Abstract
Previously it was shown that the Ets proteins, PU.1 and Spi-B, exhibit functional redundancy in B lymphocytes. To investigate the possibility that PU.1 or Spi-B or both share overlapping roles with Ets-1 or Elf-1, PU.1+/−Ets-1−/−, PU.1+/−Elf-1−/−, and Spi-B−/−Ets-1−/− animals were generated. No blood cell defects were observed in these animals except those previously reported for Ets-1−/− mice. Therefore, no genetic overlap was detected between PU.1 or Spi-B with Ets-1 or Elf-1. In contrast, the results confirmed functional redundancy for PU.1 and Spi-B in that PU.1+/−Spi-B−/− bone marrow progenitors yielded smaller colonies in methylcellulose cultures than did wild-type, PU.1+/− or Spi-B−/−progenitors. In addition, PU.1+/−Spi-B+/+, PU.1+/−Spi-B+/−, and PU.1+/− Spi-B−/− mice displayed extramedullary splenic hematopoiesis. In summary, PU.1 and Spi-B regulate common target genes required for proliferation of hematopoietic progenitors or their committed descendants, whereas Ets-1 or Elf-1 do not appear to regulate shared target genes with PU.1 or Spi-B.
Introduction
A number of Ets transcription factors, including Ets-1, PU.1 (Spi-1), Spi-B, and Elf-1, are detected in the hematopoietic system where they exhibit overlapping expression patterns.1 We have focused this study on understanding overlapping roles of these Ets family members, which are expressed at defined stages of B- and T-lymphocyte development. Expression of PU.1 is confined to hematopoietic cells including bone marrow and fetal liver progenitors, monocytes, neutrophils, and B cells.1-5PU.1−/− animals have been generated by 2 groups and show similar, but not identical, phenotypes.6 7 Mice harboring both PU.1 alleles lack lymphoid or myeloid cells during the fetal liver stage of hematopoiesis indicating that PU.1 is critical for the development of these lineages. The PU.1−/− allele used in our studies results in embryonic lethality at E16.5 to E18.5 precluding an analysis of hematopoietic cells in adult animals.
In contrast to PU.1, Spi-B expression is limited to lymphoid cells.1,6,8,9 Unlike PU.1−/− mice, Spi-B−/− mice are viable and exhibit normal numbers of all blood cell lineages.10 However, Spi-B−/−B cells are defective in their responses to B cell receptor (BCR)–mediated signals. In particular, these cells fail to produce normal titers of IgG antibodies in response to T-dependent antigens and form weak germinal centers characterized by high levels of B-cell apoptosis. Mutant B cells proliferate poorly when stimulated by anti-IgM antibodies in vitro.
Ets-1 is expressed at high levels in lymphoid tissues and is also expressed in nonhematopoietic tissues.1,11,12 In Rag-2 complementation assays, Ets-1−/− embryonic stem (ES) cells produce reduced numbers of T lymphocytes and generate peripheral B cells shifted toward an IgM+ plasma cell phenotype.13,14 Ets-1−/− T cells fail to proliferate efficiently in response to anti-CD3 antibodies or concanavalin A, indicating a defect in response to T-cell receptor (TCR) cross-linking. Ets-1−/− animals exhibit lymphoid defects similar to those observed in chimeric animals and a reduction in the number of natural killer (NK) cells.15 Therefore, Ets-1 is required for the normal development or function of all lymphoid cell types (B cells, T cells, and NK cells). Elf-1 is highly expressed in hematopoietic tissues of adult mice such as the spleen, thymus, and bone marrow and at lower levels in other tissues.16 Although Elf-1 is reported to regulate a number of genes important for normal blood cell maturation,17-19Elf-1−/− mice are viable and display normal hematopoietic cell development and function (K.P.B., N. Muthusamy, and J. M. Leiden, unpublished results, June 1997). Therefore, unlike Ets-1, Elf-1 does not play any obvious nonredundant role in hematopoiesis.
PU.1 and Spi-B share approximately 70% amino acid homology in the Ets DNA binding domain, bind to similar DNA elements, and activate many of the same target genes in vitro.5,8,20,21PU.1−/− mice die in utero and lack B cells and T cells, precluding an analysis of lymphoid function. To examine functional overlap of PU.1 and Spi-B, we generated PU.1+/−Spi-B−/−animals, which are viable and exhibit B cell defects not present in PU.1+/− or Spi-B−/− mice.22PU.1+/−Spi-B−/− mice are characterized by a high percentage of apoptotic B cells and reduced B-cell numbers in the bone marrow and peripheral tissues. Purified PU.1+/−Spi-B−/− B cells display a seriously reduced proliferation potential in response to BCR cross-linking, and overall tyrosine phosphorylation and Ca++ flux induced by BCR ligation are defective.
Although Ets-1 and Elf-1 are not as closely related to PU.1 as Spi-B, they still share about 35% amino acid homology in their respective Ets domains and recognize similar DNA sequences. Therefore, we sought to determine whether Ets-1 or Elf-1 exhibits overlapping functions with PU.1 or Spi-B in vivo. Like PU.1−/− and PU.1−/−Spi-B−/− mice, PU.1−/−Ets-1−/− and PU.1−/−Elf-1−/− mice die by E18.5. We also generated PU.1+/−Ets-1−/−, PU.1+/−Elf-1−/−, and Spi-B−/−Ets-1−/− animals and examined various blood cell lineages in these animals. Our analyses detected only minor deviations in blood cell development or function in these compound mutants, suggesting that PU.1 and Spi-B do not share significant overlap in gene regulation with Ets-1 or Elf-1. In contrast, further examination of PU.1+/−Spi-B−/− animals has revealed an additional hematopoietic defect not previously reported. Specifically, bone marrow progenitors from PU.1+/−Spi-B−/−mice yield smaller colonies in methylcellulose cultures indicating a defect in either proliferation or survival of these progenitors. We also demonstrate extramedullary hematopoiesis in mice heterozygous for PU.1, irrespective of their Spi-B genotype. In summary, we confirm that the closely related proteins PU.1 and Spi-B regulate partially overlapping target genes, whereas the more divergent Ets proteins Ets-1 or Elf-1 appear not to share significant functional overlap with PU.1 or Spi-B.
Study design
Flow cytometry analysis
Single-cell suspensions were prepared from the indicated tissues, lysed with ammonium chloride buffer, stained, and analyzed as previously reported.10
Blood cell counts
Blood was harvested from the carotid artery of mice immediately after euthanasia by CO2 inhalation and transferred into Microtainer tubes with EDTA (Becton Dickinson, Franklin Lakes, NJ) to prevent coagulation. Total white blood cell counts, red blood cell counts, hematocrits, and platelet counts were analyzed by an electronic counter. For differential counts, blood cell smears were prepared, stained, and 100 to 200 white blood cells were counted manually.
Clonogenic assays
Single-cell suspensions from bone marrow of adult animals were lysed with an ammonium chloride buffer to remove red blood cells and then were counted with a Coulter counter. Bone marrow cells (20 000) or spleen cells (200 000) were plated in 1.5 mL complete methylcellulose media containing the cytokines stem cell factor (SCF), interleukin-3 (IL-3), IL-6, erythropoietin (EPO), and pokeweed mitogen-stimulated spleen cell–conditioned media (Stem Cell Technologies, Vancouver, BC, Canada). Cultures were maintained in 5% CO2 in a humidified 37°C incubator for 6 to 7 days and then plates were scored for the numbers of erythroid (E), monocyte (M), granulocyte (G), granulocyte/monocyte (GM), and granulocyte/erythroid/monocyte/megakaryocyte (GEMM) colonies. After counting colonies, cells from the entire plate were harvested and counted using a Coulter counter to determine the total number of cells recovered.
Results and discussion
Elf-1−/− and PU.1+/−Elf-1−/−hematopoiesis is normal, whereas Ets-1−/−, PU.1+/−Ets-1−/−, and Spi-B−/−Ets-1−/−exhibit similar alterations in lymphoid cells
The generation of compound mutant animals deficient in the expression of one or more Ets transcription factors has allowed us to investigate the functional redundancy of these proteins in hematopoiesis. In this study, we wished to investigate possible overlapping functions of PU.1 and Spi-B with Ets-1 or Elf-1 by intercrossing PU.1+/− and Spi-B−/− animals with Ets-1−/− and Elf-1−/− animals. We examined fetal liver sections of PU.1−/−Ets-1−/− and PU.1−/−Elf-1−/− embryos at E16.5 and showed that they exhibited apparently normal proerythroblasts and erythroblasts (not shown). Moreover, there was no increase in the incidence of anemia or alteration in the timing of embryonic lethality in PU.1−/−Ets-1−/− and PU.1−/−Elf-1−/− embryos as compared to PU.1−/− embryos. Flow cytometry indicated that double mutant embryos, like PU.1−/− embryos, lacked lymphoid or myeloid lineages (not shown). Therefore, we were unable to detect any overlap in the function of Ets-1 and Elf-1 with PU.1 in fetal liver hematopoiesis.
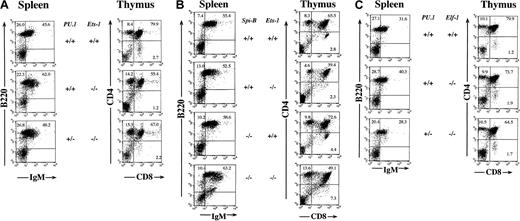
We have previously shown functional redundancy between PU.1 and Spi-B by analyzing mice containing a single copy of the PU.1 gene and no Spi-B(PU.1+/−Spi-B−/−).22Using a similar strategy, we investigated possible overlapping functions of PU.1 with Ets-1 and Elf-1 by generating viable PU.1+/−Ets-1−/− and PU.1+/−Elf-1−/− animals. We also generated viable Spi-B−/−Ets-1−/− animals. Analysis of thymus and spleen cell suspensions in PU.1+/−Ets-1−/− and Spi-B−/−Ets-1−/− animals demonstrated a phenotype identical to that previously reported for Ets-1−/− animals. In particular, there were modest reductions in the CD4+CD8+ population, a shift from CD4−CD8+ toward CD4loCD8+ phenotype, and a significant population of B220dullIgM+ plasma cells in the spleen (Figure 1A,B). On the other hand, flow cytometry showed no apparent defects in PU.1+/+Elf-1−/− or PU.1+/−Elf-1−/− animals (Figure 1C). Therefore, we did not detect any significant alterations in blood cell populations in PU.1+/−Elf-1−/−, PU.1+/−Ets-1−/−, or Spi-B−/−Ets-1−/− animals that were not previously identified in Ets-1−/− animals.
Flow cytometric analysis of blood cell lineages in mutant mice.
Single-cell suspensions of spleen and thymus from adult mice of the indicated genotypes were prepared and stained with antibodies to B220 and IgM or CD4 and CD8. Numbers indicate percentages of positive cells in each quadrant. Ten thousand events per dot plot were collected.
Flow cytometric analysis of blood cell lineages in mutant mice.
Single-cell suspensions of spleen and thymus from adult mice of the indicated genotypes were prepared and stained with antibodies to B220 and IgM or CD4 and CD8. Numbers indicate percentages of positive cells in each quadrant. Ten thousand events per dot plot were collected.
We isolated B cells and T cells from spleens of PU.1+/−Ets-1−/− and PU.1+/−Elf-1−/− mice, stimulated them in vitro with mitogenic compounds and examined their ability to proliferate. Wild-type, Ets-1−/−, PU.1+/−Ets-1−/−, Elf-1−/−, and PU.1+/−Elf-1−/− B cells all proliferated essentially normally in response to LPS and IgM cross-linking (not shown). Like Ets-1−/− T cells, PU.1+/−Ets-1−/− T cells displayed reduced proliferation in response to plate-bound α-CD3 antibody, whereas Elf-1−/− and PU.1+/−Elf-1−/− T cells proliferated normally in response to this stimulation (not shown). Therefore, we were unable to detect any defects in in vitro proliferation by compound mutant lymphocytes that were not previously identified in Ets-1−/− animals.
Elf-1 and PU.1 are both expressed in myeloid cells as well as lymphoid cells. Wild-type, Elf-1−/− and PU.1+/−Elf-1−/− mice recruited approximately equal numbers of macrophages in response to intraperitoneal thioglycollate injection (not shown). In addition, these macrophages produced equivalent amounts of nitrite (NO2−) in response to treatment with interferon-γ and lipopolysaccharide (LPS) (not shown). Increases in NO2− concentration were due to nitric oxide synthase (NOS) activity because the response could be inhibited by NG-monomethyl-l-arginine (NMA), a competitive inhibitor of the NOS enzyme. Hence, we were unable to define any statistically significant functional defects of macrophage recruitment or nitric oxide production in Elf-1−/− or PU.1+/−Elf-1−/− animals.
Our studies with these compound mutants failed to detect any significant hematopoietic deficiencies in these animals except those that were previously noted in Ets-1−/− mutants, leading us to believe that these Ets proteins largely regulate different sets of target genes. However, it is possible that our studies have failed to uncover genuine functional overlap between PU.1 and Ets-1 or Elf-1 because PU.1+/−Ets-1−/− and PU.1+/−Elf-1−/− animals still contain a single functional copy of the PU.1 locus.
Defect in bone marrow hematopoiesis in PU.1+/−Spi-B+/−and PU.1+/−Spi-B−/−mice
PU.1 is expressed in hematopoietic progenitor cells, but it is not known whether Spi-B is expressed in this lineage. To determine whether hematopoietic progenitors in PU.1+/−Spi-B−/−mice were normal, we performed colony-forming assays with bone marrow suspensions from mutant animals. Suspensions of bone marrow cells gave rise to statistically insignificant variations in the numbers of colonies of each type (Table 1). Therefore, it does not appear that there is a defect in the production of any particular hematopoietic cell type in PU.1+/−Spi-B−/− animals. We did, however, note that the size of hematopoietic colonies generated by PU.1+/−Spi-B−/− bone marrow progenitors generally appeared smaller than the size of colonies from wild-type bone marrow progenitors. After the numbers of colonies were quantified, cells were harvested from methylcellulose assays and counted. Compared to wild-type cultures, PU.1+/−Spi-B−/−cultures exhibited on average a 3-fold reduction in cell recovery, whereas PU.1+/−Spi-B+/− cultures exhibited a 30% reduction and PU.1+/−Spi-B+/+ cultures were unaffected (Table 1). These data indicate that PU.1+/−Spi-B−/− bone marrow hematopoietic cells may be deficient in proliferation or be more susceptible to cell death.
Progenitor colony counts for methylcellulose assays
| Colony type . | Bone marrow . | |||||
|---|---|---|---|---|---|---|
| E . | G . | M . | GM . | GEMM . | Cells recovered (× 106) . | |
| PU.1+/+Spi-B+/+ | 1.6 ± 0.5 | 30.6 ± 6.4 | 37.4 ± 4.5 | 6.4 ± 1.3 | 19.9 ± 5.7 | 1.2 ± 0.6 |
| PU.1+/−Spi-B+/+ | 5.4 ± 1.3 | 57.4 ± 16.3 | 56.0 ± 8.3 | 8.0 ± 3.0 | 10.0 ± 2.3 | 2.8 ± 1.3 |
| PU.1+/−Spi-B+/− | 4.8 ± 1.5 | 56.3 ± 6.4 | 48 ± 5.0 | 6.5 ± 0.9 | 21.8 ± 3.6 | 1.3 ± 0.2 |
| PU.1+/+Spi-B−/− | 2 ± 0.7 | 28.9 ± 5.4 | 50.4 ± 9.4 | 11.1 ± 3.7 | 14.4 ± 2.1 | 0.8 ± 0.1 |
| PU.1+/−Spi-B−/− | 3.5 ± 2.0 | 38 ± 10.1 | 52.3 ± 7.8 | 3.3 ± 1.0 | 13.2 ± 4.0 | 0.4 ± 0.1 |
| Colony type . | Bone marrow . | |||||
|---|---|---|---|---|---|---|
| E . | G . | M . | GM . | GEMM . | Cells recovered (× 106) . | |
| PU.1+/+Spi-B+/+ | 1.6 ± 0.5 | 30.6 ± 6.4 | 37.4 ± 4.5 | 6.4 ± 1.3 | 19.9 ± 5.7 | 1.2 ± 0.6 |
| PU.1+/−Spi-B+/+ | 5.4 ± 1.3 | 57.4 ± 16.3 | 56.0 ± 8.3 | 8.0 ± 3.0 | 10.0 ± 2.3 | 2.8 ± 1.3 |
| PU.1+/−Spi-B+/− | 4.8 ± 1.5 | 56.3 ± 6.4 | 48 ± 5.0 | 6.5 ± 0.9 | 21.8 ± 3.6 | 1.3 ± 0.2 |
| PU.1+/+Spi-B−/− | 2 ± 0.7 | 28.9 ± 5.4 | 50.4 ± 9.4 | 11.1 ± 3.7 | 14.4 ± 2.1 | 0.8 ± 0.1 |
| PU.1+/−Spi-B−/− | 3.5 ± 2.0 | 38 ± 10.1 | 52.3 ± 7.8 | 3.3 ± 1.0 | 13.2 ± 4.0 | 0.4 ± 0.1 |
| Colony type . | Spleen . | |||||
|---|---|---|---|---|---|---|
| E . | G . | M . | GM . | GEMM . | Cells recovered (× 106) . | |
| PU.1+/+Spi-B+/+ | 4.7 ± 0.6 | 35.3 ± 10.5 | 25.7 ± 5.9 | 2.7 ± 0.7 | 11.1 ± 3.3 | 2.6 ± 0.9 |
| PU.1+/−Spi-B+/+ | 2.8 ± 0.8 | 24.3 ± 8.1 | 42.5 ± 18.6 | 4.3 ± 2.1 | 23.8 ± 13.2 | 2.7 ± 0.5 |
| PU.1+/−Spi-B+/− | 2.5 ± 0.6 | 37.8 ± 16.1 | 44.0 ± 12.8 | 6.5 ± 1.7 | 41.3 ± 7.9 | 2.1 ± 0.3 |
| PU.1+/+Spi-B−/− | 3.0 ± 1.2 | 22.5 ± 6.8 | 20.3 ± 7.5 | 3.8 ± 0.5 | 7.7 ± 2.6 | 2.6 ± 0.9 |
| PU.1+/−Spi-B−/− | 4.0 ± 0.6 | 45.8 ± 18.3 | 56.6 ± 11.6 | 6.0 ± 3.0 | 38.8 ± 9.6 | 2.1 ± 1.1 |
| Colony type . | Spleen . | |||||
|---|---|---|---|---|---|---|
| E . | G . | M . | GM . | GEMM . | Cells recovered (× 106) . | |
| PU.1+/+Spi-B+/+ | 4.7 ± 0.6 | 35.3 ± 10.5 | 25.7 ± 5.9 | 2.7 ± 0.7 | 11.1 ± 3.3 | 2.6 ± 0.9 |
| PU.1+/−Spi-B+/+ | 2.8 ± 0.8 | 24.3 ± 8.1 | 42.5 ± 18.6 | 4.3 ± 2.1 | 23.8 ± 13.2 | 2.7 ± 0.5 |
| PU.1+/−Spi-B+/− | 2.5 ± 0.6 | 37.8 ± 16.1 | 44.0 ± 12.8 | 6.5 ± 1.7 | 41.3 ± 7.9 | 2.1 ± 0.3 |
| PU.1+/+Spi-B−/− | 3.0 ± 1.2 | 22.5 ± 6.8 | 20.3 ± 7.5 | 3.8 ± 0.5 | 7.7 ± 2.6 | 2.6 ± 0.9 |
| PU.1+/−Spi-B−/− | 4.0 ± 0.6 | 45.8 ± 18.3 | 56.6 ± 11.6 | 6.0 ± 3.0 | 38.8 ± 9.6 | 2.1 ± 1.1 |
All values are expressed as mean ± SE.
E indicates erythroid colonies; G, granulocyte colonies; M, monocyte colonies; GM, mixed granulocyte and monocyte colonies; GEMM, multilineage colonies containing myeloid and erythroid cells.
A previous report has shown that the number of cells in PU.1−/− progenitor colonies harvested from a similar methylcellulose assay is reduced approximately 6-fold compared to controls.23 In our assays, PU.1+/−Spi-B+/+ cultures yielded normal cell numbers, whereas PU.1+/−Spi-B−/−cultures yielded reduced numbers. Therefore,in the absence of sufficient PU.1 protein, Spi-B is critical for proliferation or survival of bone marrow hematopoietic cells in methylcellulose cultures. These results suggest that Spi-B is likely to be expressed in bone marrow hematopoietic progenitors or some of their committed progeny. Flow cytometry analysis has indicated that PU.1−/− fetal liver cells express very low levels of c-kit receptor, which is important for the expansion of hematopoietic progenitors.24 Moreover, the receptors for G-colony-stimulating factor (CSF), M-CSF, and GM-CSF are also PU.1 targets.23 It may be that Spi-B can compensate for PU.1 in regulating the expression of these or other membrane receptors.
PU.1+/−Spi-B+/+, PU.1+/−Spi-B+/−, and PU.1+/−Spi-B−/−mice exhibit extramedullary hematopoiesis
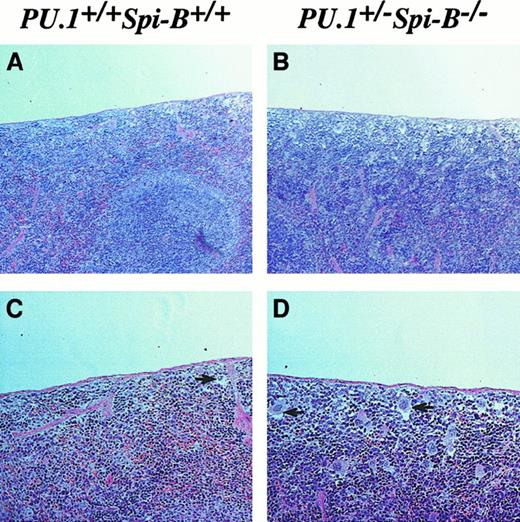
PU.1+/−Spi-B−/− animals frequently exhibit enlarged spleens, despite having fewer B lymphocytes. Hematoxylin and eosin staining of splenic sections indicated disorganized architecture with poor definition between red and white pulp areas in PU.1+/−Spi-B−/− animals (Figure 2A,B). Mature megakaryocytes could be visualized under the splenic capsule in PU.1+/−Spi-B−/− animals (Figure 2C,D). Further analysis identified cells resembling erythroid and myeloid progenitors indicative of the presence of increased extramedullary hematopoiesis in these animals.
Extramedullary hematopoiesis in the spleen.
Spleens were harvested from wild-type and PU.1+/−Spi-B−/− mice, fixed in formalin, sectioned, and stained with hematoxylin and eosin. The presence of megakaryocytes under the capsule of the spleen is indicated by the black arrows. Occasional megakaryocytes are found in wild-type spleens, but the numbers are increased greatly in PU.1+/−Spi-B−/− spleens. (Original magnifications: panels A and B, × 20; panels C and D, × 40.)
Extramedullary hematopoiesis in the spleen.
Spleens were harvested from wild-type and PU.1+/−Spi-B−/− mice, fixed in formalin, sectioned, and stained with hematoxylin and eosin. The presence of megakaryocytes under the capsule of the spleen is indicated by the black arrows. Occasional megakaryocytes are found in wild-type spleens, but the numbers are increased greatly in PU.1+/−Spi-B−/− spleens. (Original magnifications: panels A and B, × 20; panels C and D, × 40.)
To further characterize the presence of splenic hematopoiesis in mutant animals, we performed hematopoietic colony-forming assays with methylcellulose media. Spleens from mice with a single copy of thePU.1 gene, PU.1+/−Spi-B+/+, PU.1+/−Spi-B+/−, and PU.1+/−Spi-B−/−, contained increased numbers of hematopoietic progenitors compared to wild-type spleens (Table 1). In particular, there were more GM and GEMM colonies formed. Although, there was some increase in the numbers of colonies produced in cultures from PU.1+/−Spi-B+/− and PU.1+/−Spi-B−/− mice as compared to PU.1+/−Spi-B+/+, these differences were not statistically significant. The extramedullary hematopoiesis present in these animals may potentially be a response to a defect in bone marrow hematopoiesis.
Previous data have suggested a role for PU.1 in homing or engraftment of hematopoietic progenitors in the bone marrow. Although PU.1−/− ES cells formed normal erythrocytes and megakaryocytes in the fetal liver, they were unable to contribute efficiently to any adult bone marrow hematopoietic lineages.25 Moreover, fluorescently labeled PU.1−/− progenitors exhibited defects in their ability to home to and engraft properly in the bone marrow and failed to express the adhesion molecules VLA-4/CD49d or VLA-5/CD49e.24 Our data suggest that hematopoietic progenitors heterozygous for PU.1 may also be deficient in homing to and engraftment within the bone marrow microenvironment leading to significant extramedullary hematopoiesis.
To determine whether perturbations in bone marrow and splenic hematopoiesis affect peripheral blood cell counts, we analyzed peripheral blood samples obtained from wild-type and PU.1+/−Spi-B−/− mice. Despite reduced numbers of B cells detected in the peripheral blood of PU.1+/−Spi-B−/− mice by flow cytometry, total white blood cell counts were increased (wild-type, 6540 ± 1060/μL; PU.1+/−Spi-B−/−, 10 280 ± 1380/μL; P = .05). Differential cell counts of peripheral blood smears showed that the increased white blood cell counts were largely attributable to increases in granulocyte counts (wild-type, 14.7% ± 1.9%; PU.1+/−Spi-B−/−, 29.8% ± 7.9%;P = .07). As expected, PU.1+/−Spi-B−/− mice had reduced percentages of lymphocytes in the blood (wild-type, 83.1% ± 2.0%; PU.1+/−Spi-B−/− , 68.2% ± 7.8%;P = .07). The percentage of monocyes was unchanged. Red blood cell counts and hematocrit values were also similar for wild-type and PU.1+/−Spi-B−/− animals. In contrast, there was a statistically significant increase in the platelet counts in PU.1+/−Spi-B−/− mice (1 270 000 ± 150 000/μL) as compared to wild-type mice (625 000 ± 154 000/μL; P = .013). The increases in granulocytes and platelets in the peripheral blood of PU.1+/−Spi-B−/− mice may be related to alterations in bone marrow or splenic hematopoieisis.
In summary, we have analyzed functional overlap between various Ets family members by creating animals in which the PU.1 locus is heterozygous on a knockout background for another Ets protein (Ets-1, Elf-1, or Spi-B). We previously used such a system to show that PU.1 and Spi-B exhibit functional overlap in B-cell development and function.22 However, we were unable to uncover significant overlap in gene regulation between PU.1 with the more divergent family members Ets-1 or Elf-1. Nor did we find significant overlap between Spi-B and Ets-1. In contrast, we report an additional defect in PU.1+/−Spi-B−/− animals confirming that these 2 Ets proteins regulate some of the same target genes. We conclude that PU.1 and Spi-B are partially redundant in B-cell signaling and function and in the proliferation or survival of bone marrow hematopoietic progenitors. These studies indicate a clear difference in functional overlap between the closely related proteins PU.1 and Spi-B versus their overlap with the more divergent Ets proteins Ets-1 and Elf-1.
We would like to thank C. Clendenin and K. Sigrist for assistance with mouse husbandry and David Adelman for assistance in quantifying the clonogenic assays.
Supported by grant 52094 from the National Institutes of Health and by grant 10295 to L.A.G.-S. and 03668 to K.P.B. from the National Heart, Lung and Blood Institute. M.C.S. is an investigator of the Howard Hughes Medical Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
M. Celeste Simon, Howard Hughes Medical Institute, Abramson Family Cancer Research Institute, University of Pennsylvania, Rm 456, BRB II-III, 421 Curie Blvd, Philadelphia, PA 19104; e-mail:celeste2@mail.med.upenn.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal