Abstract
Aberrant methylation of multiple CpG islands has been described in acute myeloid leukemia (AML), but it is not known whether these are independent events or whether they reflect specific methylation defects in a subset of cases. To study this issue, the methylation status of 14 promoter-associated CpG islands was analyzed in 36 cases of AML previously characterized for estrogen-receptor methylation (ERM). Cases with methylation density of 10% or greater were considered positive. Seventeen cases (47%) were ERM+ while 19 cases were ERM−. Hypermethylation of any of the following,p15, p16, CACNA1G,MINT1, MINT2, MDR1,THBS1, and PTC1 (2 promoters), was relatively infrequent (6% to 31% of patients). For each of these CpG islands, the methylation density was positively correlated with ERM density (rank order correlation coefficients, 0.32-0.59; 2-tailedP ≤ .058 for each gene). Hypermethylation ofMYOD1, PITX2, GPR37, andSDC4 was frequently found in AML (47% to 64% of patients). For each of these genes as well, methylation density was positively correlated with ERM density (correlation coefficients 0.43 to 0.69, P ≤ .0087 for each gene). MLH1 was unmethylated in all cases. Hypermethylation of p15,MDR1, and SDC4 correlated with reduced levels of expression. There was an inverse correlation between age and the number of genes methylated (P = .0030). It was concluded that CpG-island methylation in AML results from methylation defects in subsets of cases. These results have potential implications for the classification and prognosis of AML and for the identification of patients who may benefit from treatment with methylation inhibitors.
Introduction
Acute myeloid leukemia (AML) is a hematopoietic malignancy that frequently exhibits nonrandom chromosomal translocations.1 Cytogenetic studies reveal a wide variety of translocations involving specific subtypes of the disease, and structurally altered genes play important roles in cell proliferation, differentiation, and gene transcription.2 However, oncogenes and tumor-suppressor genes that are frequently altered in solid tumors, such as p53 and K-RAS, are infrequently mutated in AML.3,4 In addition to these genetic changes, several studies indicate that epigenetic changes, such as aberrant DNA methylation, can also play important roles in the progression of a wide variety of human neoplasms.5,6 In particular, hypermethylation of promoter-associated CpG-rich regions, termed CpG islands, can result in gene silencing that is clonally propagated through mitosis by the action of DNA-methyltransferase enzymes. Such methylation-associated silencing plays a physiological role in X-chromosome inactivation7 and imprinting8 and a pathological role in silencing tumor-suppressor genes in neoplasia. Silencing of methylated CpG islands appears to result from the acquisition of a closed chromatin structure.9 For selected genes, allele-specific methylation suggests that this process can be an alternative mechanism to mutations or deletions in tumor-suppressor gene inactivation in human neoplasms.6
In AML, several genes, including p15,10MDR1,11ER,12 andHIC1,13 have been shown to be inactivated by methylation. Methylation patterns may have clinical implications in that ER methylation was found to be associated with improved survival.14 However, it is unclear whether suchER methylation is prognostic because of estrogen receptor function or whether it reflects a distinct pathway of tumorigenesis in AML, with distinct expression profiles of genes that could influence prognosis, such as MDR1. Indeed, it has been reported that AML cases have frequent methylation of multiple genes simultaneously.15 To study this issue further, we have analyzed the methylation status of 14 separate loci in a series of AML cases previously characterized for ER methylation. We found remarkable concordance of methylation for all the genes analyzed, suggesting the existence of distinct methylator pathways in this disease.
Methods
Samples
We selected 36 cases for this study from Southwest Oncology Group study SWOG-8600, a clinical trial for patients with previously untreated AML.16 SWOG-8600 was largely limited to patients younger than age 66 years, and selection for the present study was of course limited to those with cryopreseved samples of blood or marrow cells remaining available. In SWOG-8600, the overall complete remission rate of the 723 eligible patients younger than age 66 years on the study was 55% (58% among patients younger than 50 years).
All but one of the cases selected here had previously been studied for estrogen receptor (ER) methylation (ERM) by Southern blot analysis. The cases were selected as follows: 8 cases were selected because of high levels of ERM (greater than 90%); 8 cases were chosen on the basis of low ERM (lower than 5%); and 20 additional unselected cases were included in the study. Normal bone marrow samples were also obtained from bone marrow transplant donors. Informed consent was obtained from all patients before the specimens were collected according to institutional guidelines. Genomic DNA and RNA were extracted by means of standard procedures.
Bisulfite polymerase chain reaction
All methylation studies were done by means of bisulfite polymerase chain reaction (PCR). In this analysis, the bisulfite treatment of DNA converts all unmethylated CpG sites to UpG, leaving methylated CpGs intact. The DNA is then amplified with gene-specific primers and digested with restriction enzymes that distinguish methylated from unmethylated alleles. This approach allows for a quantitative measurement of methylation density. Bisulfite treatment of genomic DNA was done essentially as described.17 Briefly, 2 μg of genomic DNA was denatured in 0.2 N NaOH at 37°C for 10 minutes and incubated with 3 M sodium bisulfite at 50°C for 15 hours. After reaction, DNA was purified with Wizard DNA Clean-Up System (Promega, Madison, WI), and desulfonated in 0.3 N NaOH at 25°C for 5 minutes. DNA was then precipitated with ammonium acetate and ethanol, washed with 70% ethanol, and resuspended in 20 μL H2O. PCR reactions were performed in 50 μL reaction mixtures containing 2 μL bisulfite-treated DNA, 1.25 mM dNTP mixtures, 6.7 mM MgCl2, 1 nmol primers, and 1 U Taq DNA polymerase. To avoid overestimation of the methylated alleles, the following points were considered. First, primers were designed to contain a minimum number of CpG dinucleotides in the sequence to avoid the biased amplification of methylated alleles. If primers do contain CpG sites, they were designed to amplify methylated and unmethylated alleles equally (with a mixture of C or T used for sense and a mixture of G or A for antisense primers). Second, the primers were designed to contain a maximum number of thymidines converted from cytosines to avoid amplifying the nonconverted genomic sequence. Third, amplification from genomic DNA without bisulfite treatment was always carried out to monitor absence of nonspecific amplification. After amplification, 50% to 80% of the PCR products were digested with restriction enzymes as described previously18 and electrophoresed in nondenaturing polyacrylamide gels. The gels were stained with ethidium bromide, and the proportion of methylated alleles, designated the methylation density, was evaluated by densitometry. The genes selected, primer sequences, PCR condition, and restriction enzymes used are described in Table1.
Genes selected for methylation analysis in acute myeloid leukemia
| Gene . | Chromosome . | Function . | Primers (F/R) . | Annealing (temperature, no. cycles) . | Restriction enzyme . |
|---|---|---|---|---|---|
| CACNA1G | 17q21 | T-type calcium channel | GGGGGYGTTTTTTTTYGGATTTT | 58 (5), 56 (5), 54 (5), 52 (26), 30 sec | EcoRI |
| TTCCCCTACRCCCCTAAAACTTCC | |||||
| ER-alpha | 6q25 | Estrogen receptor | GGTTTTTGAGTTTTTTGTTTTG | 60 (3), 57 (4), 54 (5), 51 (25), 30 sec | TaqI |
| AACTTACTACTATCCAAATACACCTC | |||||
| GPR37 | 7q31 | G-protein–coupled receptor | GGTTAGGTGGGGTAAGAGTTT | 56, 45 sec, 35 cycles | HinfI |
| AACRTTTAATCCAATTACAAACC | |||||
| MDR1 | 7q21 | Drug efflux | GTTATAGGAAGTTTGAGTTT | 54 (3), 51 (4), 48 (5), 45 (26), 30 sec | TaqI |
| AAAAACTATCCCATAATAAC | |||||
| MINT1 | 5q13 | CpG island hypermethylated in cancer | GGGTTGGAGAGTAGGGGAGTT | 55 (35) 30 sec | TaqI |
| CCATCTAAAATTACCTCRATAACTTA | |||||
| MINT2 | 2p22 | CpG island hypermethylated in cancer | YGTTATGATTTTTTTGTTTAGTTAAT | 60 (3), 58 (4), 56 (5), 54 (26) | TaqI |
| TACACCAACTACCCAACTACCTC | |||||
| MLH1 | 3p21 | Mismatch repair | TAGTAGTYGTTTTAGGGAGGGA | 53 (35) 30 sec | RsaI |
| TCTAAATACTCAACRAAAATACCTT | |||||
| MyoD | 11p15 | Muscle-specific transcription factor | TGTTGGAGAGGTTTGGAAAGG | 53, 30 sec, 35 cycles | RsaI |
| AAAATCCRAAACCAATAAAAACAC | |||||
| p15 | 9p21 | Cyclin-dependent kinase inhibitor | GGAGTTTAAGGGGGTGGG | 50, 30 sec, 35 cycles | NruI |
| CCTAAATTACTTCTAAAAAAAAAC | |||||
| p16 | 9p21 | Cyclin-dependent kinase inhibitor | GGTTTTGGYGAGGGTTGTTT | 58 (5), 56 (5), 54 (5), 52 (26), 30 sec | TaqI |
| ACCCTATCCCTCAAATCCTCTAAAA | |||||
| PITX2 | 4q25 | Homeotic gene | TAAGTGTTTYGTTAGGTTTTTT | 50, 30 sec, 35 cycles | TaqI |
| CCAAACTCCACTACACAATAAC | |||||
| PTC-A | 9q22 | WNT signaling | GTTGGTTTGTTAATYGGAGT | 58 (5), 56 (5), 54 (5), 52 (26), 30 sec | TaqI |
| TTACCAACCRAAACCATATT | |||||
| PTC-B | 9q22 | WNT signaling | 5-AATGTGTGGAATTTAGGGA | 54, 45 sec, 35 cycles | HgaI |
| TAAAACAACCAACTACAACTTAC | |||||
| SDC4 | 20q12 | Cell surface heparan sulfate proteoglycan | GGGGATTTGTTTGGTAGTGG | 57, 45 sec, 35 cycles | HinfI |
| CCCGAAATTCCCCAATAAA | |||||
| THBS1 | 15q15 | Angiogenesis inhibitor | GGAGAGAGGAGTTTAGATTGGTT | 54, 45 sec, 38 cycles | BstUI |
| AAATAAAAATTACTCCTAAAAAAC |
| Gene . | Chromosome . | Function . | Primers (F/R) . | Annealing (temperature, no. cycles) . | Restriction enzyme . |
|---|---|---|---|---|---|
| CACNA1G | 17q21 | T-type calcium channel | GGGGGYGTTTTTTTTYGGATTTT | 58 (5), 56 (5), 54 (5), 52 (26), 30 sec | EcoRI |
| TTCCCCTACRCCCCTAAAACTTCC | |||||
| ER-alpha | 6q25 | Estrogen receptor | GGTTTTTGAGTTTTTTGTTTTG | 60 (3), 57 (4), 54 (5), 51 (25), 30 sec | TaqI |
| AACTTACTACTATCCAAATACACCTC | |||||
| GPR37 | 7q31 | G-protein–coupled receptor | GGTTAGGTGGGGTAAGAGTTT | 56, 45 sec, 35 cycles | HinfI |
| AACRTTTAATCCAATTACAAACC | |||||
| MDR1 | 7q21 | Drug efflux | GTTATAGGAAGTTTGAGTTT | 54 (3), 51 (4), 48 (5), 45 (26), 30 sec | TaqI |
| AAAAACTATCCCATAATAAC | |||||
| MINT1 | 5q13 | CpG island hypermethylated in cancer | GGGTTGGAGAGTAGGGGAGTT | 55 (35) 30 sec | TaqI |
| CCATCTAAAATTACCTCRATAACTTA | |||||
| MINT2 | 2p22 | CpG island hypermethylated in cancer | YGTTATGATTTTTTTGTTTAGTTAAT | 60 (3), 58 (4), 56 (5), 54 (26) | TaqI |
| TACACCAACTACCCAACTACCTC | |||||
| MLH1 | 3p21 | Mismatch repair | TAGTAGTYGTTTTAGGGAGGGA | 53 (35) 30 sec | RsaI |
| TCTAAATACTCAACRAAAATACCTT | |||||
| MyoD | 11p15 | Muscle-specific transcription factor | TGTTGGAGAGGTTTGGAAAGG | 53, 30 sec, 35 cycles | RsaI |
| AAAATCCRAAACCAATAAAAACAC | |||||
| p15 | 9p21 | Cyclin-dependent kinase inhibitor | GGAGTTTAAGGGGGTGGG | 50, 30 sec, 35 cycles | NruI |
| CCTAAATTACTTCTAAAAAAAAAC | |||||
| p16 | 9p21 | Cyclin-dependent kinase inhibitor | GGTTTTGGYGAGGGTTGTTT | 58 (5), 56 (5), 54 (5), 52 (26), 30 sec | TaqI |
| ACCCTATCCCTCAAATCCTCTAAAA | |||||
| PITX2 | 4q25 | Homeotic gene | TAAGTGTTTYGTTAGGTTTTTT | 50, 30 sec, 35 cycles | TaqI |
| CCAAACTCCACTACACAATAAC | |||||
| PTC-A | 9q22 | WNT signaling | GTTGGTTTGTTAATYGGAGT | 58 (5), 56 (5), 54 (5), 52 (26), 30 sec | TaqI |
| TTACCAACCRAAACCATATT | |||||
| PTC-B | 9q22 | WNT signaling | 5-AATGTGTGGAATTTAGGGA | 54, 45 sec, 35 cycles | HgaI |
| TAAAACAACCAACTACAACTTAC | |||||
| SDC4 | 20q12 | Cell surface heparan sulfate proteoglycan | GGGGATTTGTTTGGTAGTGG | 57, 45 sec, 35 cycles | HinfI |
| CCCGAAATTCCCCAATAAA | |||||
| THBS1 | 15q15 | Angiogenesis inhibitor | GGAGAGAGGAGTTTAGATTGGTT | 54, 45 sec, 38 cycles | BstUI |
| AAATAAAAATTACTCCTAAAAAAC |
Reverse transcriptase PCR
We used 6 μg of total RNA to synthesize complementary DNA (cDNA) using Superscript II reverse transcriptase (Life Technologies, Rockville, MD) according to the manufacturer's protocol. To amplify cDNA, PCR was performed in a 50-μL reaction mixture containing 1 μL cDNA, 1 × PCR buffer (Life Technologies), 1.25 mM dNTP, 1.5 mM MgCl2, primers, and 1 U Taq polymerase (Life Technologies). Sequences of the PCR primers used are as follows. p15F: 5′-TGGGGGCGGCAGCGATGAG-3; p15R: 5′-AGGTGGGTGGGGGTGGGAAAT-3′; SDC4F: 5-CCTCTAGATAACCATATCCCTGAGA-3′; SDC4R: 5′-CCTAATGTCCACCCTTCAAAAT-3′. To verify the integrity of messenger RNA (mRNA), the GAPDH gene was amplified by means of the following primers: GAPDHF: 5′-CGGAGTCAACGGATTGGTCGTAT-3′ and GAPDHR 5′-AGCCTTCTCCATGGTGGTGAAGAC-3′. After PCR, 10 μL of the samples were electrophoresed in 1.5% agarose gels and visualized by ethidium bromide staining. All reactions included negative controls where reverse transcriptase was omitted.
Statistical considerations
For each gene analyzed, the methylation frequency was defined as the proportion of subjects in whom the methylation density equaled or exceeded 10%; the selection of this criterion is described in the “Results” section. Since the distributions of methylation densities were unlikely to be Gaussian, correlation among methylation densities of the various genes was measured by means of Spearman rank order correlation coefficient (RS). Associations between quantitative clinicopathological characteristics and the number of methylated genes were examined by means of ordinary least squares regression analysis.
Results
Samples and genes analyzed
The 36 selected AML cases (17 females, 19 males) ranged in age from 19 to 69 years (median, 39 years). All but 1 had previously been characterized for ERM by Southern blot.14 To allow the analysis of multiple genes in each sample, we used a bisulfite-PCR approach that requires considerably less DNA than Southern blotting. We first determined the methylation status ofER using bisulfite-PCR. Of 36 cases analyzed, 20 (56%; 95% confidence interval, 38%-72%) showed methylation of ER, ie, methylation density of 2% or greater. Among these 20, theER methylation density ranged from 8% to 61% (median, 44%). There was a strong correlation between ER methylation density as determined by bisulfite-PCR and by Southern blot analysis (RS = 0.75, P < .0001). However, the values obtained by bisulfite-PCR were generally lower than those previously determined by Southern blot analysis. This most likely relates to a lower sensitivity of bisulfite-PCR because the reaction requires 2 adjacent CpGs to be simultaneously methylated, while Southern blot analysis requires 1 of 2 CpGs to be methylated. In addition, the sites analyzed by PCR are slightly different from those analyzed by Southern blot, which probably partly explains the lack of a perfect correlation between the 2 determinations.
Methylation of multiple CpG Islands in AML
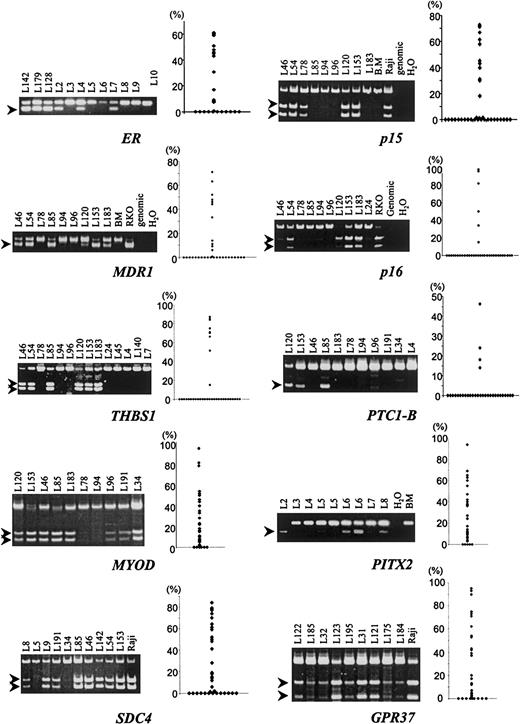
To examine whether ERM reflected genome-wide aberrant CpG-island methylation, we studied the methylation status of 14 additional loci (Table 1; examples in Figure1). In normal bone marrow, no significant (2% or greater) methylation was observed in any of the genes analyzed (Figure 1 and data not shown). The proportion of patients with significant methylation of individual CpG islands varied from 0 of 36 (0%) of the cases for MLH1 to 31 of 36 (86%) forPITX2 (Table 2). Of the 14 additional CpG islands analyzed, MLH1 was unmethylated in all samples; 9 CpG islands (CACNA1G, MINT1,MINT2, p16INK4A, THBS1,p15INK4B, PTC1A, PTC1B, and MDR1) were methylated relatively infrequently (range: 6% to 36% of patients); and 4 CpG islands (MYOD, SDC4, GRP37, and PITX2) were methylated relatively frequently (range: 61% to 86% of patients). The pairwise correlations between methylation densities of the genes examined (including ER, but omitting MLH1) were measured by means of RS. All of the correlation coefficients were positive, ranging from 0.04 to 0.83, suggesting that methylation affects multiple genes in a subset of the cases. For example,ER methylation correlated positively with methylation of all other genes (R = 0.53, P = .0008, for p15; R = 0.34, P = .04, for p16; R = 0.40,P = .017, for THBS1; R = 0.55,P = .0005, for PTC1A; R = 0.36,P = .031, for PTC1B; R = 0.59,P = .0002, for MDR1; R = 0.38,P = .023, for CACNA1G; R = 0.32,P = .058, for MINT1; R = 0.36,P = .030, for MINT2; R = 0.43,P = .0087, for SDC4; R = 0.69,P = .0001, for GPR37; R = 0.57,P = .0003, for MYOD; and R = 0.56,P = .0004, for PITX2).
Examples and summary of methylation analysis of multiple CpG islands in AML.
Aberrant methylation was detected by bisulfite-PCR (left of each panel) and quantitated by densitometry, and a scatter plot of all the data in 36 patients is shown on the right of each panel. The genes examined are shown below each panel. After bisulfite modification, DNA was amplified by means of primers that amplify methylated and unmethylated alleles equally. PCR products were then digested with the use of restriction enzymes that cleave methylated alleles only. Methylation bands are indicated by arrows.
Examples and summary of methylation analysis of multiple CpG islands in AML.
Aberrant methylation was detected by bisulfite-PCR (left of each panel) and quantitated by densitometry, and a scatter plot of all the data in 36 patients is shown on the right of each panel. The genes examined are shown below each panel. After bisulfite modification, DNA was amplified by means of primers that amplify methylated and unmethylated alleles equally. PCR products were then digested with the use of restriction enzymes that cleave methylated alleles only. Methylation bands are indicated by arrows.
Distribution of methylation densities for CpG islands of 15 selected genes among 36 acute myeloid leukemia patients
| Gene . | Methylation density . | |||||
|---|---|---|---|---|---|---|
| Below 2% . | 2% to 9.9% . | At least 10% . | ||||
| No. . | % . | No. . | % . | No. . | % . | |
| Infrequently methylated genes | ||||||
| MLH1 | 36 | 100% | 0 | 0 | ||
| CACNA1G | 33 | 92% | 0 | 3 | 8% | |
| MINT2 | 34 | 94% | 0 | 2 | 6% | |
| MINT1 | 29 | 81% | 1 | 3% | 6 | 17% |
| THBS1 | 27 | 75% | 0 | 9 | 25% | |
| p16 | 30 | 83% | 0 | 6 | 17% | |
| MDR1 | 23 | 64% | 2 | 6% | 11 | 31% |
| PTC1A | 30 | 83% | 0 | 6 | 17% | |
| PTC1B | 32 | 89% | 0 | 4 | 11% | |
| p15 | 25 | 69% | 0 | 11 | 31% | |
| Frequently methylated genes | ||||||
| MyoD | 8 | 22% | 6 | 17% | 22 | 61% |
| SDC4 | 14 | 39% | 2 | 6% | 20 | 56% |
| GPR37 | 10 | 28% | 9 | 25% | 17 | 47% |
| PITX2 | 5 | 14% | 8 | 22% | 23 | 64% |
| ER (PCR) | 16 | 44% | 3 | 8% | 17 | 47% |
| Gene . | Methylation density . | |||||
|---|---|---|---|---|---|---|
| Below 2% . | 2% to 9.9% . | At least 10% . | ||||
| No. . | % . | No. . | % . | No. . | % . | |
| Infrequently methylated genes | ||||||
| MLH1 | 36 | 100% | 0 | 0 | ||
| CACNA1G | 33 | 92% | 0 | 3 | 8% | |
| MINT2 | 34 | 94% | 0 | 2 | 6% | |
| MINT1 | 29 | 81% | 1 | 3% | 6 | 17% |
| THBS1 | 27 | 75% | 0 | 9 | 25% | |
| p16 | 30 | 83% | 0 | 6 | 17% | |
| MDR1 | 23 | 64% | 2 | 6% | 11 | 31% |
| PTC1A | 30 | 83% | 0 | 6 | 17% | |
| PTC1B | 32 | 89% | 0 | 4 | 11% | |
| p15 | 25 | 69% | 0 | 11 | 31% | |
| Frequently methylated genes | ||||||
| MyoD | 8 | 22% | 6 | 17% | 22 | 61% |
| SDC4 | 14 | 39% | 2 | 6% | 20 | 56% |
| GPR37 | 10 | 28% | 9 | 25% | 17 | 47% |
| PITX2 | 5 | 14% | 8 | 22% | 23 | 64% |
| ER (PCR) | 16 | 44% | 3 | 8% | 17 | 47% |
Hypermethylator phenotypes in AML
The strong concordance between different methylation events described above suggested the presence of hypermethylator phenotypes in AML, as described in colon cancer. In order to begin defining a classification of AML based on methylation profiling, we grouped the cases according to the number of loci methylated in each case, using a threshold of 10% or greater for all loci. The use of a threshold is suggested by the fact that, in colon cancer, the presence of a hypermethylator phenotype is characterized by both more frequent methylation and more dense methylation.19 As shown in Table 2, the infrequently methylated loci (ie, the first 9 loci) seldom had methylation densities of 2% to 9.9%. Such intermediate densities were much more common among the 4 frequently methylated genes. Overall, only 36 of 540 methylation densities were between 2% and 10% and are therefore affected by the use of a threshold.
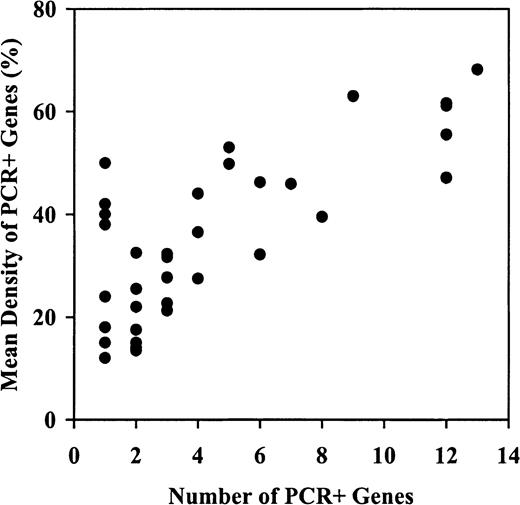
Using the 10% threshold, we found that the proportion of methylated genes ranged from 0 of 15 loci to 13 of 15 loci. As Table3 shows, 16 patients (44%) had methylation of fewer than 3 genes (including 1 patient with none), while 13 (36%) and 7 (19%) had methylation of 3 to 7 and 8 to 13 genes, respectively. Moreover, the mean methylation density of the methylated genes tended to increase with the number of methylated genes, from a median of 22% in the 15 patients with 1 or 2 methylated genes, to 61% in the 7 patients with 8 to 13 methylated genes (Table3, Figure 2). These data suggest the presence of a hypermethylator phenotype in AML that affects both methylation frequency and density.
Methylation of multiple CpG islands in acute myeloid leukemia
| No. PCR+ genes3-150 . | No. patients . | Mean methylation density (%) of all 14 genes . | Mean methylation density (%) of PCR+ genes3-150 . | ||
|---|---|---|---|---|---|
| Median . | Range . | Median . | Range . | ||
| 0-2 | 16 | 3.1 | 0.3-5.2 | 22.0 | 12.0-50.0 |
| 3-7 | 13 | 10.4 | 5.1-22.9 | 32.3 | 21.3-53.0 |
| 8-13 | 7 | 47.6 | 22.6-63.3 | 61.1 | 39.5-68.2 |
| No. PCR+ genes3-150 . | No. patients . | Mean methylation density (%) of all 14 genes . | Mean methylation density (%) of PCR+ genes3-150 . | ||
|---|---|---|---|---|---|
| Median . | Range . | Median . | Range . | ||
| 0-2 | 16 | 3.1 | 0.3-5.2 | 22.0 | 12.0-50.0 |
| 3-7 | 13 | 10.4 | 5.1-22.9 | 32.3 | 21.3-53.0 |
| 8-13 | 7 | 47.6 | 22.6-63.3 | 61.1 | 39.5-68.2 |
PCR indicates polymerase chain reaction.
PCR+ is defined as methylation density of at least 10%.
Hypermethylator phenotypes affect both the frequency and density of methylation in AML.
Shown is the mean methylation density of PCR+ genes (ie, genes defined as having methylation of 10% or greater) plotted against the number of PCR+ genes in a particular patient. The mean methylation density of the methylated genes progressively increases as the number of genes methylated increases, suggesting that both methylation frequency and density are affected in these patients.
Hypermethylator phenotypes affect both the frequency and density of methylation in AML.
Shown is the mean methylation density of PCR+ genes (ie, genes defined as having methylation of 10% or greater) plotted against the number of PCR+ genes in a particular patient. The mean methylation density of the methylated genes progressively increases as the number of genes methylated increases, suggesting that both methylation frequency and density are affected in these patients.
This analysis raised the possibility that the correlations between the methylation of ER and the 13 additional loci could have been due largely to the group of 7 cases with particularly dense and frequent methylation. To address this issue, we repeated the correlation analyses excluding these 7 cases. Of the remaining 29 cases, none were methylated at CACNA1G, MINT2,PTC1-A, or PTC1-B. However, there were still at least marginally statistically significant correlations between methylation densities of ER and each of the following:p15 (RS = 0.42, P = .023);MDR1 (RS = 0.40, P = .030);PITX2 (RS = 0.33, P = .083);GPR37 (RS = 0.58, P = .0010); andMyoD (RS = 0.38, P = .045).
Clinicopathological features of hypermethylation in AML
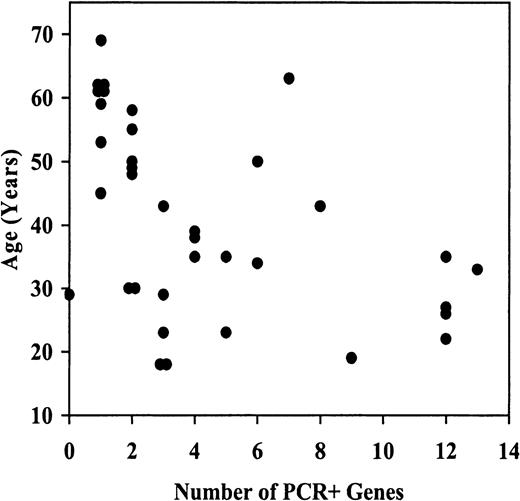
The clinical features of all 36 cases grouped into 3 methylation categories are shown in Table4. Age, white blood count (WBC), absolute peripheral blast count, MRK16 expression, and cyclosporin A (CsA)–inhibited DiOC2 efflux appear to decrease with increasing methylation, while marrow blast percentage tends to increase with increasing methylation. However regression analysis of each variable in relation to the number of genes PCR+ for methylation indicates that the association between age and methylation is statistically significant (2-tailed P = .0040) (Figure3), with older patients tending to have fewer methylated genes. None of the other patient or disease characteristics are significantly associated with methylation. However, in view of the small sample size, the absence of significant associations should be viewed as inconclusive. The inverse correlation between age and methylation is consistent with our prior study ofER methylation.14 Centrally reviewed karyotypes were available for only 7 of these patients and did not reveal clustering of abnormalities in specific groups. The number of cases studied is too low to allow meaningful correlations with outcome. Nevertheless, it is interesting to note that the group of 7 cases with high levels of methylation had a strikingly poor complete response rate after induction chemotherapy (29%, compared with 62% and 63% in the other groups), despite the relatively young age of the patients (median, 27 years). Definitive estimation of correlations between methylation category and therapeutic results will have to await larger studies.
Characteristics of 36 acute myeloid leukemia patients, by number of genes that are polymerase chain reaction-positive for methylation
| . | 0-2 PCR+ genes (N = 16) . | 3-7 PCR+ genes (N = 13) . | 8-13 PCR+ genes (N = 7) . | All patients (N = 36) . | ||||
|---|---|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| Gender | ||||||||
| Female | 8 | 50% | 6 | 46% | 3 | 43% | 17 | 47% |
| FAB | ||||||||
| M1 | 4 | 25% | 2 | 15% | 3 | 43% | 9 | 25% |
| M2 | 5 | 31% | 7 | 54% | 3 | 43% | 15 | 42% |
| M3 | 2 | 13% | 1 | 8% | 0 | 4 | 11% | |
| M4, M5 | 5 | 32% | 2 | 16% | 0 | 6 | 17% | |
| M0/AML, not otherwise specified | 0 | 1 | 8% | 1 | 14% | 2 | 6% | |
| MDR1, based on MRK164-150,4-151 | ||||||||
| Negative | 7 | 64% | 6 | 55% | 7 | 100% | 20 | 69% |
| Dim | 1 | 9% | 3 | 27% | 0 | 4 | 14% | |
| Moderate/bright | 3 | 27% | 2 | 18% | 0 | 5 | 17% | |
| Unknown | 5 | — | 2 | — | 0 | — | 7 | — |
| CsA-inhibited DiOC2efflux4-150,‡ | ||||||||
| Negative | 7 | 58% | 7 | 58% | 7 | 100% | 21 | 68% |
| Dim | 1 | 8% | 0 | 0 | 1 | 3% | ||
| Moderate/bright | 4 | 33% | 5 | 42% | 0 | 9 | 29% | |
| Unknown | 4 | — | 1 | — | 0 | — | 5 | — |
| Age, y, median (range) | 55 (29-69) | NA | 35 (18-63) | NA | 27 (19-43) | NA | 39 | NA |
| Marrow blasts, %, median (range) | 83 (25-98) | NA | 81 (13-97) | NA | 95 (8-97) | NA | 87 | NA |
| WBC, 1 × 109/L (1000 μL), median (range) | 54.3 (1-270) | NA | 38.3 (6-308) | NA | 19.7 (2-103) | NA | 37.7 | NA |
| Hemoglobin, g/dL, | 9.3 (7-14) | NA | 10.0 (6-12) | NA | 8.2 (6-15) | NA | 9.1 | NA |
| Platelets, 1000 μL, median (range) | 41 (3-120) | NA | 43 (10-141) | NA | 56 (24-167) | NA | 43 | NA |
| . | 0-2 PCR+ genes (N = 16) . | 3-7 PCR+ genes (N = 13) . | 8-13 PCR+ genes (N = 7) . | All patients (N = 36) . | ||||
|---|---|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| Gender | ||||||||
| Female | 8 | 50% | 6 | 46% | 3 | 43% | 17 | 47% |
| FAB | ||||||||
| M1 | 4 | 25% | 2 | 15% | 3 | 43% | 9 | 25% |
| M2 | 5 | 31% | 7 | 54% | 3 | 43% | 15 | 42% |
| M3 | 2 | 13% | 1 | 8% | 0 | 4 | 11% | |
| M4, M5 | 5 | 32% | 2 | 16% | 0 | 6 | 17% | |
| M0/AML, not otherwise specified | 0 | 1 | 8% | 1 | 14% | 2 | 6% | |
| MDR1, based on MRK164-150,4-151 | ||||||||
| Negative | 7 | 64% | 6 | 55% | 7 | 100% | 20 | 69% |
| Dim | 1 | 9% | 3 | 27% | 0 | 4 | 14% | |
| Moderate/bright | 3 | 27% | 2 | 18% | 0 | 5 | 17% | |
| Unknown | 5 | — | 2 | — | 0 | — | 7 | — |
| CsA-inhibited DiOC2efflux4-150,‡ | ||||||||
| Negative | 7 | 58% | 7 | 58% | 7 | 100% | 21 | 68% |
| Dim | 1 | 8% | 0 | 0 | 1 | 3% | ||
| Moderate/bright | 4 | 33% | 5 | 42% | 0 | 9 | 29% | |
| Unknown | 4 | — | 1 | — | 0 | — | 5 | — |
| Age, y, median (range) | 55 (29-69) | NA | 35 (18-63) | NA | 27 (19-43) | NA | 39 | NA |
| Marrow blasts, %, median (range) | 83 (25-98) | NA | 81 (13-97) | NA | 95 (8-97) | NA | 87 | NA |
| WBC, 1 × 109/L (1000 μL), median (range) | 54.3 (1-270) | NA | 38.3 (6-308) | NA | 19.7 (2-103) | NA | 37.7 | NA |
| Hemoglobin, g/dL, | 9.3 (7-14) | NA | 10.0 (6-12) | NA | 8.2 (6-15) | NA | 9.1 | NA |
| Platelets, 1000 μL, median (range) | 41 (3-120) | NA | 43 (10-141) | NA | 56 (24-167) | NA | 43 | NA |
PCR indicates polymerase chain reaction; FAB, French-American-British; CsA, ; DiOC2WBC, white blood count.
Categories based on Kolmogorov-Smirnov D-value as defined in Leith et al.36
MRK16: negative D < 0.15, dim 0.15 ≤ D < 0.20, moderate 0.20 ≤ D < 0.30, bright D ≥ 0.30.
Efflux: negative D < 0.20, dim 0.20 ≤ D < 0.25, moderate 0.25 ≤ D < 0.40, bright D ≥ 0.40.
Inverse correlation between age and CpG-island methylation in AML.
For each patient studied, the age at diagnosis vs the number of genes PCR+ is plotted for methylation genes.
Inverse correlation between age and CpG-island methylation in AML.
For each patient studied, the age at diagnosis vs the number of genes PCR+ is plotted for methylation genes.
Correlation between hypermethylation and differential expression of multiple genes
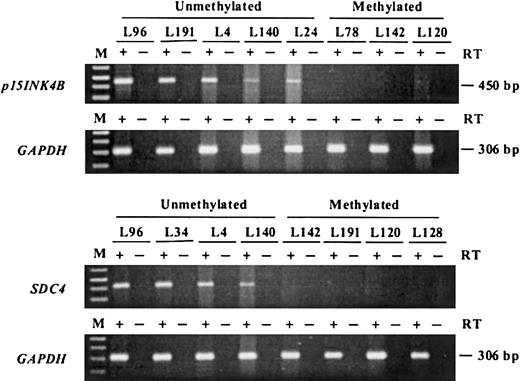
To study whether the aberrant methylation detected in AML actually correlates with gene silencing, the expression status ofp15INK4B and SDC4 was determined by reverse transcriptase (RT) PCR for 15 cases with or without methylation of these loci. Previous data had demonstrated differential ERgene expression in these same cases.14 Representative results for p15 and SDC4 are shown in Figure4. The tumors that showed aberrant methylation of p15 did not express mRNA or expressed significantly lower levels compared with unmethylated cases. Similarly,SDC4 was expressed weakly or not at all in methylated cases. Expression of MDR1 was previously determined for 29 of the 36 cases by various methods, including immunohistochemistry and functional analyses.20 MRK16 expression tended to be lower among patients with high levels of MDR1 methylation. The 5 patients with MDR1 methylation densities greater than 40% were all MRK16−, ie, had D-values lower than 0.15. Similarly, of the 5 patients with moderate or bright MRK16 expression (D greater than 0.20), 4 were PCR− for MDR1methylation. However there were discrepant cases. Of 17 patients with no MDR1 methylation (0% methylation density), 10 were MRK16−, suggesting that mechanisms other than methylation may prevent MRK16 expression. Also 1 patient with 33% MDR1methylation density expressed MRK16 at a high level (D = 0.50), perhaps reflecting tumor heterogeneity in this patient.
Expression analysis of p15 andSDC4 by RT-PCR.
Complementary DNA was amplified by means of gene-specific primers and electrophoresed in 1.5% agarose gels; p15 mRNA was detectable in all the unmethylated samples but none of the methylated samples. Similar results were obtained for SDC4. The methylation status of each sample is shown above each lane. The integrity of RNA was verified by RT-PCR by means of primers specific for GAPDH. M is the marker lane. + indicates lanes where reverse transcriptase was added while − indicates lanes where reverse transcriptase was omitted (negative controls).
Expression analysis of p15 andSDC4 by RT-PCR.
Complementary DNA was amplified by means of gene-specific primers and electrophoresed in 1.5% agarose gels; p15 mRNA was detectable in all the unmethylated samples but none of the methylated samples. Similar results were obtained for SDC4. The methylation status of each sample is shown above each lane. The integrity of RNA was verified by RT-PCR by means of primers specific for GAPDH. M is the marker lane. + indicates lanes where reverse transcriptase was added while − indicates lanes where reverse transcriptase was omitted (negative controls).
Discussion
In the present study, we examined the methylation status of multiple CpG islands in a series of AML patients with or without methylation of ER. Our results indicate that ERmethylation occurs concomitantly with aberrant methylation of other loci. On the basis of the number of genes methylated, AML cases can be divided into 3 groups that differ substantially in the frequency, nature, and extent (density) of hypermethylation events. These results suggest that CpG-island methylation is related to specific methylation defects in subsets of AMLs, rather than that methylation of each individual island represents a random event followed by selection for the affected cell.
It should be emphasized that this study was based on a group of 36 previously untreated AML patients with comparatively young ages (median 39 years, with only 1 patient over 65 years old) and high WBCs (median 37.7 × 109/L [37 700/μL]). These selection factors may affect the generalizability of these results. Moreover, since these patients were selected in part on the basis of their ERM results, their distribution of methylation density and frequency may not be representative of the more general population of AML patients. In addition, the number of patients studied here did not allow us to make definitive correlations between methylation and clinico-pathologic correlates. These issues must now be addressed in studies that include larger numbers of patients.
Several studies now point to the existence of hypermethylator phenotypes in various human neoplasms. In colorectal cancer,21 gastric cancer,22 and pancreatic cancer,23 a profound hypermethylator phenotype termed CpG-island methylator phenotype was described in subsets of cases, and was shown to involve such genes as p16INK4A,MLH1, and THBS1. Apparently similar methylator phenotypes were described in breast,24 brain, bladder, and prostate cancers.25 Preliminary data suggest the presence of such hypermethylator phenotypes in hepatocellular carcinomas and acute lymphoblastic leukemia. Overall, then, hypermethylator phenotypes have been observed in most of the major types of human cancers. Because hypermethylation frequently correlates with differential gene expression compared with unmethylated tumors (and normal tissues), these data point to the existence of subsets of cancers with markedly different expression profiles.
In AML, the different methylation categories appear to identify clinically distinct groups. The inverse correlation between methylation and age is particularly interesting because much previous work has indicated that, in epithelial cells, CpG-island methylation actually increases with age.26 One possible explanation for this finding is that methylation reflects specific carcinogenic insults that lead to neoplastic transformation. Indeed, previous studies have suggested different methylation profiles in experimental lung tumors induced by various carcinogens,27 and preliminary studies suggest correlations between CpG-island methylation and specific carcinogenic exposures in hepatocellular carcinomas. If this hypothesis is correct, it would suggest that AML in the elderly is a substantially different disease from AML in young people; this is supported by observations of vastly different cytogenetic changes and clinical courses in these patients. Our results suggest then that methylation could be useful in the further molecular classification of AML in the hope of identifying clinically distinct subgroups.
The cause of hypermethylation in AML remains unclear. One proposed mechanism of this is up-regulation of DNA methyltransferase. Melki et al28 reported an elevated level of expression of DNA methyltransferase in acute leukemias, but were unable to relate it to degrees of aberrant methylation.15 These results were consistent with other studies that showed aberrant methyltransferase activity in various types of tumors,6 but little evidence of correlation between either mRNA levels or enzyme activity and CpG island methylation.29 Other factors, such as loss of trans-acting factors protecting CpG island,30 aberrant recruitment of DNA-methyltransferases to CpG islands,31 or alterations in demethylase gene/activity,32 may also participate in aberrant CpG-island methylation. Finally, as mentioned above, hypermethylation may reflect specific carcinogenic insults. Of special relevance to AML, radiation exposures have been associated with high rates of ER methylation in rodent models of lung cancer.27
Given that CpG-island methylation is deregulated globally in subsets of AML, it becomes difficult to assign functional significance to each of these events. Indeed, while it has been argued that hypermethylation in cancer suggests a tumor-suppressor role for the affected genes,10 many genes affected by hypermethylation in AML are not expressed in hematopoietic cells, and even genes with clear-cut oncogene function have been shown to be hypermethylated in selected cases. Thus, it is probably more accurate to think of hypermethylation in neoplasia as a deregulated process akin to microsatellite instability, whereby many loci are affected but only a few have truly functional significance. It is also worth noting that, although the frequency of methylation of SDC4, GPR37,PITX2, and MYOD was not different in ERM+ and ERM− AML cases, the density of methylation in the 2 groups were different. These results indicate that the hypermethylator phenotype affects both frequency and density of methylation (Figure 2). Density of methylation is in fact a critical feature of the extent of gene silencing in cancer.33
One gene that may have functional significance in AML isp15/INK4B. AML is one of the few neoplasms that show methylation of p15/INK4B.10 The p15/INK4B gene plays an important role in transforming growth factor β (TGF-β)–induced growth arrest,34 and abrogation of TGFβ-signaling pathway in hematopoietic tumor cell lines reverses the growth-inhibitory effect ofTGF-β.35 However, in the absence of clear-cut evidence of mutational inactivation of p15/INK4Bin some cases, it remains difficult to assign a definitive tumor-suppressor role for this gene. In our study, p15/INK4Bmethylation frequency was relatively lower than had been previously reported. This difference may be partly caused by the relative overrepresentation of AML cases with low global levels of methylation. Also, we have examined the methylation status of CpG sites close to the transcription start site; these sites appear to be well correlated with expression status, but may have lower levels of methylation in AML. Another interesting hypermethylated gene in AML is MDR1. In this study, MDR1 functional expression was absent in the patients with high levels of methylation. However, several cases did not express MDR1 despite the absence of methylation. One possible explanation for this is that MDR1 is an inducible gene that is not uniformly expressed in the absence of noxious stimuli. Thus, it would be interesting to determine whether methylation correlates better with MDR1 expression after chemotherapy than at diagnosis.
Supported by a Translational Research Grant from the Leukemia and Lymphoma Society of America, a Research Grant from the American Cancer Society, and National Institutes of Health grants CA-38926 and CA-32102 to the Southwest Oncology Group. M.T. is a postdoctoral fellow of the Japan Society for the Promotion of Science.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jean-Pierre Issa, The MD Anderson Cancer Center, Box 428, 1515 Holcombe Blvd, Houston, TX 77030; e-mail:jpissa@mdanderson.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal