Abstract
For the treatment of β-globin gene defects, a homologous recombination-mediated gene correction approach would provide advantages over random integration-based gene therapy strategies. However, “neighborhood effects” from retained selectable marker genes in the targeted locus are among the key issues that must be taken into consideration for any attempt to use this strategy for gene correction. An Ala-to-Ile mutation was created in the β6 position of the mouse β-major globin gene (β6I) as a step toward the development of a murine model system that could serve as a platform for therapeutic gene correction studies. The marked β-major gene can be tracked at the level of DNA, RNA, and protein, allowing investigation of the impact of a retained phosphoglycerate kinase (PGK)-neo cassette located between the mutant β-major and β-minor globin genes on expression of these 2 neighboring genes. Although the PGK-neo cassette was expressed at high levels in adult erythroid cells, the abundance of the β6I mRNA was indistinguishable from that of the wild-type counterpart in bone marrow cells. Similarly, the output from the β-minor globin gene was also normal. Therefore, in this specific location, the retained, transcriptionally active PGK-neo cassette does not disrupt the regulated expression of the adult β-globin genes.
Introduction
Since the molecular cloning and sequencing of the globin genes more than 20 years ago, many investigators have explored gene therapy as a potential mechanism for treating β-globin gene defects.1 The gene therapy approach that has been most carefully studied makes use of retroviral vectors to introduce β-globin genes into hematopoietic stem or progenitor cells, where the β-globin gene randomly integrates into the host cell genome (reviewed in 2). This approach has met with limited success for a variety of reasons, including low efficiency of progenitor cell transduction,3,4 variegation of expression of the integrated β-globin genes, and silencing of the integrated β-globin genes that occurs as a function of time.5,6 When the locus control region was identified more than a decade ago, the core regions of the hypersensitive sites were added to retroviral vectors to reduce or eliminate the integration site-specific variegation of β-globin gene expression.7-9 Unfortunately, this change in the design of these vectors led to instability in the viral genomes on integration, which limited the usefulness of the locus control region (LCR) in retroviral systems.7,8 Although improvements in the system have recently been described using lentivirus-based vectors, fundamental problems with regulated β-globin gene expression still exist.10 In addition, retroviral vectors necessarily cause mutations at sites of integration, and the results of these mutations in any given recipient cell cannot be predicted. The mutagenesis caused by random integration remains a problem for most gene therapy strategies.
Alternative approaches for the gene therapy of β-globin disorders include “gene correction”11,12 and homologous recombination.13-15 Shesely et al13 first described the production of a targeted mutation of the human β-globin locus with homologous recombination in mouse erythroleukemia (MEL) cells containing a single copy of human chromosome 11. In that experiment, the authors used a targeting vector that contained 4.7 kb nonisogenic human DNA in the targeting arms. One integration event in 10 000 was shown to be homologous. More recently, using vectors containing 2 to 3 kb isogenic DNA in each targeting arm and positive selection only, we and others have shown that many vectors homologously recombine 1% to 2% of the time.
An additional unexpected effect of homologous recombination has been described by many laboratories—retained selectable marker cassettes can have strong effects on the expression of neighboring genes.16-24 Groudine et al16,17 first reported that when selectable marker cassettes are targeted to the LCR of the β-globin gene cluster, the LCR appears to preferentially interact with the selectable marker gene so that the expression of downstream globin genes is dramatically reduced. “Neighborhood effects” have also been described in many other multigene loci, and the effect can be noticeable for hundreds of kilobases.20-23 The neighborhood effect apparently has a directional component in some loci,21 but general rules for neighborhood effects have not yet been established.
The low efficiency of homologous recombination in MEL cells and the powerful neighborhood effects of retained selectable marker genes in the β-globin locus suggested that homologous recombination-mediated gene correction of the β-globin gene would not be useful. In this study, however, we created a subtle mutation in the β6 position of the mouse β-major globin gene (β6I), a mutation that resembles the βS mutation in humans. This mutation allows us to track the mutant β gene at the level of DNA, RNA, and protein. The mutant locus contains a transcriptionally active phosphoglycerate kinase (PGK)-neo cassette between the β-major and β-minor globin genes. Remarkably, the retained PGK-neo cassette did not affect the output of either β-globin gene, suggesting that neighborhood effects may not limit the ability of homologous recombination to be used as a gene correction strategy for mutant β-globin genes.
Materials and methods
Construction of the β6I targeting vector
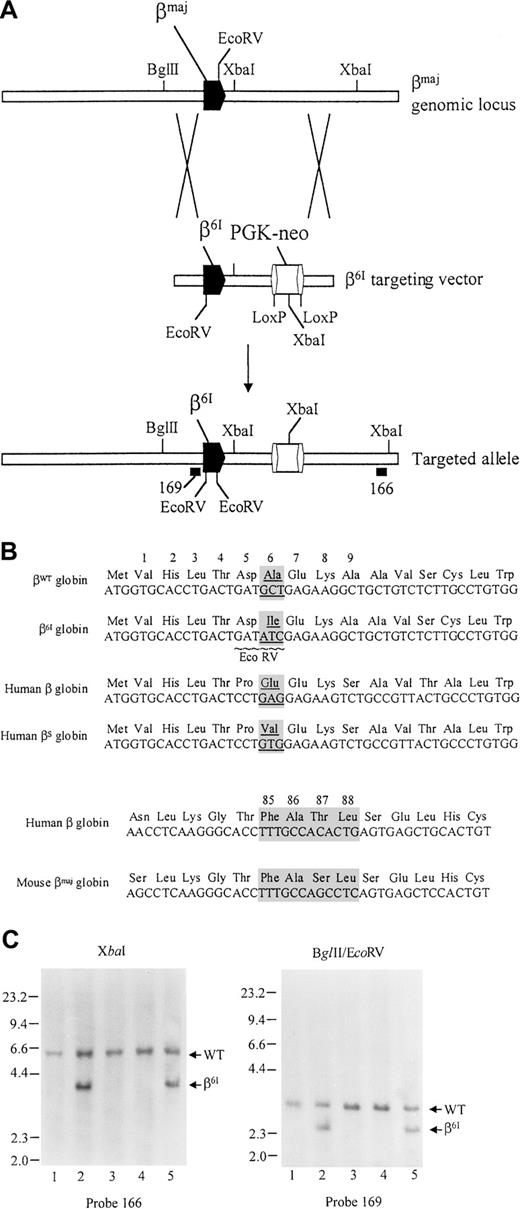
The targeting strategy is depicted in Figure1A. The targeting vector consists of a 6-kb left-targeting arm, a LoxP-flanked phosphoglycerate kinase I gene promoter-driven neomycin resistance gene cassette (PGK-neo), and a 2.2-kb right-targeting arm. The left-targeting arm, containing a 6-kb AvaI fragment spanning the mouse β-major gene, was isolated from a 110-kb 129/SvOla DNA-derived P1 clone (designated 1935), containing the entire mouse β-globin cluster. The left arm was subcloned into pGEM3zf+ (Promega, Madison, WI). Site-directed mutagenesis of codon 6 (GCT→ATC) was then performed using the Quickchange mutagenesis kit (Stratagene, La Jolla, CA), and the presence of the “β6I” mutation was confirmed by sequence analysis. The entire β-major gene and flanking DNA were also sequenced and were found normal. Using P1 1935 as the template, the right-targeting arm was generated by polymerase chain reaction (PCR) using the forward primer 5′-ACGTGGATCCACTGCGAGGCAGAGGCAGGC-3′ and the reverse primer 5′-ACGTAAGCTTTATGCCTCAGTACAGGGGAT-3′, which generates a 2.2-kb BamHI-HindIII fragment from sequences located between 4131 and 6370 bp downstream from β-major cap site. The left arm, PGK-neo cassette, and right arm were cloned in the proper orientation in pCR 2.1 (Invitrogen, Carlsbad, CA), as shown in Figure1A. No DNA sequences were removed from the targeted locus.
Targeting strategy for generating the β6Imutation.
(A) Top line depicts the structure of wild-type β-major genomic locus; the β6I targeting vector is shown in the middle. The targeted allele with a retained PGK-neo cassette is depicted on the bottom line. Locations of external probe 166 and internal probe 169 are shown. (B) Comparison of DNA and amino acid sequences of mouse β-major globin and human β globin genes. (C) Southern blot analysis of genomic tail DNA derived from the progeny of β6Iheterozygous mutant mice. (left) DNA digested with XbaI and hybridized with probe 166. (right) DNA digested with EcoRV and BglII and hybridized with probe 169.
Targeting strategy for generating the β6Imutation.
(A) Top line depicts the structure of wild-type β-major genomic locus; the β6I targeting vector is shown in the middle. The targeted allele with a retained PGK-neo cassette is depicted on the bottom line. Locations of external probe 166 and internal probe 169 are shown. (B) Comparison of DNA and amino acid sequences of mouse β-major globin and human β globin genes. (C) Southern blot analysis of genomic tail DNA derived from the progeny of β6Iheterozygous mutant mice. (left) DNA digested with XbaI and hybridized with probe 166. (right) DNA digested with EcoRV and BglII and hybridized with probe 169.
Creation of β6I mice
Forty micrograms targeting vector was linearized by ApaI digestion and electroporated into RW4 embryonic stem (ES) cells (derived from a 129/SvJ mouse) as previously described.19 G418-resistant ES clones were isolated and screened by Southern blot analysis using the 3′ external probe designated 166. Two correctly targeted ES clones (83 and 140) were confirmed by Southern blot analysis to contain the β6Imutation. C57BL/6 blastocysts were then microinjected with both clones and implanted into pseudopregnant Swiss Webster foster females to generate chimeras, as previously described.19 Chimeric males were bred with wild-type 129/SvJ female mice, and germline transmission of the β6I mutation was confirmed by Southern blot analysis.
RNA expression analysis
Total RNA from mouse bone marrow and peripheral blood was isolated as described.25 Two micrograms total RNA from each sample was reverse-transcribed into complementary DNA (cDNA) exactly as previously described25 and analyzed by PCR (see below). The relative messenger RNA (mRNA) expression of β6I and the wild-type β-major gene was measured by a real-time PCR assay using the TaqMan system (PerkinElmer, Norwalk, CT). An exon 1 forward primer specific for either wild-type mouse β-major sequence 5′-TGGTGCACCTGACTGATGCT-3′) or β6I sequence 5′-TGGTGCACCTGACTGATATC-3′) was used in combination with a universal exon 2 reverse primer 5′-CCATGGGCCTTCACTTTGGC-3′). The output from the wild-type β-major allele or the mutant β6I allele was measured using either the wild-type primer set or β6I primer set, respectively. The PCR reaction was performed in the presence of a DNA intercalating dye, SYBR green. A PCR amplification profile was derived by recording SYBR green fluorescence intensity, which was in linear relation to the amount of PCR product formed, as a function of PCR cycle number. A standard curve was generated by plotting the PCR cycle number at which a reaction entered exponential amplification versus the amount of input DNA and was then used for a determination of sample template concentration.26
A semiquantitative reverse transcription (RT)-PCR assay was used to measure murine peripheral blood β-minor mRNA expression. A PCR primer set specific for murine β-minor gene was designed to span intron 1 (forward primer, 5′-GAGTCTGTTGTGTTGACTTG-3′; reverse primer, 5′-TTGTCCAGGTTTTTCAGGCCC-3′). The PCR product from the mouse β-minor cDNA measured 294 bp. A murine β-actin gene primer set was included as a control for RNA loading (forward primer, 5′-GCTGTATTCCCCTCCATCGTG-3′; reverse primer, 5′-CACGGTTGGCCTTAGGGTTCAG-3′), which generated a 265-bp PCR fragment. The PCR reaction was performed in the presence of 20 μCi/mL α-32P]dATP for 25 cycles. PCR products were resolved by electrophoresis on tris borate EDTA (TBE)-5% polyacrylamide gels, and radioactivity of individual bands was quantitated using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA). Relative β-minor mRNA expression was normalized for β-actin mRNA abundance and expressed as a percentage of wild-type expression.
Globin chain analysis
Triton-acid-urea–polyacrylamide gel electrophoresis (PAGE) was used to quantitate globin chain composition of peripheral red blood cells (RBCs) as previously described27 with minor modifications.
Hematopoietic progenitor cell assay
Mice were killed, and bone marrow cells were harvested from the tibiae and femurs. Mononuclear cells were isolated using Histopaque 1077 (Sigma, St Louis, MO). Viable cells were quantified using trypan blue staining. For analysis of colony-forming unit–granulocyte (CFU-G) and colony-forming unit–granulocyte-macrophage (CFU-GM), the cells were plated at a concentration of 50 000 cells/mL in Methocult M 3434 (Stemcell Technologies, Vancouver, BC, Canada), containing 10 ng/mL recombinant murine interleukin-3 (rmIL-3), 50 ng/mL recombinant murine stem cell factor (rmSCF), 10 ng/mL rhIL-6, and 3 U/mL recombinant human erythropoietin (rhEPO), with or without 900 μg G418 (active)/mL media. For analysis of burst-forming unit-erythroid (BFU-E), bone marrow cells were plated in Methocult M3334 (Stemcell Technologies) at a concentration of 200 000 viable cells/mL (with rhEPO at 3 U/mL), with or without G418 at a concentration of 900 μg (active)/mL. Colonies were quantified 8 days after plating.
Detection of neomycin phosphotransferase
Splenocytes, bone marrow, and peripheral blood cells were harvested from wild-type mice and β6I homozygous mice, cytospun onto coverslips, fixed in methanol, rehydrated in phosphate-buffered saline (PBS), and blocked for 30 minutes in blocking solution (0.1% Triton X-100, 1% bovine serum albumin in PBS). Coverslips were incubated in rabbit anti–Tn5 Neo polyclonal antibody (US Biological, Swampscott, MA) in blocking solution for 1 hour. The coverslips were then washed twice in washing solution (0.1% Triton X-100 in PBS), stained with fluorescein-conjugated goat antirabbit secondary antibody for 1 hour, and washed twice in washing solution. Cells were costained with phycoerythrin (PE)-conjugated monoclonal antibodies directed against various mouse cell surface markers, as previously described.28 Nuclear DNA was visualized with 4′,6-diamidino-2-phenylindole (DAPI). Samples were viewed with a Nikon Microphot-SA fluorescence microscope.
Neo protein was also detected using flow cytometric analysis. Bone marrow cells were first stained with PE-conjugated monoclonal antibodies against various mouse cell surface markers (Ter-119, Gr-1, or CD3). Then the cells were fixed in Cytofix/Cytoperm solution (Pharmingen, San Diego, CA) at 4°C overnight, washed twice in Perm/Wash buffer (Pharmingen), and incubated in Perm/Wash buffer containing rabbit anti-Neo antibody at 4°C for 30 minutes. The cells were washed twice in Perm/Wash buffer, incubated with fluorescein-conjugated antirabbit secondary antibody for 30 minutes, and finally washed twice in Perm/Wash buffer. Dual-color flow cytometric analysis was performed using a FACScan flow cytometer (Becton Dickson, San Jose, CA), as previously described.28
Blood cell morphology and sickling test
Blood smears were prepared from oxygenated mouse peripheral blood, followed by May-Grünwald-Giemsa staining. For the sickling test, 10 μL freshly collected blood was mixed with 10 μL 2% sodium metabisulfite solution on a slide and incubated under sealed coverslips at room temperature for 30 minutes to induce deoxygenation.
Hemoglobin solubility test
Solubility of hemolysates was studied by measuring turbidity in a concentrated potassium phosphate buffer on deoxygenation, exactly as previously described.29
Hematologic parameters
Peripheral blood was collected from the retro-orbital sinuses of adult mice and analyzed using a Hemavet 850 system (CDC Technologies, Oxford, CT). Reticulocytes were stained with acridine orange and analyzed using a FACScan flow cytometer (Becton Dickson) as previously described.30
Hemoglobin oxygen-binding curve
Results
Design of the β6I mutation in the mouse β-major globin gene
In this study, we developed mice with a mutation in position 6 of the mouse β-major globin gene. This mutation, which changes GCT (encoding Ala) to ATC (encoding Ile), was designed for several reasons (Figure 1B). First, this mutation creates a novel EcoRV site in the β-major gene and serves as a genetic mark that can be followed by restriction enzyme analysis. Second, the β6 Ala-to-Ile (β6I) mutation should produce a mutant β-globin chain with an altered hydrophobicity, and it should be distinguishable from wild-type β-major globin using routine globin chain separation methods. Third, the β6I mutation was predicted to produce a murine hemoglobin that might polymerize on deoxygenation.
Creation of β6I mutant mice
We transfected RW-4 ES cells with the targeting vector shown in Figure 1A. Four correctly targeted RW4 ES clones were identified out of 220 screened. Two clones (83 and 140) were arbitrarily selected for further analysis and were confirmed on Southern blot to contain the β6I mutation. Both cell lines were then microinjected into C57BL/6 blastocysts to generate chimeras. Both lines transmitted the β6I mutation through the germline. Figure 1C shows a Southern blot analysis of genomic tail DNA from 4 F1 littermates produced by mating a chimera with a wild-type 129/SvJ female. Shown in the left panel is a Southern blot of XbaI-digested tail DNA derived from one wild-type 129/SvJ control mouse (lane 1) and the 4 F1 mice (lanes 2-5) and hybridization with the external probe 166. The wild-type allele produced a 5.6-kb fragment. The mutant allele, containing an internal XbaI site within the PGK-neo cassette, produced a diagnostic 3.8-kb fragment. Two of the mice (lanes 2 and 5) are heterozygous for the targeted allele. In the right panel, the same group of DNA samples was hybridized with internal probe 169 after EcoRV/BglII double-digestion. The wild-type fragment measured 3.4 kb. The β6I mutation created a newEcoRV site that reduced the size of the hybridizing band to 2.7 kb. The presence of the predicted 2.7-kb EcoRV/BglII fragment confirmed that mice represented in lanes 2 and 5 were heterozygous for the β6I mutation. Hybridization of blots with a neo-specific probe revealed a single hybridizing band of the predicted size for each enzyme tested; a single PGK-neo cassette was, therefore, integrated in the genome (data not shown). Heterozygous mice were bred with wild-type 129/SvJ mice, and heterozygous F2 mice were then intercrossed. The ratio of βWT/βWT:βWT/β6I:β6I/β6I mice produced by these matings was 25:46:39.
Expression of adult β-globin genes in β6Imutant mice
The relative expression of the β6I and wild-type β-major genes was compared in bone marrow RNA obtained from heterozygous β6I mice using a real-time PCR assay. In this assay, an exon 1 forward primer specific for either wild-type mouse β-major sequence or β6I sequence was used in combination with a universal exon 2 reverse primer. PCR reactions were performed in the presence of a DNA intercalating dye, SYBR green. A PCR amplification curve was derived in a real-time by plotting PCR product fluorescence intensity (ΔRn) on the y-axis versus PCR cycle number on the x-axis. In Figure 2A, 10-fold serial dilutions (100 pg, 10 pg, 1 pg, and 0.1 pg DNA) of pβWT, a plasmid containing the wild-type β-major gene, were amplified using either the βWT primer set (reactions 1-4) or β6I primer set (reactions 5-8). Reaction 1 entered the exponential phase of amplification at cycle 11. Decreasing the amount of input template DNA resulted in a progressive delay in entry into the exponential phase. In contrast, the β6I-specific primer did not amplify the wild-type β-major template, as evidenced by a lack of exponential rise in fluorescence (reactions 5-8), demonstrating the specificity of this assay. Next, as shown in Figure 2B, 10 ng plasmid DNA containing the mutant β6I(pβ6I) or the wild-type β-major sequence (pβWT) was used in a real-time PCR assay. Each plasmid sample (or a control with no DNA) was amplified using either the βWT primer set (reactions 1-3) or β6Iprimer set (reactions 4-6). Exponential amplification was only observed when the templates were appropriately matched with their specific primer sets (reactions 1 and 5 vs. reactions 2 and 4). Amplification curves of reactions 1 and 5 were superimposable, suggesting that wild-type β-major and β6I sequences were amplified with equal efficiency. Bone marrow RNA harvested from a β6Iheterozygous mouse or a wild-type control mouse was reverse-transcribed to cDNA and analyzed by real-time PCR (Figure 2C). The output from the wild-type β-major allele or mutant β6I allele was measured, respectively, using either the βWT primer set (reactions 1 and 2) or β6I primer set (reactions 3 and 4). A reaction amplifying the wild-type peripheral blood cDNA with the β6I primer set was included as a negative control (reaction 5). Amplification curves generated by reactions 1 to 4 were indistinguishable from one another, indicating that the β6I allele was expressed at a level equivalent to that of the wild-type β-major gene. These experiments were repeated 3 times with similar results.
Targeted insertion of a PGK-neo cassette does not disrupt the regulated expression of 2 neighboring murine β-major and β-minor globin genes.
(A) Real-time PCR assay for the wild-type mouse β-major genomic DNA. PCR product fluorescence intensity (ΔRn) is plotted on the y-axis versus PCR cycle number on the x-axis. Tenfold serial dilutions of pβWT, a plasmid encoding the wild-type β-major gene, were amplified using either the βWT primer set (reactions 1-4) or β6I primer set (reactions 5-8). Plotting the PCR cycle number at which the reaction entered exponential amplification, versus the logarithm of the amount of input DNA, generated a standard curve (not shown). (B) Ten nanograms plasmid DNA encoding the mutant β6I (pβ6I) or the wild-type β-major sequence (pβWT) was used for real-time PCR. Each plasmid sample or a no DNA control (No DNA) was amplified using either the βWT primer set (reactions 1-3) or the β6Iprimer set (reactions 4-6). (C) Real-time PCR analysis of bone marrow RNA. Bone marrow RNA was harvested from a heterozygous β6I mouse (βWT/β6I) or a wild-type control mouse (βWT/βWT), reverse-transcribed to cDNA, and analyzed by real-time PCR using either the βWT primer set (reactions 1-2) or β6Iprimer set (reactions 3-4). A reaction amplifying the wild-type peripheral blood cDNA with the β6I primer set was included as a negative control (reaction 5). (D) Semiquantitative RT-PCR analysis of peripheral blood RNA. Peripheral blood RNA was harvested from homozygous β6I mice (β6I/β6I) or wild-type control mice (βWT/βWT), reverse-transcribed, and analyzed by PCR using a β-minor primer set or a β-actin gene primer set. Reactions were performed in the presence of α-32P]dATP and stopped before amplifications reached a product plateau (not shown). Amplification products were resolved on 5% TBE-polyacrylamide gels and quantitated using a PhosphorImager. For each experiment, the relative β-minor mRNA expression was normalized for β-actin mRNA level and expressed as a percentage of wild-type expression. Shown are the mean values, along with standard deviations, from 3 separate experiments in which 2 samples were used for each genotype.
Targeted insertion of a PGK-neo cassette does not disrupt the regulated expression of 2 neighboring murine β-major and β-minor globin genes.
(A) Real-time PCR assay for the wild-type mouse β-major genomic DNA. PCR product fluorescence intensity (ΔRn) is plotted on the y-axis versus PCR cycle number on the x-axis. Tenfold serial dilutions of pβWT, a plasmid encoding the wild-type β-major gene, were amplified using either the βWT primer set (reactions 1-4) or β6I primer set (reactions 5-8). Plotting the PCR cycle number at which the reaction entered exponential amplification, versus the logarithm of the amount of input DNA, generated a standard curve (not shown). (B) Ten nanograms plasmid DNA encoding the mutant β6I (pβ6I) or the wild-type β-major sequence (pβWT) was used for real-time PCR. Each plasmid sample or a no DNA control (No DNA) was amplified using either the βWT primer set (reactions 1-3) or the β6Iprimer set (reactions 4-6). (C) Real-time PCR analysis of bone marrow RNA. Bone marrow RNA was harvested from a heterozygous β6I mouse (βWT/β6I) or a wild-type control mouse (βWT/βWT), reverse-transcribed to cDNA, and analyzed by real-time PCR using either the βWT primer set (reactions 1-2) or β6Iprimer set (reactions 3-4). A reaction amplifying the wild-type peripheral blood cDNA with the β6I primer set was included as a negative control (reaction 5). (D) Semiquantitative RT-PCR analysis of peripheral blood RNA. Peripheral blood RNA was harvested from homozygous β6I mice (β6I/β6I) or wild-type control mice (βWT/βWT), reverse-transcribed, and analyzed by PCR using a β-minor primer set or a β-actin gene primer set. Reactions were performed in the presence of α-32P]dATP and stopped before amplifications reached a product plateau (not shown). Amplification products were resolved on 5% TBE-polyacrylamide gels and quantitated using a PhosphorImager. For each experiment, the relative β-minor mRNA expression was normalized for β-actin mRNA level and expressed as a percentage of wild-type expression. Shown are the mean values, along with standard deviations, from 3 separate experiments in which 2 samples were used for each genotype.
A semiquantitative RT-PCR assay was next used to measure murine peripheral blood β-minor mRNA expression. Peripheral blood RNA was harvested from homozygous β6I mice (β6I/β6I) or wild-type mice (βWT/βWT), reverse-transcribed to cDNA, and analyzed by PCR using a murine β-minor primer set or a murine β-actin gene primer set as a control for RNA quality and abundance. PCR reactions were performed in the presence of [α-32P]dATP and stopped before amplifications reached a plateau. PCR products were resolved by PAGE, and the radioactivity of individual bands was quantitated with the Phosphorimager (Molecular Dynamics). As shown in Figure 2D, the output from the β-minor globin gene was equivalent in β6I/β6I and βWT/βWT mice.
Globin chain composition in the red blood cells of β6I mutant mice
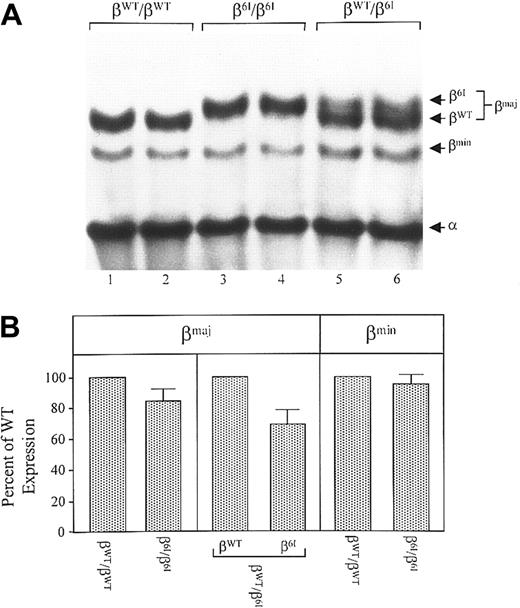
We next measured the abundance of β6I globin chains in peripheral RBCs. Figure 3A shows Triton-acid-urea–PAGE analysis of a globin chain composition of hemolysates derived from wild-type controls (βWT/βWT; lanes 1 and 2), β6Ihomozygotes (β6I/β6I; lanes 3 and 4), and heterozygotes (βWT/β6I; lanes 5 and 6). The levels of βWT, β6I, βmin, and α globin chains were quantitated by densitometry; a mean value was derived from duplicates run on each gel and expressed as a percentage of βWT. As shown in Figure 3A, β6I globin exhibited retarded electrophoretic mobility compared with the βWT chain. In the erythrocytes of heterozygous mice, β6I globin represented 40% of all β-major globin chains (Figure 3B). A similar reduction in the abundance of β6I mutant chains was also detected in homozygous mice relative to that of βWT chains in wild-type mice (84% that of βWT). The abundance of β-minor (Figure 3B) and α-globin chains in the RBCs of β6I homozygotes, heterozygotes, and wild-type animals were identical. Therefore, despite the equal abundance of β6Ι and wild-type mRNAs, the mutant β-globin chains are less abundant than WT β chains in peripheral red cells, suggesting that a posttranscriptional mechanism(s) contributes to this difference in vivo.
Globin-chain composition in mouse RBCs.
(A) Hemolysates were prepared from the peripheral blood of β6I/β6I mice, βw/β6I mice, or βWT/βWT mice. Globin chains from fresh hemolysates were resolved on TAU-polyacrylamide gels and visualized with Coomassie blue. Shown is a representative gel from 4 that displayed virtually identical protein profiles. Positions of βWT, β6I, βmin, and α globin chains are indicated. (B) The βWT, β6I, and βmin chains were quantitated by densitometry. An average was calculated from duplicates run on each gel and expressed as a percentage of wild-type level. Shown are the means, along with standard deviations, from 4 different gels.
Globin-chain composition in mouse RBCs.
(A) Hemolysates were prepared from the peripheral blood of β6I/β6I mice, βw/β6I mice, or βWT/βWT mice. Globin chains from fresh hemolysates were resolved on TAU-polyacrylamide gels and visualized with Coomassie blue. Shown is a representative gel from 4 that displayed virtually identical protein profiles. Positions of βWT, β6I, βmin, and α globin chains are indicated. (B) The βWT, β6I, and βmin chains were quantitated by densitometry. An average was calculated from duplicates run on each gel and expressed as a percentage of wild-type level. Shown are the means, along with standard deviations, from 4 different gels.
Expression of the retained PGK-neo cassette in β6I mutant mice
We next examined the expression of the PGK-neo cassette in various adult β6I/β6I mouse organs using an S1 nuclease protection assay (data not shown). Correctly initiated PGK-neo mRNA was detected at low levels in RNA derived from the hearts, lungs, kidneys, or livers of these mice, but it was easily detected in RNA derived from the bone marrow and spleens of β6I/β6I mice.
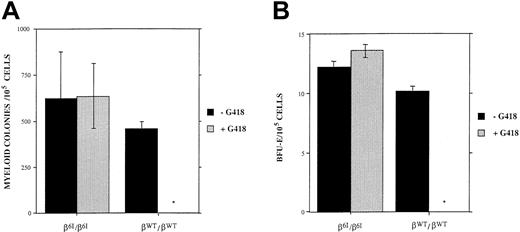
To determine whether the PGK-neo cassette was expressed during hematopoietic development, we plated bone marrow cells from wild-type mice or from β6I/β6I mice in a methylcellulose-based culture system. Bone marrow cells were cultured in the presence or absence of 900 μg/mL (active) G418 (Figure4). In the absence of G418, there was no significant difference in the number, size, or morphology of CFU-G–, CFU-GM–, or BFU-E–derived colonies either from wild-type mice or from β6I/β6I mice. The presence of G418 in the media almost entirely inhibited erythroid and myeloid colony formation from bone marrow progenitors derived from wild-type mice but had no effect on the number of colonies derived from β6I/β6I mice (Figure 4). Moreover, the presence of G418 had no effect on the size or morphology of the hematopoietic colonies derived from the β6I/β6I mice (data not shown). These results suggest that the neomycin phosphotransferase gene in the β-globin gene cluster is active in early erythroid and myeloid progenitor cells derived from β6I/β6Imutant mice and that the cassette remains active throughout both erythroid and myeloid differentiation.
Colony quantitation.
(A) Quantitation of myeloid colonies (CFU-G and CFU-GM) derived from the bone marrow cells of either wild type or β6I/β6I mice using growth conditions that permitted myeloid differentiation after 8 days of culture. (B) Quantitation of erythroid colonies (BFU-E) using conditions that permitted only erythroid growth after 8 days of culture. There was no significant difference in the numbers of myeloid or erythroid colonies derived from either the β6I/β6I or the βwt/βwt mice in the absence of G418. The addition of 900 μg/mL active G418 caused near-complete suppression of colony formation from wild-type bone marrow cells. In contrast, G418 did not suppress myeloid or the erythroid colony numbers (or the sizes of colonies) derived from the bone marrow cells of β6I/β6I mice. Graphs represent means and standard deviations from 3 separate experiments.
Colony quantitation.
(A) Quantitation of myeloid colonies (CFU-G and CFU-GM) derived from the bone marrow cells of either wild type or β6I/β6I mice using growth conditions that permitted myeloid differentiation after 8 days of culture. (B) Quantitation of erythroid colonies (BFU-E) using conditions that permitted only erythroid growth after 8 days of culture. There was no significant difference in the numbers of myeloid or erythroid colonies derived from either the β6I/β6I or the βwt/βwt mice in the absence of G418. The addition of 900 μg/mL active G418 caused near-complete suppression of colony formation from wild-type bone marrow cells. In contrast, G418 did not suppress myeloid or the erythroid colony numbers (or the sizes of colonies) derived from the bone marrow cells of β6I/β6I mice. Graphs represent means and standard deviations from 3 separate experiments.
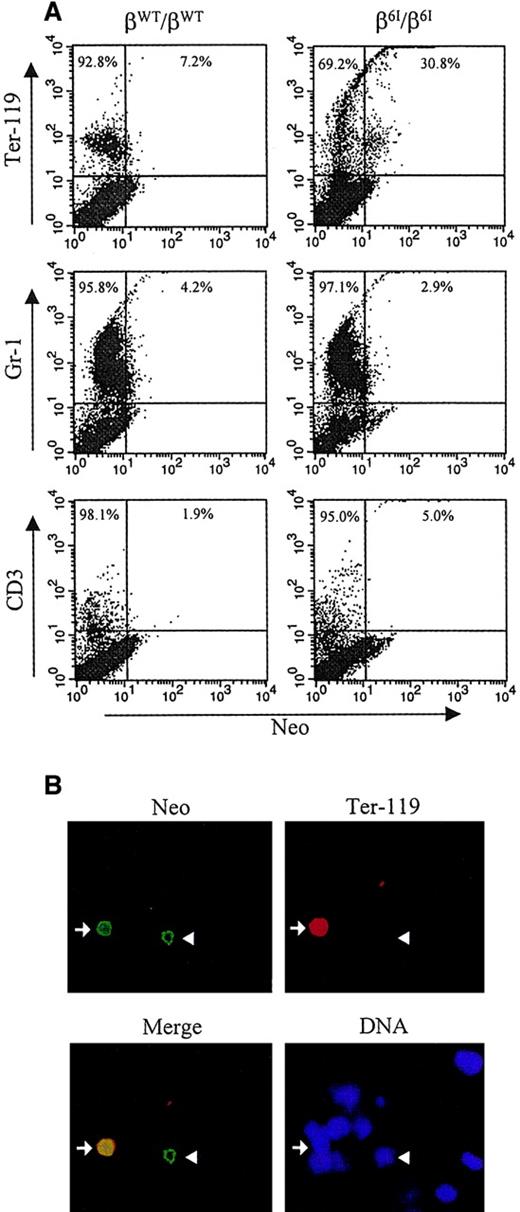
We next sought to detect neomycin phosphotransferase in individual bone marrow cells derived from adult β6I/β6Imice. Figure 5A shows a 2-color flow cytometric analysis of Neo expression in erythroid cells (Ter-119+), late myeloid cells (Gr-1+), and CD3+ T lymphocytes derived from the bone marrow cells of adult βWT/βWT or β6I/β6I mice. Neo-labeling was not detected in the late myeloid compartment or in T cells. In contrast, a fraction of β6I/β6I Ter-119+erythroid cells stained positively for Neo (Figure 5A).
Expression of PGK-neo in β6I mice.
(A) Flow cytometric analysis was used to quantitate cells expressing immunoreactive neomycin phosphotransferase. Bone marrow cells were first labeled with PE-conjugated monoclonal antibodies specific for cell surface antigen Ter-119, Gr-1, or CD3. Cells were then fixed in Cytofix/Cytoperm solution and incubated with rabbit anti-Neo polyclonal antibody and fluorescein-conjugated antirabbit secondary antibody. Neo expression is plotted on the x-axis, and surface markers are plotted on the y-axis. (B) Bone marrow cells from β6I/β6I mice were analyzed for the presence of immunoreactive neomycin phosphotransferase by indirect immunofluorescence microscopy. Cells were first stained with PE-conjugated antibody specific for murine erythroid lineage marker Ter-119, spun onto coverslips, fixed in methanol, and incubated with rabbit anti-Neo polyclonal antibody and fluorescein-conjugated antirabbit secondary antibody. Nuclear DNA was visualized with DAPI. A representative microscopic field is shown for Neo (Neo), Ter-119 (Ter-119), and DNA staining (DNA). A merged micrograph of the Neo and Ter-119 labeling patterns is also shown (Merge). Arrows indicate, Ter-119+/Neo+ cells; arrowheads, Ter-119-/Neo+ cells. Magnification × 200.
Expression of PGK-neo in β6I mice.
(A) Flow cytometric analysis was used to quantitate cells expressing immunoreactive neomycin phosphotransferase. Bone marrow cells were first labeled with PE-conjugated monoclonal antibodies specific for cell surface antigen Ter-119, Gr-1, or CD3. Cells were then fixed in Cytofix/Cytoperm solution and incubated with rabbit anti-Neo polyclonal antibody and fluorescein-conjugated antirabbit secondary antibody. Neo expression is plotted on the x-axis, and surface markers are plotted on the y-axis. (B) Bone marrow cells from β6I/β6I mice were analyzed for the presence of immunoreactive neomycin phosphotransferase by indirect immunofluorescence microscopy. Cells were first stained with PE-conjugated antibody specific for murine erythroid lineage marker Ter-119, spun onto coverslips, fixed in methanol, and incubated with rabbit anti-Neo polyclonal antibody and fluorescein-conjugated antirabbit secondary antibody. Nuclear DNA was visualized with DAPI. A representative microscopic field is shown for Neo (Neo), Ter-119 (Ter-119), and DNA staining (DNA). A merged micrograph of the Neo and Ter-119 labeling patterns is also shown (Merge). Arrows indicate, Ter-119+/Neo+ cells; arrowheads, Ter-119-/Neo+ cells. Magnification × 200.
The expression of the PGK-neo cassette in the erythroid compartment was confirmed by immunofluorescence analysis, as shown in Figure 5B. As expected, positive Neo staining was readily detected in a significant proportion of Ter-119+ erythroid cells (Figure 5B, arrow) but not in the Gr-1+ or the CD3+ population (data not shown). We also observed a small percentage of Neo+ cells in the adult bone marrow that were Ter-119− (Figure 5B, arrowhead); the lineage of these Neo+ cells is unknown. However, in view of the results from the colony assays (Figure 4), these Neo-expressing cells may include earlier erythroid progenitor cells (BFU-E, CFU-E, or proerythroblasts) that do not express the late erythroid marker Ter-119.
As revealed by immunofluorescence microscopy, β6I/β6I mice also contained a small percentage (less than 2%) of Neo+ RBCs in their peripheral blood (data not shown). These observations strongly suggest that Neo is present in reticulocytes, but it has a short half-life in mature peripheral red cells.
Erythrocyte and hemoglobin characteristics of β6I mice
There was no significant difference between the RBC counts, hemoglobin concentrations (Hb), hematocrit values (Hct), or other red cell indices of β6I/β6I mice compared with their wild-type counterparts (Table 1). The reticulocyte counts of all mutant mice were also normal (3% or less; data not shown). Similarly, heterozygous mice (βWT/β6I) also had normal hematologic values (Table 1) and reticulocyte counts (data not shown).
Hematologic parameters of wild-type mice and of β61 heterozygous and homozygous mutant mice
| Genotype . | n . | RBC (106/μl) . | Hb (g/dL) . | Hct (%) . | MCV (fL) . | MCH (pg) . | MCHC (g/dL) . |
|---|---|---|---|---|---|---|---|
| βWT/βWT | 12 | 10.4 ± 1.4 | 14.6 ± 1.8 | 51.1 ± 7.0 | 49.3 ± 1.8 | 14.1 ± 1.1 | 28.7 ± 1.9 |
| βWT/β61 | 12 | 10.3 ± 0.9 | 14.5 ± 1.3 | 50.6 ± 4.6 | 49.3 ± 1.3 | 14.1 ± 0.7 | 28.7 ± 1.4 |
| β61/β61 | 12 | 11.4 ± 0.9 | 15.7 ± 1.1 | 57.6 ± 3.7 | 50.8 ± 2.1 | 13.9 ± 0.8 | 27.4 ± 1.6 |
| Genotype . | n . | RBC (106/μl) . | Hb (g/dL) . | Hct (%) . | MCV (fL) . | MCH (pg) . | MCHC (g/dL) . |
|---|---|---|---|---|---|---|---|
| βWT/βWT | 12 | 10.4 ± 1.4 | 14.6 ± 1.8 | 51.1 ± 7.0 | 49.3 ± 1.8 | 14.1 ± 1.1 | 28.7 ± 1.9 |
| βWT/β61 | 12 | 10.3 ± 0.9 | 14.5 ± 1.3 | 50.6 ± 4.6 | 49.3 ± 1.3 | 14.1 ± 0.7 | 28.7 ± 1.4 |
| β61/β61 | 12 | 11.4 ± 0.9 | 15.7 ± 1.1 | 57.6 ± 3.7 | 50.8 ± 2.1 | 13.9 ± 0.8 | 27.4 ± 1.6 |
All values are means ± SD.
N indicates number of analysis; RBC, red blood cells; Hb, hemoglobin concentrations; Hct, hematocrit; MCV, mean corpuscular volume; MCH, mean cellular hemoglobin content; MCHC, mean cellular hemoglobin concentration.
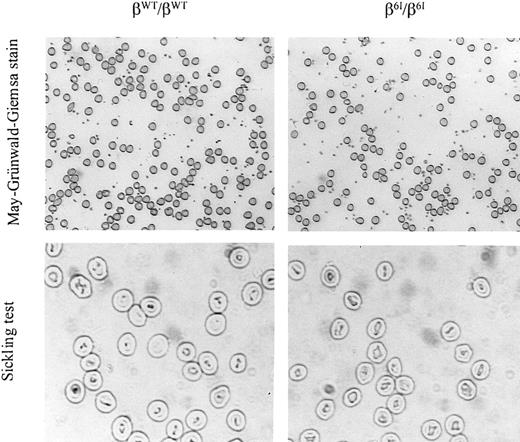
To determine whether the mutant mice contained irreversibly sickled RBCs in vivo, fully oxygenated peripheral blood smears were prepared from wild-type mice and homozygous β6I mice. A representative microscopic field for each group is shown in the top 2 panels of Figure 6. No irreversibly sickled cells were observed in the periphery of β6I/β6I mice (Figure 6). The same blood samples were treated in vitro under deoxygenating conditions. The morphology of erythrocytes from β6I/β6Imice did not change on deoxygenation (Figure 6). Similarly, the mutant hemoglobin did not precipitate with oxidant stress (data not shown).
Homozygous β6I mice have normal peripheral red blood cell morphology and normal hematologic parameters.
An oxygenated blood smear was prepared from a βWT/βWT mouse or a β6I/β6I mouse followed by May-Grünwald-Giemsa staining, magnification × 100. A sickling test was performed on the same fresh blood samples by viewing cell morphology under deoxygenating conditions after incubation in a 1% solution of sodium metabisulfite under coverslips, magnification × 200. A representative photomicrograph of each sample is shown.
Homozygous β6I mice have normal peripheral red blood cell morphology and normal hematologic parameters.
An oxygenated blood smear was prepared from a βWT/βWT mouse or a β6I/β6I mouse followed by May-Grünwald-Giemsa staining, magnification × 100. A sickling test was performed on the same fresh blood samples by viewing cell morphology under deoxygenating conditions after incubation in a 1% solution of sodium metabisulfite under coverslips, magnification × 200. A representative photomicrograph of each sample is shown.
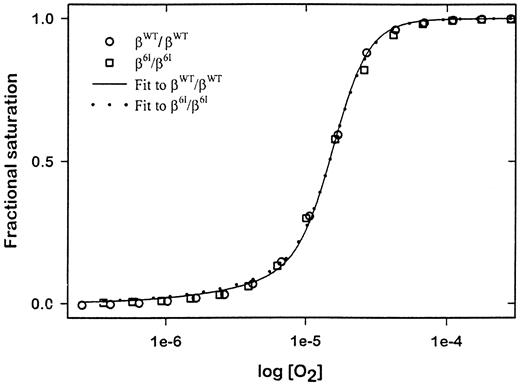
Finally, as shown in Figure 7, the hemoglobin oxygen-binding curve of hemolysates from β6I homozygous mice was indistinguishable from that of normal blood, demonstrating that β6I mutant hemoglobin has a normal P50 value (βWT/βWT:8.2 mm Hg; β6I/β6I:8.0 mm Hg) and the same Hill coefficient.
Hemoglobin-oxygen dissociation curves.
Oxygenated hemolysates from β6I homozygotes (β6I/β6I), β6I heterozygotes (βWT/β6I), and wild-type mice (βWT/βWT), were deoxygenated incrementally with humidified O2-free nitrogen gas. O2fractional saturation of a hemolysate was determined by monitoring the change in absorbance at 555 nm versus O2 partial pressure. A fitted oxygen dissociation isotherm for each genotype (n = 3 for all groups) is plotted for derivation of a P50value.
Hemoglobin-oxygen dissociation curves.
Oxygenated hemolysates from β6I homozygotes (β6I/β6I), β6I heterozygotes (βWT/β6I), and wild-type mice (βWT/βWT), were deoxygenated incrementally with humidified O2-free nitrogen gas. O2fractional saturation of a hemolysate was determined by monitoring the change in absorbance at 555 nm versus O2 partial pressure. A fitted oxygen dissociation isotherm for each genotype (n = 3 for all groups) is plotted for derivation of a P50value.
Discussion
In this study, we produced a mutation in the mouse β-major globin gene using homologous recombination in embryonic stem cells. The mutation, located at the β6 position, changed the Ala residue to Ile, which allowed us to track the mutation at the level of DNA, RNA, and protein. The mutant locus also contained a retained PGK-neo cassette between the β-major and β-minor globin genes. PGK-neo expression was detected in the maturing erythroid cells from the bone marrow and in the RBCs from the peripheries of the mutant mice. The mutant β-major globin gene had an output that was identical to its wild-type counterpart in the bone marrow, and similarly the output of the β-minor globin gene was also normal. Analysis of β6Iprotein abundance revealed that red blood cells derived from heterozygous mice had approximately 40% β6I and 60% βWT chains, suggesting that posttranscriptional events contribute to the final abundance of the mutant β globin chain in RBCs. Homozygous mice bearing the mutation had normal blood counts, reticulocyte counts, and peripheral smears and had no evidence for a sickling phenotype.
Based on previous experiments performed in several laboratories, we expected the retained PGK-neo cassette to down-regulate the expression of the β-minor globin gene because the PGK-neo cassette is interposed between the 5′ LCR and the β-minor gene. Groudine et al16,17 first demonstrated that selectable marker cassettes interposed between the 5′ LCR and the β-globin cluster could down-regulate expression of the linked β-globin gene. Smithies et al33,34 also showed that mice homozygous for an insertional disruption of the β-major gene with a Neo-resistance cassette are more anemic than mice homozygous for a naturally occurring deletion of the same gene. This discrepancy in the severity of the phenotypes in these 2 mutations was thought to result from a “knock-down” of the β-minor globin gene expression caused by the retained selectable marker gene. In a large series of subsequent experiments, it became clear that retained PGK-neo or PGK-hygro cassettes near the positions of HS1, HS2, and HS3 could reduce expression of the embryonic and the adult globin genes.18,19,24 These results suggested that if a selectable marker cassette was retained between the functional LCR and the target gene with which it interacts, expression of the target gene could be reduced. Other examples of this phenomenon have been reported.20-23 For example, a retained PGK-neo cassette in the granzyme B gene down-regulates expression of a number of granzyme genes located downstream from granzyme B, but it does not disrupt expression of the cathepsin G gene located at the 3′ end of the granzyme cluster.21 Similarly, a retained PGK-neo cassette in the cathepsin G gene down-regulated the expression of a chymase gene downstream from it but did not affect expression of the granzyme genes located upstream.21 These results suggest that the neighborhood effect can be unidirectional, but the “rules” for neighborhood effects have yet to be established.
We do not yet understand why the β-minor globin gene is not down-regulated by the retained PGK-neo cassette. Our results demonstrate that the lack of a neighborhood effect is not due to silencing of the PGK-neo gene in the maturing red cells. We observed high-level PGK-neo expression in the erythroid compartment, indicating that “capture” of PGK-neo expression by the LCR does not necessarily disrupt the interaction between the LCR and the neighboring globin genes. A simplified LCR competition model, therefore, does not explain our data. One alternative possibility is that because the β-minor gene is located at the furthest distance from the 5′ LCR, the LCR effect may be diminished. It is also possible that 3′ HS-1, located downstream from the β-globin gene cluster and closest to the β-minor gene, may be able to compensate for functions provided by the upstream LCR elements. Finally, it is possible that there is simply some unique topological chromatin constraint for the neighborhood effect to occur but that this constraint is not operative with this particular mutation. Regardless, retention of the PGK-neo cassette between the β-major and the β-minor globin genes in this position has no measurable effect on the output of either gene.
The β-globin mutation engineered in this study changed an alanine to an isoleucine residue at position 6 of the β-major globin gene. Amino acids in the β6 position of the mouse and human genes are different (Ala vs Glu), as are several surrounding residues (Figure 1). The β6 valine of deoxygenated human βS chains interacts with a region in the EF loop of adjacent β-globin chains, permitting subsequent polymerization of hemoglobin molecules containing the βS subunit.35 The EF region of the mouse β-globin chain is different from the EF region of the human; therefore, designing a mutation that would create a murine sickling hemoglobin is a difficult task. The substitution of Ile in the β6 position was predicted to make a hemoglobin that might have the potential to polymerize on deoxygenation, but analysis of mice homozygous for this mutation revealed that the mutant protein did not cause hemoglobin polymerization or hemolytic anemia. Therefore, β6I is not a “sickling” hemoglobin in an otherwise normal mouse. There are several potential explanations for this observation. One is there may be fundamental differences between the β6 region and the EF loop between humans and mice that do not permit polymerization to occur. Another is that polymerization may be significantly inhibited by the persistent expression of the β-minor globin gene, which comprises nearly 25% of the total adult β-globin in these mice. Yet another holds that mouse α-globin has significant antisickling properties,36-41 which suggests that β6I could sickle if it were to interact with human α-globin but not with mouse α-globin. Experiments designed to address some of these issues are in progress.
Real-time PCR analysis of the wild-type versus mutant β-globin alleles in heterozygous mice revealed that the abundance of the mutant β6I mRNA in bone marrow–derived red cells is normal. However, when we evaluated the abundance of the mutant β6I chains by Triton-acid-urea–PAGE, the mutant chains were less abundant than their wild-type counterparts in heterozygous mice. Therefore, a posttranscriptional event(s) may contribute to the reduced β6I protein in the peripheral blood of these animals. The β6I mRNA may be less translatable than its wild-type counterpart, or the β6I protein may be less stable in adult red blood cells. Significantly, mice that are homozygous for the β6I mutation are not anemic and do not have elevated reticulocyte counts. The oxygen-hemoglobin binding curve of the mutant hemoglobin is identical to wild-type, and mutant red blood cells do not exhibit abnormal behavior when placed under oxidative stress in vitro. Regardless, it is interesting to note that humans heterozygous for the βS mutation normally have 30% to 40% HbS and 60% to 70% HbA in peripheral blood red blood cells, despite the fact that βS and wild type β-mRNAs are produced in equal abundance. A similar posttranslational defect in βS and β6I globin metabolism may therefore occur in vivo.
The discovery of a position in which a retained PGK-neo cassette does not affect β-major globin gene function permits a reconsideration of homologous recombination strategies for the correction of mutant β-globin genes. Until now, we and others have been concerned that the β-globin gene could not be corrected by this strategy because of potential neighborhood effects caused by retained selectable markers in the “corrected” locus. The observation reported here, however, suggests that it may be possible to position a selectable marker cassette near the human β-globin gene in a manner that does not disrupt its expression; direct experiments to test this hypothesis using ES cells containing a copy of the human β-globin gene cluster should now be performed. The recent observation by Hatada et al42 that hematopoietic progenitor cells have the machinery required to perform homologous recombination further supports this approach. However, significant obstacles remain. Homologous recombination in embryonic stem cells and hematopoietic progenitors is an inefficient process.42 The ability to stably transfer genetic material into the genomes of quiescent pluripotent hematopoietic stem cells remains a significant problem. However, if these obstacles could be removed, a gene-correction strategy based on homologous recombination would have many advantages over random integration models of β-globin gene correction, where random mutagenesis occurs at each integration site.
We thank Alexander Kilinger and Sheila Bijoor for technical assistance and Pam Goda and Kelly Schrimpf for expert blastocyst injections and animal husbandry. Nancy Reidelberger provided expert assistance with the manuscript preparation.
Supported by National Institutes of Health grants DK38682 (T.J.L.), GM24486 (G.K.A.), NSF MCB 0077596 (G.K.A., J.M.H.), and F32 DK09584 (R.K.).
R.M.K. and Z.H.L. contributed equally to this manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Timothy J. Ley, Division of Oncology, Section of Stem Cell Biology, Washington University School of Medicine, Campus Box 8007, 660 South Euclid Ave, St Louis, MO 63110-1093; e-mail:timley@im.wustl.edu.

![Fig. 2. Targeted insertion of a PGK-neo cassette does not disrupt the regulated expression of 2 neighboring murine β-major and β-minor globin genes. / (A) Real-time PCR assay for the wild-type mouse β-major genomic DNA. PCR product fluorescence intensity (ΔRn) is plotted on the y-axis versus PCR cycle number on the x-axis. Tenfold serial dilutions of pβWT, a plasmid encoding the wild-type β-major gene, were amplified using either the βWT primer set (reactions 1-4) or β6I primer set (reactions 5-8). Plotting the PCR cycle number at which the reaction entered exponential amplification, versus the logarithm of the amount of input DNA, generated a standard curve (not shown). (B) Ten nanograms plasmid DNA encoding the mutant β6I (pβ6I) or the wild-type β-major sequence (pβWT) was used for real-time PCR. Each plasmid sample or a no DNA control (No DNA) was amplified using either the βWT primer set (reactions 1-3) or the β6Iprimer set (reactions 4-6). (C) Real-time PCR analysis of bone marrow RNA. Bone marrow RNA was harvested from a heterozygous β6I mouse (βWT/β6I) or a wild-type control mouse (βWT/βWT), reverse-transcribed to cDNA, and analyzed by real-time PCR using either the βWT primer set (reactions 1-2) or β6Iprimer set (reactions 3-4). A reaction amplifying the wild-type peripheral blood cDNA with the β6I primer set was included as a negative control (reaction 5). (D) Semiquantitative RT-PCR analysis of peripheral blood RNA. Peripheral blood RNA was harvested from homozygous β6I mice (β6I/β6I) or wild-type control mice (βWT/βWT), reverse-transcribed, and analyzed by PCR using a β-minor primer set or a β-actin gene primer set. Reactions were performed in the presence of α-32P]dATP and stopped before amplifications reached a product plateau (not shown). Amplification products were resolved on 5% TBE-polyacrylamide gels and quantitated using a PhosphorImager. For each experiment, the relative β-minor mRNA expression was normalized for β-actin mRNA level and expressed as a percentage of wild-type expression. Shown are the mean values, along with standard deviations, from 3 separate experiments in which 2 samples were used for each genotype.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/1/10.1182_blood.v98.1.65/6/m_h81311243002.jpeg?Expires=1768071048&Signature=SMSoARwgD1sT5jBlhJliIS-S2naR8isH2piYtszqYUFP7PCHRqoJkkgxtDAI6oUBYVqzXpNY9s9fBzfqR-mu08tKcuTwAvEklGeA6A4i6m1ZEb7SSS1rpjMjLFdzqCLUNqzyWXgs6cYWzYf3BbjOuEwSkXE9tajBrA-BhR~zGRfOgFXSkKfDC3SPEKLKDinRismkO8jP9qM~x3ZsihqyVkxTyYCYsz44rwc~cCOP309apbguFTosO~Djw~GCMjgLZGz2YUAJTqKrvHEdhE8qEOhb3i~MuEJooyvtYz03p9j6tzIX~8obN1Kg730Xs9sbEUw18Ijrg~HwM9Kz7PsEAA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal