 9B).
IgH, TCR, and
neo gene rearrangements in FGFR3-induced tumors and cell lines. (A) Top panel: Southern blot analysis ofEcoRI-digested spleen cell genomic DNA and B220+cell line DNA with an IgH JH probe. As controls, a normal littermate is shown in lane 1 and a recipient of MINV-transduced BM in lane 2 (MINV). WT represents mice receiving MFINV-WT transplants (lane 3) and TD represents mice receiving MFINV-TD transplants. Mice in each experiment were numbered 1 through 5; passage number was also labeled 1 through 5. Thus cells derived from the second and fifth primary MFINV-TD BMT recipients (labeled TD2 and TD5, respectively) were injected into the secondary mice denoted TD2.2, TD2.3, TD2.4, or TD5.1 and TD5.2. Cells from a secondary animal passaged into a fourth generation recipient are labeled TD2.2.1.2. Cell lines derived from animals are designated TD2.1-L and TD2.2.1.2-L. JHrearrangements of the IgH locus were detected only in the fourth generation recipient TD2.2.1.2 as well as the TD 2.2.1.2-L and TD2.1-L cell lines. Middle panel: Southern blot analysis ofPvuII-digested spleen cell genomic DNA from MFINV-TD recipients with a TCR Cβ2 probe. DNA derived from the second and fifth primary FGFR3-TD BM recipients (labeled TD2 and TD5, respectively) were injected into the secondary mice denoted TD2.2, TD2.4, TD5.1, and TD5.2. TCR gene rearrangements were detected in the TD2.2 and TD5.1 samples, both from mice displaying abnormal T-cell populations by flow cytometry. Bottom panel: Genomic DNA digested with EcoRI (which cuts MFINV-TD once) and hybridized with a neo probe was used to enumerate the proviral copy number and determine the clonality of the tumors. MINV represents vector only control mice. TD represents FGFR3-TD BMT mice. Cells from mouse TD2 or TD5 were injected into secondary and tertiary recipients denoted TD2.2, TD2.2.1, or TD5.1 and TD5.2, respectively. In both cases, a predominant clone within a diffuse background was observed in early recipients, whereas multiple bands representing unique clones emerged in later generation mice receiving serial transplants. (B) JH and neo were hybridized as above to MINV control animals and to the primary (WT6, WT1, WT2), secondary (WT1.1, WT2.1), tertiary (WT1.1.1, WT2.1.1), and quarternary mice receiving FGFR3-WT transplants (WT1.1.1.1). No emerging clonal populations were observed in any of the mice.
IgH, TCR, and
neo gene rearrangements in FGFR3-induced tumors and cell lines. (A) Top panel: Southern blot analysis ofEcoRI-digested spleen cell genomic DNA and B220+cell line DNA with an IgH JH probe. As controls, a normal littermate is shown in lane 1 and a recipient of MINV-transduced BM in lane 2 (MINV). WT represents mice receiving MFINV-WT transplants (lane 3) and TD represents mice receiving MFINV-TD transplants. Mice in each experiment were numbered 1 through 5; passage number was also labeled 1 through 5. Thus cells derived from the second and fifth primary MFINV-TD BMT recipients (labeled TD2 and TD5, respectively) were injected into the secondary mice denoted TD2.2, TD2.3, TD2.4, or TD5.1 and TD5.2. Cells from a secondary animal passaged into a fourth generation recipient are labeled TD2.2.1.2. Cell lines derived from animals are designated TD2.1-L and TD2.2.1.2-L. JHrearrangements of the IgH locus were detected only in the fourth generation recipient TD2.2.1.2 as well as the TD 2.2.1.2-L and TD2.1-L cell lines. Middle panel: Southern blot analysis ofPvuII-digested spleen cell genomic DNA from MFINV-TD recipients with a TCR Cβ2 probe. DNA derived from the second and fifth primary FGFR3-TD BM recipients (labeled TD2 and TD5, respectively) were injected into the secondary mice denoted TD2.2, TD2.4, TD5.1, and TD5.2. TCR gene rearrangements were detected in the TD2.2 and TD5.1 samples, both from mice displaying abnormal T-cell populations by flow cytometry. Bottom panel: Genomic DNA digested with EcoRI (which cuts MFINV-TD once) and hybridized with a neo probe was used to enumerate the proviral copy number and determine the clonality of the tumors. MINV represents vector only control mice. TD represents FGFR3-TD BMT mice. Cells from mouse TD2 or TD5 were injected into secondary and tertiary recipients denoted TD2.2, TD2.2.1, or TD5.1 and TD5.2, respectively. In both cases, a predominant clone within a diffuse background was observed in early recipients, whereas multiple bands representing unique clones emerged in later generation mice receiving serial transplants. (B) JH and neo were hybridized as above to MINV control animals and to the primary (WT6, WT1, WT2), secondary (WT1.1, WT2.1), tertiary (WT1.1.1, WT2.1.1), and quarternary mice receiving FGFR3-WT transplants (WT1.1.1.1). No emerging clonal populations were observed in any of the mice.
Genomic DNA digested with EcoRI (cuts MFINV-WT once) and hybridized with a neo probe was used to enumerate the proviral copy number and further examine the clonality of the tumors. This analysis revealed that initial FGFR3-TD recipients had a single predominant population of retrovirus-transduced cells within a more diffuse polyclonal background. With serial transplantation, further clonal bands appeared, confirming the emergence of an oligoclonal population of retrovirus-engineered cells (Figure 9A). The presence of multiple clones was confirmed by the observation that a TCRgene rearrangement was present in TD5.1 but not TD5.2 mice. Both mice received an infusion of the same tumor cells from the TD5 mouse. In FGFR3-WT recipient mice, no emerging clonality was observed (Figure9B).
Discussion
It has been reported that translocations into the IgH switch region on chromosome 14 are ubiquitous in MM.1-3Fgfr3 is one of the more common translocation partners being identified in about 25% of patients with MM.2Interestingly, in some cases the translocated fgfr3 contains activating mutations.2,4 We have previously demonstrated that ectopic in vitro expression of activated mutant FGFR3 in myeloma cells leads to IL-6 independence, enhanced IL-6–induced proliferation, and a reduction in myeloma cell apoptosis in the absence of IL-6.14 These effects correlate closely with the level of activation of FGFR3. We therefore postulated that the ectopic overexpression of FGFR3 in primary hematopoietic cells might result in B-cell malignancy, perhaps MM. To examine this issue we transplanted FGFR3-expressing BM cells into lethally irradiated recipient mice.
Our experiments established that activated mutant FGFR3 is capable of rapidly transforming hematopoietic cells, resulting in the formation of leukemia/lymphomas within 1 month after BMT. It was also established that tumors that arose in FGFR3-TD mice could be serially passaged to secondary, tertiary, and quaternary recipients. Molecular analysis suggests that tumors that originated in primary recipients were polyclonal but become oligoclonal or monoclonal with subsequent passage to syngeneic recipients.
In contrast to the results obtained with FGFR3-TD, wild-type FGFR3 did not initially lead to tumor formation in recipient mice. It has previously been shown that wild-type FGFR3 is generally not capable of transforming cells in short-term assays. For example, Li and colleagues26 demonstrated that the transmembrane region of wild-type FGFR3 is incapable of transforming NIH3T3 cells. Furthermore, expression of the cytoplasmic domain of wild-type FGFR3 as a chimeric receptor did not result in cellular transformation of NIH3T3 cells.27 The inability of wild-type FGFR3 to transform cells in such transient assays may reflect the weaker kinase activity of FGFR3 in comparison to other FGFRs and to mutant FGFR3.13 28-30 However, our in vivo data demonstrate that wild-type FGFR3 is capable of transforming hematopoietic cells but only after a period of prolonged latency. Thus other events may be required to complement transformation, for example, acquisition of activating mutations of the gene as identified in myeloma patients. Nevertheless, no activating mutations of FGFR3 in late-onset tumors were evident in the mice analyzed here.
Previous studies of activated mutant FGFR3-TD have produced conflicting reports concerning its transforming potential. It has been suggested that full-length activated mutant FGFR3-TD is not capable of transforming cells.13 However, even in the absence of ligand, NIH3T3 cells expressing activated mutant FGFR3 have a greater transformation potential than controls.26 It has also been shown that only the intracellular portion of FGFR3-TD, expressed as a chimeric receptor, is capable of transforming NIH3T3 cells, suggesting that the extracellular and transmembrane portions of FGFR3 may act as negative regulators of the kinase domain.27 Our in vivo model demonstrates unequivocally that activated mutant FGFR3 is indeed capable of transforming cells. In our BMT model, activated mutant FGFR3 expression in hematopoietic cells led to polyclonal cell expansion and outgrowth of clonal leukemia/lymphoma cells predominantly of pre-B-cell lineage.
The ability of activated mutant FGFR3 to transform cells in comparison to the latent transformation potential of wild-type FGFR3 likely relates to the activation state of the receptor. The TDII mutant form of FGFR3 has been shown to have higher intrinsic kinase activity than wild-type FGFR3.31 The activating mutation K650E (FGFR3-TD) results in the receptor being capable of autophosphorylating at a level 100-fold higher than wild-type FGFR3.13Similarly, the severity of the hereditary chondrodysplasia phenotypes resulting from FGFR3 mutations correlates with the activation state of the receptor.13 15 This suggests that the enhanced activation state (or the increased degree of kinase activity) of the FGFR3-TD receptor is responsible for the accelerated rate of transformation.
Of interest, accumulating evidence suggests that various FGFs or FGFRs may play a role in human cancer formation or progression. Many cancer cells overexpress FGFs; for example, FGF8 has been found to be overexpressed in breast cancer samples,32 whereas bFGF expression in pancreatic cancers relates to shorter postoperative survival.33 In addition to FGF overexpression in primary tumors, it has been found that FGFRs are overexpressed in several tumor cell lines, including some breast cancer34 and pancreatic cancer lines.35 Moreover, expression of a dominant negative FGFR1 in pancreatic tumor cells decreases the ability of these cells to form tumors in nude mice.36 One study examining FGF2 and FGFR expression in 9 tumor types demonstrated that at least one FGFR was expressed in 90% of the tumors analyzed.37 FGFR3 messenger RNA (mRNA) has previously been found to be expressed mainly in ovarian, non–small-cell lung carcinomas, colon, and breast cancers.37 In a study on human thyroid carcinoma it was determined that 6 of 7 papillary carcinomas expressed FGFR3.38 In vitro it was shown that overexpression of FGFR3 in a human papillary thyroid carcinoma cell line led to overgrowth of the cells, suggesting that FGFR3 may modulate cell contact recognition in these cells.38 Of particular interest, activating mutations of the gene have been found not only in MM2 but also in bladder and cervical carcinomas.39 The overexpression of FGFs and FGFRs in several types of cancers thus suggests that these molecules play a central role in the in vivo formation of many different tumors.
Our previous work on FGFR3 overexpression in MM suggests an important contribution of the receptor to the development of this disease.14 When activated mutant FGFR3 was expressed in B9 cells, an IL-6–dependent myeloma cell line, the cells became capable of proliferating in the absence of IL-6. Furthermore, a marked reduction in cellular death in the absence of IL-6 was evident and was associated with phosphorylation of STAT3 and up-regulation of bcl-xL, indicating that the receptor might be involved in both mitogenic and antiapoptotic signaling cascades. Thus, overexpression of FGFR3 in MM is likely a significant event in the formation of this malignancy, enabling the affected B cells to expand and survive independently of growth factor stimulation.
In summary, the transformation of primary B and T cells by ectopic FGFR3 expression reported here provides compelling evidence in support of the notion that translocation of fgfr3 into the IgH switch region in MM is likely to be pivotal in tumor development. Nonetheless, wild-type FGFR3 is not dominantly transforming, requiring additional cooperating oncogenic events. In particular, mutation offgfr3, subsequent to translocation into the IgH locus, would appear to have devastating consequences because all mice expressing FGFR3-TD rapidly developed tumors.
Supported by grants from the Medical Research Council of Canada, National Cancer Institute of Canada, the Nelson Arthur Hyland Foundation, and The McCarty Cancer Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
A. Keith Stewart, Hematology-Oncology, 5th Fl, Rm 126, The Princess Margaret Hospital, 610 University Ave, Toronto, Ontario M5G 2M9, Canada; e-mail: k.stewart@utoronto.ca.








This feature is available to Subscribers Only
Sign In or Create an Account Close Modal