Abstract
Cross-tolerization of T lymphocytes after apoptotic cell uptake by dendritic cells may be involved in self-tolerance maintenance. Furthermore, immunosuppressive properties are attributed to apoptotic cells. This study evaluated the consequences of apoptotic leukocyte administration in a restrictive engraftment model of murine bone marrow (BM) transplantation. Sublethally irradiated recipients received a limited number of allogeneic BM, with or without irradiated apoptotic leukocytes of different origins. No graft-versus-host disease was observed. Whereas only a low proportion of mice receiving BM cells alone engrafted, addition of apoptotic irradiated leukocytes, independently of the origin (donor, recipient, third-party mice, as well as xenogeneic peripheral blood mononuclear cells), significantly enhanced engraftment. Similar results were obtained after infusion of leukocytes rendered apoptotic by UVB irradiation or by anti-Fas monoclonal antibody stimulation, thus confirming the role of apoptotic cells in engraftment facilitation. Overall, these results suggest that apoptotic leukocytes can nonspecifically facilitate allogeneic BM engraftment. Such a simple approach could be of interest in BM transplantation settings involving an important HLA donor/recipient disparity, a T-cell–depleted graft, or reduced conditioning regimen intensity.
Introduction
Allogeneic hematopoietic stem cell (HSC) transplantation is a major therapeutic option to treat malignant and hereditary hematologic diseases. However, the high curative potential as well as the expansion of such a treatment modality are limited by the high rate of immunologic complications. Such side effects are mainly due to the presence of immunocompetent cells of both host and donor origins and the consequent alloreactive conflict. Graft rejection is mainly mediated by recipient T lymphocytes that resist the conditioning regimen. The main factors influencing the engraftment are the type and intensity of the myeloablative treatment, graft characteristics (number of stem cells and donor T cells) as well as the magnitude of the major histocompatibility complex (MHC) disparity between the donor and the recipient.1 Prevention of graft rejection by the use of T-cell–containing grafts is unfortunately associated with a higher incidence of graft-versus-host disease (GvHD). This complication occurs after the recognition of host alloantigens by mature donor T lymphocytes present in the graft, in the context of inflammatory cytokine release.2 An effective way to prevent GvHD is to deplete the donor T-cell population present in the graft, which is, however, associated with increased malignancy relapse and graft rejection.3 Therefore, the development of new strategies to modulate alloreactivity in the setting of allogeneic HSC transplantation is necessary.
An alternative approach to T-cell depletion is to selectively eliminate alloreactive T cells while retaining T-lymphocyte subsets with other specificities (eg, antiviral).4 Recently, this interesting approach has been used to anergize allogeneic T lymphocytes by blocking the CD28 interaction with B7 molecules.5 In that study, all recipients engrafted and experienced a lower than expected incidence of GvHD after haploidentical HSC. In addition to anergic donor T lymphocytes, a high number of apoptotic (or committed to apoptosis) cells were injected, as indicated by the increase of the absolute number of CD3+ cells infused.5 These apoptotic cells are not immunologically inert.6Immunosuppressive cytokine production by phagocytes having engulfed apoptotic cells have been described.7-10 Furthermore, it has been recently suggested that the permanent uptake of apoptotic cells in the periphery allows dendritic cells (DCs) to induce and maintain tolerance to self after migration to draining lymph nodes.11
Using a restrictive engraftment model of murine bone marrow transplantation (BMT), we therefore decided to evaluate the consequences of apoptotic leukocyte administration on the engraftment of an allogeneic BM graft. We found that apoptotic leukocytes co-infused with BM cells have a graft-facilitating effect without causing GvHD. This effect was not restricted to the donor origin of the apoptotic cells, because recipient, third-party, as well as xenogeneic apoptotic leukocytes facilitated engraftment. The graft-facilitating effect observed after the injection of apoptotic cells suggested a hyporeactivity against allogeneic donor BM cells but not apoptotic cells. The use of irradiated apoptotic cells could be a relatively simple way to modulate alloreactivity in HSC transplantation settings such as an important HLA donor/recipient disparity, a T-cell–depleted graft, or reduced conditioning regimen intensity (eg, older patients, nonmalignant disorders, or tolerance induction for organ transplantation).
Materials and methods
Mice
Pathogen-free, male, 5- to 6-week-old FVB (H-2q), C57BL/6 (H-2b), and BALB/c (H-2d) mice were obtained from IFFA-Credo (L'Abresle, France) and kept in quarantine for at least 1 week before BM transplantation. Mice were given ad libitum access to food and water. Neomycin sulfate (1.1 g/L; Demavic, Longvic, France) was added to water from day −1 of bone marrow transplantation (BMT) to reduce the risk of infection.
Bone marrow transplantation
Donor bone marrow (BM) cells from C57BL/6 or FVB mice were flushed from tibiae and femora in RPMI 1640 (BioWhittaker, Verviers, Belgium). Donor spleens were removed and homogenized, and single cell suspensions were obtained. After erythrocyte lysis using a buffered ammonium chloride solution, BM cells and splenocytes (SCs) were resuspended in phosphate-buffered saline (PBS; BioWhittaker). Human peripheral blood mononuclear cells (PBMCs) were prepared from freshly collected blood from 4 different healthy donors after Ficoll (Sigma, Saint Quentin Fallavier, France) density gradient centrifugation, washed twice, and resuspended in PBS. After the isolation procedures, viability of BM cells, SCs, and PBMCs was always more than 90% by trypan blue dye exclusion. BALB/c and C57BL/6 mice, used as recipients, received a total body irradiation (TBI) 16 hours before BMT. A 6- or 7-Gy single dose TBI was applied with a dose rate of 2.7 Gy/minute. Recipients then received a single intravenous (in a lateral tail vein) injection (300 μL) containing BM cells with or without apoptotic leukocytes from different origins. No immunosuppressive agents were given.
Apoptosis induction
Apoptosis was induced either by γ- or UVB-irradiation or by exposure of activated SCs to a lytic anti-Fas monoclonal antibody (mAb). SCs and PBMCs were adjusted to 2 × 106 cells/mL (in PBS) and submitted to γ-irradiation (40 Gy) 6 hours before injection of the mice to allow apoptotic changes to occur. Alternatively, SCs were dispersed into Petri dishes (100-mm diameter) and exposed to 200 J/m2 UVB radiation (Sankyo Denki, Tokyo, Japan). For Fas-induced apoptosis, SCs were adjusted to 2 × 106 cells/mL and activated by the addition of 2.5 μg/mL concanavalin A (Con-A, Seromed, Berlin, Germany) and recombinant human interleukin-2 (IL-2; 500 IU/mL; Cetus, Rueil-Malmaison, France) in Dulbecco modified Eagle medium (BioWhittaker) culture medium supplemented with 10% heat-inactivated fetal calf serum (Boehringer Mannheim, Meylan, France), 5 × 10−5 M 2-mercaptoethanol, 2 mM L-glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin (Sigma) (complete medium) and incubated at 37°C in a humidified atmosphere with 5% CO2. After 3 days of activation, apoptosis was induced by the addition of a lytic anti-Fas mAb (1 μg/mL, clone Jo2; Pharmingen, San Diego, CA) during an additional culture period of 6 or 24 hours in the absence of IL-2. The cells were then washed in PBS and adjusted to the desired concentration before injection. At the time of injection, only a small proportion of the leukocytes were in an advanced death stage as determined by trypan blue exclusion (≤ 10%). No aggregation was observed.
Flow cytometry analysis for the detection of engraftment
Detection of the engraftment was performed between day 45 and day 50 post-BMT. H-2 phenotypes of SCs from recipient mice were determined by flow cytometry using fluorescein isothiocyanate (FITC)-labeled anti-H-2q (KH114, mouse immunoglobulin G2a [IgG2a]) and phycoerythrin (PE)-labeled anti-H-2d(SF1-1.1, mouse IgG2a) antibodies (Pharmingen). Analysis was performed on a FACSCalibur (Becton Dickinson, Mountain View, CA) using CellQuest software (Becton Dickinson). Engraftment was routinely evaluated in spleen cells and circulating leukocytes. In one experiment, engraftment was determined in additional immunologic sites: thymus, BM, and cervical lymph node. Engraftment was considered as positive if at least 15% of recipient cells had the BM donor H-2 phenotype.12Engraftment in different lineage (lymphoid or myeloid) was determined using forward light scatter and side light scatter gating.
GvHD evaluation
In each experiment, GvHD was assessed by body weight loss (weekly) and skin lesions (daily). In killed animals, GvHD was also evaluated at day 45 to day 50 histologically as described.13 The following organs were systematically examined: stomach, small and large intestines, rectum, and skin (neck and abdominal wall) as targets for GvHD. Liver, heart, lungs, spleen, kidney, testis, and brain were evaluated for tissue injuries due to irradiation.
Apoptosis detection by Annexin-V staining
Following induction of apoptosis, SCs were resuspended at 2 × 106 cells/mL in complete medium and dispensed into 24-well plates. After different periods of culture at 37°C and 5% CO2, 5 × 105 cells were harvested, washed twice in cold PBS, and resuspended in cold Annexin-V buffer (Immunotech, Marseille, France). The cells were then stained with FITC-conjugated Annexin-V (Immunotech) for 10 minutes and analyzed using a FACSCalibur flow cytometer. In some experiments, detection of secondary necrotic cells was assessed using propidium iodide (Sigma) staining.
Ex vivo splenocyte interferon-γ production in response
to alloantigens
Specificity of the induced tolerance was determined by using a one-way mixed leukocyte reaction. At day 45 to day 50 post-BMT, splenic T cells from naive BALB/c and FVB mice, as well as recipient BALB/c mice were purified by passage through nylon wool columns (> 91% CD3+) and then used as responders (2 × 105/well in round-bottom 96-well plates) against γ-irradiated (20 Gy) mature DCs generated from BM (×105/well). Mature DCs were derived from naive C57BL/6 mice as described.14 Mixed T lymphocyte/DC cultures were maintained in complete medium at 37°C in 5% CO2. Supernatants were collected from 48-hour cultures. Mouse interferon (IFN)-γ production was measured using an enzyme-linked immunosorbent assay kit (R&D Systems, Abingdon, United Kingdom) according to manufacturer's instructions.
Statistical analysis
Statistical analysis was performed using SigmaStat and SigmaPlot software version 4.0 (Jandel Scientific, Erkrath, Germany). Chi-square and Fisher exact tests were used when indicated. P values < .05 were considered as statistically significant.
Results
Splenocytes irradiated at 40 Gy are apoptotic
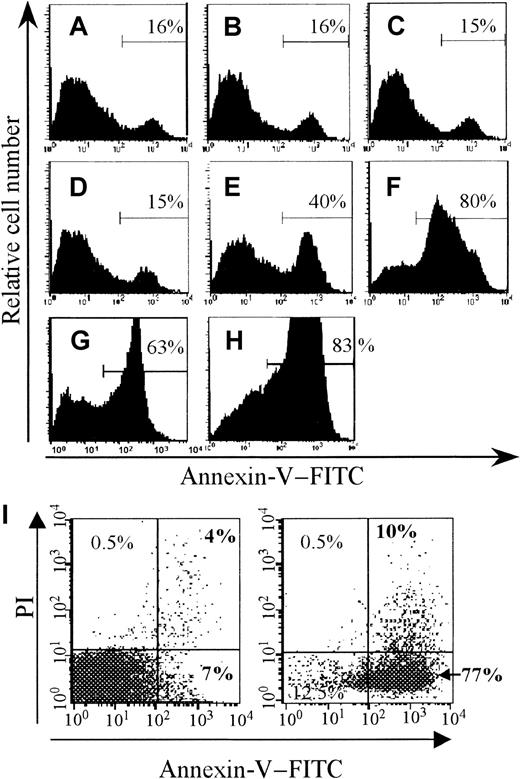
Gamma-irradiation of murine SCs or human PBMCs leads to apoptotic death of such cells.10 15-17 Using FITC–Annexin-V staining and FACS analysis, we evaluated the kinetics of apoptosis induction by 40 Gy γ-irradiation in murine SCs. As soon as 2 hours after irradiation, 40% of irradiated cells were labeled by FITC–Annexin-V (Figure 1E), and by 6 hours after irradiation (when irradiated cells were injected in recipient mice), as much as 80% of cells were apoptotic (Figure 1F), indicating that a majority of irradiated SCs in our model were apoptotic at the time of injection.
Induction of splenocyte apoptosis.
Splenocytes (SCs) were submitted to γ-irradiation (40 Gy). Alternatively, SCs were cultivated in complete medium in the presence of Con-A and IL-2 for 3 days and then exposed to a lytic anti-Fas mAb (Jo2). Treated and control (untreated) cells were then washed, resuspended at the same concentration in complete medium, and kept at 37°C and 5% CO2. After different periods of culture, the cells were stained with FITC-conjugated Annexin-V to detect apoptosis induction. The percentage of Annexin-V+ cells is indicated in each histogram. Upper panels represent nonirradiated control cells after 0, 2, and 6 hours of culture (A, B, and C, respectively). Intermediate panels represent γ-irradiated SCs after 0, 2, and 6 hours of culture (D, E, and F, respectively). Lower panels represent SCs after 6 and 24 hours exposure to Jo2 (G and H, respectively). Before infusion, the absence of aggregate and secondary necrotic cells (< 10%) was determined by the trypan blue exclusion method. In some experiments, cells in an advanced stage of death were assessed by using propidium iodide (PI) gating. Panel I shows control cells after 6 hours of culture (left dot plot) and γ-irradiated SCs at the time of infusion (right panel, 77% of cells are apoptotic, PI− and Annexin-V+).
Induction of splenocyte apoptosis.
Splenocytes (SCs) were submitted to γ-irradiation (40 Gy). Alternatively, SCs were cultivated in complete medium in the presence of Con-A and IL-2 for 3 days and then exposed to a lytic anti-Fas mAb (Jo2). Treated and control (untreated) cells were then washed, resuspended at the same concentration in complete medium, and kept at 37°C and 5% CO2. After different periods of culture, the cells were stained with FITC-conjugated Annexin-V to detect apoptosis induction. The percentage of Annexin-V+ cells is indicated in each histogram. Upper panels represent nonirradiated control cells after 0, 2, and 6 hours of culture (A, B, and C, respectively). Intermediate panels represent γ-irradiated SCs after 0, 2, and 6 hours of culture (D, E, and F, respectively). Lower panels represent SCs after 6 and 24 hours exposure to Jo2 (G and H, respectively). Before infusion, the absence of aggregate and secondary necrotic cells (< 10%) was determined by the trypan blue exclusion method. In some experiments, cells in an advanced stage of death were assessed by using propidium iodide (PI) gating. Panel I shows control cells after 6 hours of culture (left dot plot) and γ-irradiated SCs at the time of infusion (right panel, 77% of cells are apoptotic, PI− and Annexin-V+).
Donor-irradiated apoptotic SCs enhance the engraftment of an allogeneic HSC graft
To study the host-versus-graft reactivity and modulation, we designed a restrictive murine BMT model. BALB/c mice (H-2d) were exposed to a 6-Gy TBI before receiving a limited number of 106 BM cells from FVB mice (H-2q). In this model, the low dose of irradiation allows the recipient immune system to reject the low number of allogeneic BM cells injected. An autologous hematopoietic recovery was observed, as only 11% of recipient mice engrafted under such conditions (Table1). To evaluate the graft-facilitating potential of donor apoptotic SCs, 5 × 106 irradiated SCs from FVB mice were added to the BM cell suspension. This addition of apoptotic cells resulted in a significant increase in the percentage of engrafted mice (49% versus 11%, P < .001; Table 1). Furthermore, a higher percentage of donor-type cells was found in the engrafted mice that received apoptotic SCs in both lymphoid and myeloid lineage (Figure 2 and Table2). This favorable effect of donor apoptotic SCs was also observed when the number of BM cells was reduced (3 × 105 instead of 10,6P < .001; Table 1).
Irradiated donor splenocytes co-infused with bone marrow cells enhance allogeneic bone marrow engraftment
| Recipient mice . | FVB (H-2q) BM cells . | FVB (H-2q) irradiated SCs . | Mice engrafted (H-2q phenotype) at day 45 to day 50 post-BMT (%)* . |
|---|---|---|---|
| C57BL/6 (H-2b) | 3 × 105 | None | 3 (1/28) |
| 3 × 105 | 5 × 106 | 57 (15/26)† | |
| BALB/c (H-2d) | 3 × 105 | None | 2 (1/44) |
| 3 × 105 | 5 × 106 | 38 (17/44)† | |
| BALB/c (H-2d) | 106 | None | 11 (5/44) |
| 106 | 5 × 106 | 49 (25/51)† |
| Recipient mice . | FVB (H-2q) BM cells . | FVB (H-2q) irradiated SCs . | Mice engrafted (H-2q phenotype) at day 45 to day 50 post-BMT (%)* . |
|---|---|---|---|
| C57BL/6 (H-2b) | 3 × 105 | None | 3 (1/28) |
| 3 × 105 | 5 × 106 | 57 (15/26)† | |
| BALB/c (H-2d) | 3 × 105 | None | 2 (1/44) |
| 3 × 105 | 5 × 106 | 38 (17/44)† | |
| BALB/c (H-2d) | 106 | None | 11 (5/44) |
| 106 | 5 × 106 | 49 (25/51)† |
BM, bone marrow; SCs, splenocytes; BMT, bone marrow transplantation.
Pooled results of 3 to 5 independent experiments. Number of engrafted mice/number of analyzed mice at day 45 to day 50 post-BMT are indicated in parentheses.
P < .001.
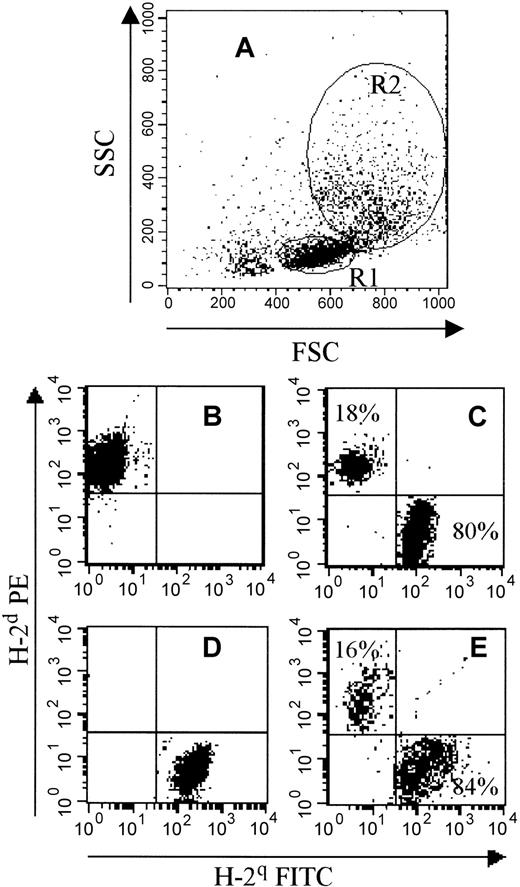
Flow cytometric representation of the phenotype of spleen cells 45 to 50 days after BMT.
BALB/c mice (H-2d) were submitted to a 6-Gy TBI and grafted 24 hours later with 106 BM cells from FVB (H-2q) mice alone or with apoptotic SCs. Between day 45 and day 50 post-BMT, engraftment was evaluated by FACS analysis. (A) This panel shows forward light scatter and side light scatter gating with 2 different regions: R1 (corresponding to lymphocytes that were confirmed by anti-CD3 mAb staining) and R2 (corresponding to monocytes and granulocytes, confirmed by anti-Gr1 and CD14 mAb staining). (B,D) These panels show H-2 expression on BALB/c (B, H-2dPE+) and FVB (D, H-2q FITC+) SCs. (C,E) These panels show a representative flow cytometry profile from an engrafted mouse that received 106 FVB BM cells plus 5 × 106 apoptotic irradiated FVB SCs. A donor phenotype (H-2q) was observed in both lymphoid (C, R1 gating) and myeloid (E, R2 gating) lineages. The percentage of H-2qdonor cells is indicated in each panel.
Flow cytometric representation of the phenotype of spleen cells 45 to 50 days after BMT.
BALB/c mice (H-2d) were submitted to a 6-Gy TBI and grafted 24 hours later with 106 BM cells from FVB (H-2q) mice alone or with apoptotic SCs. Between day 45 and day 50 post-BMT, engraftment was evaluated by FACS analysis. (A) This panel shows forward light scatter and side light scatter gating with 2 different regions: R1 (corresponding to lymphocytes that were confirmed by anti-CD3 mAb staining) and R2 (corresponding to monocytes and granulocytes, confirmed by anti-Gr1 and CD14 mAb staining). (B,D) These panels show H-2 expression on BALB/c (B, H-2dPE+) and FVB (D, H-2q FITC+) SCs. (C,E) These panels show a representative flow cytometry profile from an engrafted mouse that received 106 FVB BM cells plus 5 × 106 apoptotic irradiated FVB SCs. A donor phenotype (H-2q) was observed in both lymphoid (C, R1 gating) and myeloid (E, R2 gating) lineages. The percentage of H-2qdonor cells is indicated in each panel.
Irradiated leukocytes co-infused with bone marrow cells enhance the engraftment in both myeloid and lymphoid lineages
| Irradiated leukocytes . | Mice* . | Donor-derived cells (FVB mice, H-2q) (%) . | Degree2-153 . | |
|---|---|---|---|---|
| Lymphocytes mean ± SEM†(range)‡ . | Granulocytes + monocytes mean ± SEM† (range)‡ . | |||
| None | 5/44 | 63 ± 15 (28-98) | 67 ± 12 (25-91) | 2/5 |
| FVB (H-2q) | 29/51 | 92 ± 3 (61-100) | 93 ± 3 (53-100) | 25/29 |
| BALB/c (H-2d) | 14/25 | 77 ± 7 (18-100) | 82 ± 6 (27-100) | 12/14 |
| C57BL/6 (H-2b) | 11/25 | 92 ± 3 (72-100) | 88 ± 3 (67-100) | 9/11 |
| PBMC | 12/23 | 90 ± 4 (43-100) | 90 ± 2 (76-100) | 10/12 |
| Irradiated leukocytes . | Mice* . | Donor-derived cells (FVB mice, H-2q) (%) . | Degree2-153 . | |
|---|---|---|---|---|
| Lymphocytes mean ± SEM†(range)‡ . | Granulocytes + monocytes mean ± SEM† (range)‡ . | |||
| None | 5/44 | 63 ± 15 (28-98) | 67 ± 12 (25-91) | 2/5 |
| FVB (H-2q) | 29/51 | 92 ± 3 (61-100) | 93 ± 3 (53-100) | 25/29 |
| BALB/c (H-2d) | 14/25 | 77 ± 7 (18-100) | 82 ± 6 (27-100) | 12/14 |
| C57BL/6 (H-2b) | 11/25 | 92 ± 3 (72-100) | 88 ± 3 (67-100) | 9/11 |
| PBMC | 12/23 | 90 ± 4 (43-100) | 90 ± 2 (76-100) | 10/12 |
Sublethally irradiated BALB/c (H-2d) recipient mice were grafted with 106 bone marrow cells from FVB (H-2q) mice alone or with irradiated leukocytes from different origins. The percentage of donor cells in lymphocytes and granulocytes + monocytes was determined by flow cytometry as described in the “Methods and materials” section and shown in Figure 2. PBMC, peripheral blood mononuclear cell.
Number of engrafted mice/number of analyzed mice at day 45 to day 50 post-BMT.
Data are from engrafted mice only of 3 to 5 independent experiments.
Data indicate the percentage range of donor cells in engrafted mice.
Number of mice with a full donor phenotype/number of engrafted mice.
Donor apoptotic SCs were also capable of increasing the engraftment rate in another donor/recipient pair, in which only 3% of C57BL/6 (H-2b) mice irradiated at 7 Gy and injected with 3 × 105 BM cells from FVB mice engrafted. This finding was in contrast with engraftment in 57% of mice that received 3 × 105 BM cells plus 5 × 106 irradiated FVB SCs (P < .001; Table 1). Administration of irradiated FVB SCs alone (with no BM cells) to BALB/c mice did not result in donor hematopoietic reconstitution, therefore excluding the possibility that our results were due to the presence in the splenic cell suspension of HSCs resistant to γ-irradiation. Last, GvHD (clinically and histologically evaluated) was never observed after administration of irradiated SCs.
Graft-facilitating effect of irradiated SCs is not mediated by residual antirecipient cytotoxic activity retained by such cells
A recent report also described that engraftment of allogeneic BM cells is facilitated by the infusion of γ-irradiated donor SCs.18 In that report, the investigators suggested that the graft-promoting effect was mediated by the irradiated T cells, due to an enhancement of the donor T-cell antirecipient allospecific cytotoxicity after 7.5 Gy γ-irradiation.18 In our experiments, some cells were not stained by FITC–Annexin-V at the time of infusion (Figure 1F). We therefore decided to determine whether the facilitating effect we observed with irradiated donor SCs was linked to their residual antirecipient cytotoxic activity by using irradiated host SCs (ie, syngeneic and devoid of antirecipient cytotoxic activity). We found that irradiated SCs from recipient BALB/c mice co-injected with FVB BM cells were also able to favor FVB donor-type engraftment (P < .05; Tables 2 and3).
Irradiated apoptotic leukocytes enhance allogeneic bone marrow engraftment independently of their major histocompatibility complex identity with bone marrow cells
| Recipient mice . | FVB (H-2q) BM cells . | Irradiated SCs (5 × 106) . | Mice engrafted (H-2q phenotype) at day 45 to day 50 post-BMT (%)3-150 . |
|---|---|---|---|
| BALB/c (H-2d) | 106 | None | 11 (3/27) |
| BALB/c (H-2d) | 106 | BALB/c (H-2d) | 56 (14/25)3-151 |
| BALB/c (H-2d) | 106 | C57BL/6 (H-2b) | 44 (11/25)3-151 |
| BALB/c (H-2d) | 106 | FVB (H-2q) | 44 (15/34)3-151 |
| Recipient mice . | FVB (H-2q) BM cells . | Irradiated SCs (5 × 106) . | Mice engrafted (H-2q phenotype) at day 45 to day 50 post-BMT (%)3-150 . |
|---|---|---|---|
| BALB/c (H-2d) | 106 | None | 11 (3/27) |
| BALB/c (H-2d) | 106 | BALB/c (H-2d) | 56 (14/25)3-151 |
| BALB/c (H-2d) | 106 | C57BL/6 (H-2b) | 44 (11/25)3-151 |
| BALB/c (H-2d) | 106 | FVB (H-2q) | 44 (15/34)3-151 |
BM, bone marrow; SCs, splenocytes; BMT, bone marrow transplantation.
Pooled results of 3 independent experiments. Number of engrafted mice/number of analyzed mice at day 45 to day 50 post-BMT are indicated in parentheses.
P < .05.
We confirmed the potential of recipient-irradiated SCs to facilitate donor hematopoietic reconstitution in a different donor/recipient combination by using a more restrictive model. Recipient BALB/c mice were exposed to lethal 8-Gy TBI and then grafted with a limited dose of 106 BM cells from C57BL/6 mice. Under these conditions, all recipient mice were dead by day 11 after transplantation. In contrast, only 20% of recipient BALB/c mice died after infusion of C57BL/6 BM cells plus irradiated BALB/c SCs. All remaining recipient mice engrafted (P < .05), with a complete donor reconstitution (100% of cells with donor [H-2b] phenotype). These results suggest that the graft-promoting effects of irradiated SCs are not due to increased antirecipient alloreactivity of such cells.
SCs rendered apoptotic by other stimuli also have graft-facilitating effects
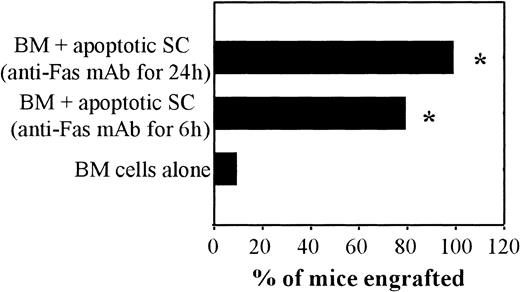
To confirm that the apoptotic status of the administered SCs was indeed responsible for the observed graft-facilitating activity, alternative stimuli (lytic anti-Fas mAb Jo2 treatment and UVB-irradiation) were used to induce apoptosis. As shown in Figure3, 80% and 100% of recipient mice that received BM cells plus donor SCs exposed to Jo2 mAb engrafted (6 and 24 hours exposure to Jo2, respectively). In contrast, only 10% of recipient mice engrafted after administration of BM cells alone (P < .001; Figure 3). Furthermore, the addition of UVB-irradiated apoptotic SCs to BM cells also resulted in graft facilitation (40% engraftment versus 14% in the absence of apoptotic cells, P = .10). These results further support the demonstration that apoptotic cells have graft-promoting effects.
Graft-facilitating effect of SCs rendered apoptotic after Fas triggering.
BALB/c (H-2d) mice were submitted to a 6-Gy TBI and grafted 24 hours later with 106 BM cells from FVB (H-2q) mice alone or with activated SCs rendered apoptotic after exposure to a lytic anti-Fas mAb (Jo2) for 6 or 24 hours (see Figure 1G, H, respectively). Between day 45 and day 50 post-BMT, engraftment was evaluated by FACS analysis. The percentage of engrafted mice (ie, mice with at least 15% of spleen lymphocytes with donor BM phenotype, H-2q) is shown. *P < .005.
Graft-facilitating effect of SCs rendered apoptotic after Fas triggering.
BALB/c (H-2d) mice were submitted to a 6-Gy TBI and grafted 24 hours later with 106 BM cells from FVB (H-2q) mice alone or with activated SCs rendered apoptotic after exposure to a lytic anti-Fas mAb (Jo2) for 6 or 24 hours (see Figure 1G, H, respectively). Between day 45 and day 50 post-BMT, engraftment was evaluated by FACS analysis. The percentage of engrafted mice (ie, mice with at least 15% of spleen lymphocytes with donor BM phenotype, H-2q) is shown. *P < .005.
Apoptotic third-party SCs or xenogeneic human PBMCs retain graft-facilitating effects
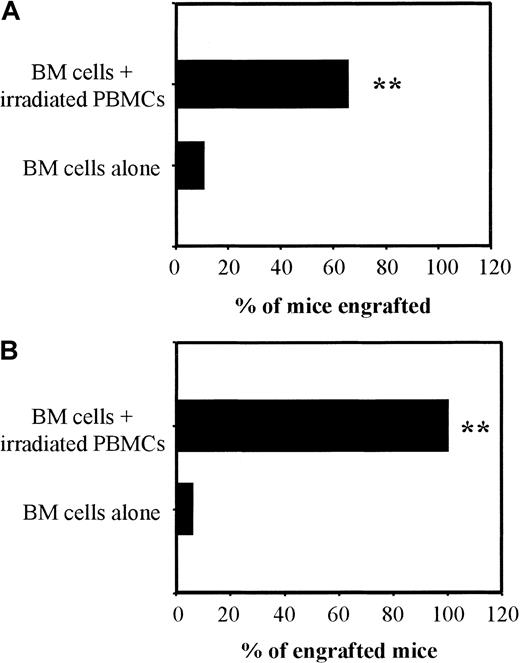
We then tested whether apoptotic SCs needed to be MHC-matched to BM cells or recipient mice to exert their graft-facilitating effect. We found that the addition of irradiated SCs from third-party C57BL/6 (H-2b) mice to FVB (H-2q) BM cells also increased the proportion of recipient BALB/C (H-2d) mice with FVB donor-type phenotype (Tables 2 and 3). To confirm that this effect was independent of MHC matching between BM cells and co-injected irradiated cells, a pool of γ-irradiated human PBMCs was infused with the BM cell suspension. The lack of influence of MHC specificity in this model was supported by the findings that co-injection of FVB BM cells plus a pool of 40 Gy irradiated human PBMCs similarly increased the proportion of recipient BALB/c mice with a FVB phenotype (Figure4A). The graft-facilitating effect of irradiated human PBMCs was also observed in another donor/recipient combination by using C57BL/6 mice as the donors of BM cells and BALB/c as recipients: 6% of mice receiving BM cells alone engrafted versus 25% after addition of 5 × 106 irradiated PBMCs and 100% after addition of 107 irradiated PBMCs (P < .005; Figure 4B).
Graft-facilitating effect of irradiated human PBMCs.
BALB/c (H-2d) mice were submitted to 6-Gy TBI and grafted 24 hours later with 106 BM cells from FVB (H-2q; panel A, 2 independent experiments with 12 mice per group) or C57BL/6 (H-2b; panel B, one experiment with 12 mice per group) mice alone or with 107 γ-irradiated human PBMCs. Between day 45 and day 50 post-BMT, engraftment was measured by FACS analysis. Results are expressed as previously described in Figure3. **P < .005.
Graft-facilitating effect of irradiated human PBMCs.
BALB/c (H-2d) mice were submitted to 6-Gy TBI and grafted 24 hours later with 106 BM cells from FVB (H-2q; panel A, 2 independent experiments with 12 mice per group) or C57BL/6 (H-2b; panel B, one experiment with 12 mice per group) mice alone or with 107 γ-irradiated human PBMCs. Between day 45 and day 50 post-BMT, engraftment was measured by FACS analysis. Results are expressed as previously described in Figure3. **P < .005.
Injection of apoptotic leukocytes simultaneously to BM cells favors a full-donor chimerism
To better characterize the graft-facilitating effect induced by apoptotic leukocyte infusion, the presence of cells with a donor phenotype was assessed in thymus, BM, and cervical lymph node at day 45 to day 50 post-BMT. None of the 5 mice that received BM cells alone engrafted (Table 4). In contrast, in 14 recipient mice that received apoptotic cells plus BM cells, 9 presented a full-donor phenotype in each evaluated organ (Table 4). Furthermore, in 5 previous experiments, the majority of the engrafted mice that received apoptotic cells in addition to BM cells had a full-donor phenotype in myeloid lineage (Table 2), suggesting that the reconstitution of the hematopoietic system was derived from the donor BM cells infused. In addition, the disappearance of recipient T lymphocytes in the immunologic organs (particularly in lymph node and thymus) in such mice (Table 4) also sustained this hypothesis. All these findings support persistent donor-derived engraftment.
Irradiated leukocytes co-infused with bone marrow cells enhance the engraftment in different immunologic organs
| Cells added to BM . | Percentage of donor-derived cells (FVB mice, H-2q) . | |||
|---|---|---|---|---|
| Thymus . | Bone marrow . | Lymph node . | Spleen . | |
| None | 0, 0, 0, 0, 0, 0 | 0, 0, 0, 0, 0, 0 | 0, 0, 0, 0, 0, 0 | 0, 0, 0, 0, 0, 0 |
| Irrd. FVB | 100, 99, 100, 0, 0 | 100, 100, 98, 0, 0 | 100, 100, 100, 0, 0 | 100, 100, 100, 0, 0 |
| Irrd. BALB/c | 100, 0, 0, 0, 100 | 100, 0, 0, 0, 100 | 100, 0, 0, 0, 100 | 100, 0, 0, 0, 100 |
| Irrd. PBMC | 99, 100, 100, 100 | 98, 100, 100, 100 | 100, 100, 100, 100 | 100, 100, 100, 100 |
| Viable T lymphocytes | ||||
| 104 | 0, 0, 0, 19, 9 | 0, 0, 0, 12, 9 | 0, 11, 15, 91, 33 | 0, 0, 0, 78, 33 |
| 1 × 105 | 100, 100, 100 | 100, 100, 100 | 100, 100, 100 | 100, 100, 100 |
| Cells added to BM . | Percentage of donor-derived cells (FVB mice, H-2q) . | |||
|---|---|---|---|---|
| Thymus . | Bone marrow . | Lymph node . | Spleen . | |
| None | 0, 0, 0, 0, 0, 0 | 0, 0, 0, 0, 0, 0 | 0, 0, 0, 0, 0, 0 | 0, 0, 0, 0, 0, 0 |
| Irrd. FVB | 100, 99, 100, 0, 0 | 100, 100, 98, 0, 0 | 100, 100, 100, 0, 0 | 100, 100, 100, 0, 0 |
| Irrd. BALB/c | 100, 0, 0, 0, 100 | 100, 0, 0, 0, 100 | 100, 0, 0, 0, 100 | 100, 0, 0, 0, 100 |
| Irrd. PBMC | 99, 100, 100, 100 | 98, 100, 100, 100 | 100, 100, 100, 100 | 100, 100, 100, 100 |
| Viable T lymphocytes | ||||
| 104 | 0, 0, 0, 19, 9 | 0, 0, 0, 12, 9 | 0, 11, 15, 91, 33 | 0, 0, 0, 78, 33 |
| 1 × 105 | 100, 100, 100 | 100, 100, 100 | 100, 100, 100 | 100, 100, 100 |
Sublethally irradiated BALB/c (H-2d) recipient mice were grafted with 106 bone marrow (BM) cells from FVB (H-2q) mice alone, with irradiated (Irrd.) leukocytes from different origins or with a limited number (104 or 105) of viable donor T lymphocytes purified from spleen using nylon wool columns. Two concentrations of viable donor alloreactive T lymphocytes were shown as control: the highest one favored a persistent full-donor phenotype, the lowest concentration induced only a transient mixed chimerism. At day 45 to day 50 post-BMT, the percentage of donor cells (including both lymphocytes and granulocytes + monocytes) in the different immunologic organs was determined by flow cytometry. Results are from individual recipients.
Injection of third-party (C57BL/6) apoptotic cells does not modify the alloreactivity of T lymphocytes from engrafted
mice against C57BL/6 stimulatory cells
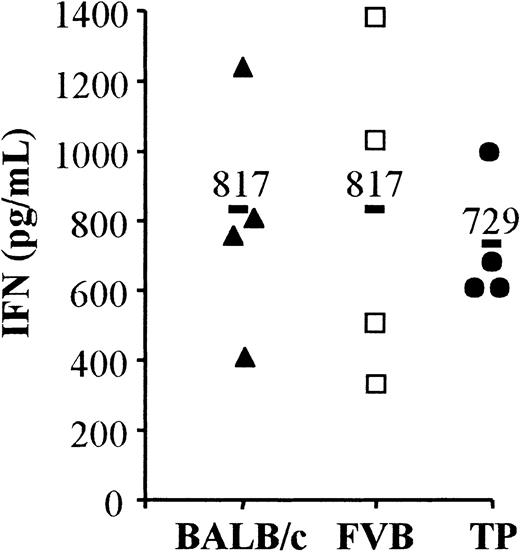
The graft-facilitating effect observed after the injection of apoptotic cells suggested a hyporeactivity against allogeneic donor BM cells. We took advantage of the fact that apoptotic cells enhance engraftment no matter their origin to determine whether this graft-facilitating effect was induced only against viable donor BM cells or also against apoptotic cells. Thus, T lymphocytes from engrafted BALB/c mice that had received third-party apoptotic cells (from C57BL/6 mice) in addition to donor FVB BM were isolated from the spleen at day 45 to day 50 post-BMT, co-cultured with irradiated mature DCs raised from C57BL/6, before IFN-γ production was measured. This cytokine has been shown to be necessary to initiate acute rejection of MHC incompatible allografts.19 The capacity of IFN-γ production by T lymphocytes from these engrafted mice was compared with the IFN production capacity of T lymphocytes from naive BALB/c and FVB mice. IFN-γ production by T lymphocytes in response to C57BL/6 DC was similar for the 3 different groups of mice (Figure5). These results suggest that T lymphocytes from engrafted mice that have received apoptotic third-party cells were fully responsive to these same third-party cells, at least in vitro. In addition, mice that did not engraft produced cytotoxic antibodies against BM cells but never against apoptotic cells. Indeed, no antibodies against third-party SCs were found in the sera of mice that had received apoptotic third-party irradiated SCs (n = 21), whereas cytotoxic anti-BM donor antibodies were identified in such mice that did not engraft (data not shown).
IFN production of ex vivo T lymphocytes in response to allogeneic third-party DCs.
Purified spleen T lymphocytes were obtained from naive BALB/c (H-2d, black triangles), FVB (H-2q, open squares) mice, or BALB/c mice grafted with 106 BM cells from FVB mice plus apoptotic SCs of C57BL/6 (H-2b) mice (noted TP, black circles) at day 45 to day 50 post-BMT. These T lymphocytes were used as responders and C57BL/6 mature DC as stimulants in a one-way mixed leukocyte reaction. The IFN-γ production was measured 48 hours later in the supernatant of cultures by enzyme-linked immunosorbent assay. The grafted mice (noted TP) included in this analysis had a complete FVB phenotype at day 45 to day 50 post-BMT. Four individual mice were tested in each group. A bold bar indicates the mean for each group. P = .739.
IFN production of ex vivo T lymphocytes in response to allogeneic third-party DCs.
Purified spleen T lymphocytes were obtained from naive BALB/c (H-2d, black triangles), FVB (H-2q, open squares) mice, or BALB/c mice grafted with 106 BM cells from FVB mice plus apoptotic SCs of C57BL/6 (H-2b) mice (noted TP, black circles) at day 45 to day 50 post-BMT. These T lymphocytes were used as responders and C57BL/6 mature DC as stimulants in a one-way mixed leukocyte reaction. The IFN-γ production was measured 48 hours later in the supernatant of cultures by enzyme-linked immunosorbent assay. The grafted mice (noted TP) included in this analysis had a complete FVB phenotype at day 45 to day 50 post-BMT. Four individual mice were tested in each group. A bold bar indicates the mean for each group. P = .739.
Discussion
Increased use of allogeneic HSC transplantation is limited by the high toxicity of this approach. The complex immunologic setting of allogeneic HSC transplantation is due to the possibility of direct and/or indirect presentation of allogeneic peptides by antigen-presenting cells (APCs) of donor and host origins to T cells of both origins. Interrelated consequences of this immunologic reactivity, such as graft rejection, GvHD, and leukemic relapse, significantly affect survival after HSC transplantation. The intensity of the pretransplant conditioning regimen has an important influence on the outcome of HSC transplantation, because of its toxicity and immunologic consequences. Reduction of the conditioning regimen is associated with increased graft rejection.20 In contrast, increased intensity of conditioning regimen can favor the GvHD occurrence, in part by the release of pro-inflammatory cytokines.21 Here we demonstrate that, in the context of suboptimal TBI regimen, simultaneous intravenous administration of apoptotic leukocytes with the BM graft has graft-facilitating effects. Using a restrictive model and 2 donor/recipient combinations, we found that SCs rendered apoptotic by different stimuli (γ-, UVB-irradiation, and anti-Fas mAb treatment) increased the proportion of recipient mice with a donor-type hematopoietic reconstitution. Interestingly, this graft-promoting effect was nonspecific, because it was also observed with the administration of apoptotic syngeneic, third-party, or xenogeneic leukocytes.
Several mechanisms may explain the graft-promoting effect induced by apoptotic cell infusion. One possibility is that irradiated SCs contained sufficient radioresistant hematopoietic progenitor cells capable of engraftment. This was not the case, as evidenced by the absence of a donor hematopoietic reconstitution after infusion of donor-irradiated SCs alone. Furthermore, a donor-type hematopoietic reconstitution was also observed when irradiated cells of recipient, third-party, or xenogeneic origins were injected.
Another possibility involves the deletion of recipient antidonor cytotoxic T-lymphocyte precursors and T-helper cells by “veto cells.”22 Indeed, such veto cells have been reported to facilitate engraftment.23 Several distinct cell populations can mediate such an effect, including BM cells24 and low-dose irradiated SCs.18 A “veto effect” directly mediated by the irradiated cells appears improbable, because the graft-promoting effect was also observed with apoptotic cells syngeneic to the recipient. However, the possibility that apoptotic cells could indirectly provide a microenvironment favoring putative veto cells present in the BM inoculum remains. Alternatively, Fas-mediated death of bystander leukocytes by macrophages phagocytizing apoptotic cells25 may contribute to the depletion of recipient antidonor T lymphocytes.
The nonspecificity of the graft-promoting effect (ie, independent of the MHC disparity between BM and apoptotic cells) suggests that immunosuppressive cytokines such as transforming growth factor β (TGF-β) or IL-10 might be involved. Interestingly, phagocytosis of apoptotic cells by APCs can induce secretion of such cytokines by these cells.7-10 Furthermore, apoptotic cells can themselves produce high quantities of IL-10.26 Cross-reactivity because of amino acid sequence conservation between mouse and human TGF-β and IL-10 (nearly 100% and 73%, respectively)27could at least in part explain our results with human irradiated PBMCs. We did not detect an up-regulation of IL-10 or TGF-β production by irradiated cells (results not shown), but we cannot exclude the possibility of immunosuppressive cytokine production after apoptotic cell phagocytosis in vivo.
Cells rendered apoptotic by different stimuli also had a graft-facilitating effect, implying that the apoptotic status of cells co-injected with BM cells plays a significant role in our findings. Therefore, an additional (and possibly synergistic) mechanism is the cross-tolerization of host antidonor T cells by APCs after a massive infusion of apoptotic cells. This process mimics the successive physiologic events limiting inflammation during the elimination of unwanted cells in tissue homeostasis.28 It has been shown that DCs can engulf, process apoptotic cells,29 and, under certain conditions, tolerize T cells,30,31 and, more specifically, induce alloantigen-specific hyporesponsiveness in vitro and in vivo.32 An immature DC subset trafficking through diverse tissues can continuously phagocytose cells undergoing normal turnover by apoptosis and then induce tolerance of autoreactive naive T cells in draining lymph nodes.11,33 One can therefore hypothesize that massive infusion of apoptotic leukocytes can overcome a DC stimulatory signal (inflammation induced by TBI21 34) with a tolerogenic signal favoring recipient antidonor T cells cross-tolerization. Whether this DC-mediated donor hyporeactivity would be mediated directly by donor DCs present in the BM or indirectly by recipient DCs is a question that remains to be answered.
A further explanation for these results is the potential immunosuppressive properties of MHC-derived peptides. Apoptotic cells co-injected with BM cells are a potential source of relative high quantities of MHC peptides. Peptides derived from the polymorphic or nonpolymorphic regions of MHC class I and II molecules have been described to possess immunomodulatory capacities both in vitro and in vivo.35 Human HLA-DQ1–derived peptide can indeed inhibit CD4+ lymphocyte rat alloimmune responses, supporting the immunomodulatory potential of xenogeneic MHC peptides.36
In conclusion, our findings show that it is possible to overcome the MHC barriers and to easily facilitate allogeneic HSC engraftment by the addition of apoptotic cells to the BM inoculum. Such an approach could be used to reduce the conditioning regimen intensity and its associated toxicity in some settings in which it is not desirable (ie, older patients, nonmalignant disorders). This approach would also permit the expansion of HSC transplantation to tolerance induction protocols for solid organ transplantation.37 Further studies are necessary to evaluate other important issues such as possible impairment of the graft-versus-leukemia response38 or triggering of autoimmunity39after the infusion of high quantities of apoptotic cells in the setting of HSC transplantation.
We thank Dany Trestchenkoff for help in preparing the manuscript, Eric Robinet and David E. Chalmers for critical reading of the manuscript, and Dominique Paris for his expertise in animal care and management.
Supported by grants from Fondation pour la Recherche Médicale (FRM) to M.C.B., from Association pour la Recherche sur le Cancer (No. 5567) to P.S., and from Comité Départemental de la Ligue contre le Cancer du Doubs to S.P. (Comité de Montbéliard), P.T., and P.S.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Philippe Saas, Laboratoire Thérapeutique Immuno-Moléculaire, Etablissement Français du Sang Bourgogne Franche-Comté, 1 boulevard A Fleming, BP 1937, F-25020 Besançon, France; e-mail: philippe.saas@efs.sante.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal