Abstract
There is no reliable laboratory indicator of the onset of chronic graft-versus-host disease (cGVHD). This study looks at whether the expression of OX40, a member of the tumor necrosis factor receptor family, is related to the development of cGVHD in patients who underwent allogeneic hematopoietic stem cell transplantation. Peripheral blood mononuclear cells from 22 patients after day 100 were subjected to multicolor flow cytometry. The percentages of both OX40+CD4+ and OX40+CD8+T cells were significantly higher in patients with cGVHD than those without (P < .0001 and P = .001, respectively). Serial analyses showed that OX40+CD4+ T cells elevated before the onset of cGVHD and closely correlated with the therapeutic response. The expression of CD25, CD69, and HLA-DR was partially detectable on OX40+ T cells. These results indicate that serial measurement of OX40+ T cells is useful for predicting the onset as well as the therapeutic response of cGVHD and raise a possibility that the OX40/gp34 system is involved in the pathogenesis of cGVHD.
Introduction
The increase in allogeneic hematopoietic stem cell transplantations (allo-HSCTs), particularly those using peripheral blood stem cells and involving donors other than HLA-identical siblings, has made the management of graft-versus-host disease (GVHD) a continuing problem. Although there have been advances in the prevention of acute (a) GVHD with the introduction of tacrolimus (FK506) and T-cell depletion, the incidence of chronic (c) GVHD has not decreased1 and the absence of reliable markers for predicting and monitoring cGVHD makes its control difficult.
The clinical manifestations of aGVHD and cGVHD are somewhat different. aGVHD is characterized by a triad of dermatitis, gastroenteritis, and hepatitis, whereas cGVHD is a more diverse syndrome, usually presenting with multiorgan involvement and closely related to autoimmune disease. Unlike aGVHD, T cells, which newly differentiate from donor hematopoietic stem cells, are thought to play more important roles in the development of cGVHD.2
OX40, a member of the tumor necrosis factor receptor family, is expressed on activated T cells. The interaction of OX40 with its ligand, gp34, is shown to enhance T-cell proliferation.3In murine models, several reports showed that the OX40/gp34 system is involved in the process of GVHD4-7 as well as several autoimmune disease.8-10 In addition, studies with OX40- or gp34-deficient mice revealed that this system has a critical costimulatory function in dendritic cell/CD4+ T-cell interactions.11
In this study, we measured the expression of OX40 on T cells after allo-HSCT to determine its correlation with the manifestations of cGVHD.
Study design
Twenty-two patients with hematologic malignancies and severe aplastic anemia undergoing bone marrow transplantation between 1998 and 2000 were included in this study. All patients were conditioned with myeloablative regimens. No patients received T-cell–depleted bone marrow or prophylactic anti-thymocyte globulin. Ten donors were related and 12 donors were unrelated, and HLA types were serologically 5- or 6-antigen matched between all pairs of patients and donors. Other characteristics of patients with cGVHD are shown in Table1. Peripheral blood mononuclear cells obtained more than 100 days after transplantation were subjected to 3-color flow cytometry by using FACScan (Becton Dickinson, San Jose, CA).
Clinical data of patients with cGVHD and the ratio of OX40+ T cells among total CD4 or CD8 T cells
| UPN . | aGVHD . | cGVHD . | ||||
|---|---|---|---|---|---|---|
| Grade . | Onset (mo after SCT) . | Affected organ . | Therapeutic response for primary therapy† . | OX40+CD4+* . | OX40+CD8+* . | |
| 1 | IV | Progressive (5) | Skin, liver | Worsened | 66% | 58% |
| 2 | II | Quiescent (22) | Myasthenia gravis | Unchanged | 47% | 9% |
| 3 | III | Quiescent (3) | Stomach | Unchanged | 60% | 43% |
| 4 | III | Progressive (4) | Skin, liver, stomach | Unchanged | 40% | 13% |
| 5 | III | Progressive (3) | Skin, liver | Unchanged | 87% | 97% |
| 6 | I | Quiescent (14) | Liver, skin | Improved | 43% | 23% |
| 7 | II | Quiescent (8) | Lung, skin | Improved | 22% | 26% |
| 8 | I | Quiescent (4) | Liver | Improved | 31% | 24% |
| 9 | I | Quiescent (4) | Skin, liver | Improved | 49% | 37% |
| 10 | III | Quiescent (4) | Lung, liver | Improved | 37% | 40% |
| 11 | III | Quiescent (3) | Liver | Improved | 39% | 18% |
| UPN . | aGVHD . | cGVHD . | ||||
|---|---|---|---|---|---|---|
| Grade . | Onset (mo after SCT) . | Affected organ . | Therapeutic response for primary therapy† . | OX40+CD4+* . | OX40+CD8+* . | |
| 1 | IV | Progressive (5) | Skin, liver | Worsened | 66% | 58% |
| 2 | II | Quiescent (22) | Myasthenia gravis | Unchanged | 47% | 9% |
| 3 | III | Quiescent (3) | Stomach | Unchanged | 60% | 43% |
| 4 | III | Progressive (4) | Skin, liver, stomach | Unchanged | 40% | 13% |
| 5 | III | Progressive (3) | Skin, liver | Unchanged | 87% | 97% |
| 6 | I | Quiescent (14) | Liver, skin | Improved | 43% | 23% |
| 7 | II | Quiescent (8) | Lung, skin | Improved | 22% | 26% |
| 8 | I | Quiescent (4) | Liver | Improved | 31% | 24% |
| 9 | I | Quiescent (4) | Skin, liver | Improved | 49% | 37% |
| 10 | III | Quiescent (4) | Lung, liver | Improved | 37% | 40% |
| 11 | III | Quiescent (3) | Liver | Improved | 39% | 18% |
cGVHD, chronic graft-versus-host disease; aGVHD, acute graft-versus-host disease; SCT, stem cell transplantation.
Data just before the beginning of intensive immunosuppressive therapy for cGVHD.
Primary therapy for cGVHD was increasing of tacrolimus or cyclosporin or beginning of prednisone.
Cells were stained with fluorescein isothiocyanate–conjugated monoclonal antibodies (mAbs) against CD4, CD8, CD25, CD69, or HLA-DR (Becton Dickinson), peridinin chlorophyll protein–conjugated mAb recognizing CD3 (Becton Dickinson), and biotinylated 31512(anti-human OX40) in combination with phycoerythrin-conjugated streptavidin (Becton Dickinson). After gating T cells according to CD3 positivity, the ratio of OX40+ cells in CD4+ or CD8+ cells were calculated, and the relations of OX40+ cells with CD25+ cells, CD69+cells, and HLA-DR+ cells were determined. All data were analyzed with STATCEL program (Hisae Yanai). Two-group comparison was done on the analysis of variance and Student t test. Differences with P < .05 were considered to be statistically significant.
Results and discussion
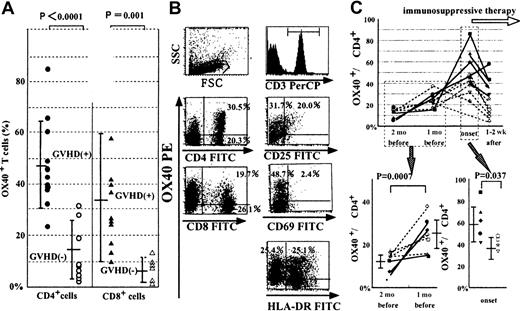
Of 22 patients enrolled, 11 patients (UPN 1-11) developed cGVHD and 6 of them were successfully treated with conventional immunosuppressive therapy such as prednisone. Among the remaining 5 patients, one patient (UPN 1) died of progressive cGVHD. Although the absolute numbers of CD4+ and CD8+ T cells varied considerably in each assay (data not shown), the percentages of both OX40+CD4+ T cells and OX40+CD8+ T cells in patients with cGVHD just before the introduction of immunosuppressive therapy were significantly higher than the peak value of OX40+ cells in patients without cGVHD (47.5% ± 17.9% versus 14.8% ± 11.6%,P < .0001; 35.3% ± 25.0% versus 5.8% ± 4.2%,P = .001; Figure 1A). The mean interval between the analysis and stem cell transplantation (SCT) is 233 days for patients with cGVHD and 274 days for those without cGVHD. Neither the severity of cGVHD (extensive type or limited type) nor the organ involved was correlated with the percentages of OX40+ cells (data not shown). CD25, CD69, and HLA-DR, which are expressed on activated T cells, were hardly or partially expressed on OX40+CD3+ T cells (Figure 1B). Neither the interval after transplantation, presence of aGVHD, nor immunosuppressive drugs for GVHD prophylaxis affected the expression of OX40 statistically (data not shown).
OX40 expression with cGVHD.
(A) Comparison of OX40 expression between patients with cGVHD (n = 11; closed circles and triangles) and without GVHD (n = 11; open circles and triangles). The mean interval between the analysis and SCT is 233 days for patients with cGVHD and 274 days for those without cGVHD. Each bar indicates the mean ± SD in each patient group. (B) Surface phenotype of T cells of a representative case with cGVHD. CD3+ T cells were gated and analyzed for expression of OX40, CD25, CD69, and HLA-DR. (C) Serial changes in the percentage of OX40+CD4+ T cells at 4 different time points around the onset of cGVHD. The first point is the day when patients visited the outpatient clinic 2 months before the onset of GVHD. The second point is the latest visit about 1 month before the onset. The third point is the time point at the onset of cGVHD just before immunosuppressive therapy. The fourth point is the next visit 1 to 2 weeks after the immunosuppressive therapy. The changes between the first 2 points and the comparison between the nonresponders (n = 5; closed point) and the responders (n = 5; open point) at the onset are depicted separately.
OX40 expression with cGVHD.
(A) Comparison of OX40 expression between patients with cGVHD (n = 11; closed circles and triangles) and without GVHD (n = 11; open circles and triangles). The mean interval between the analysis and SCT is 233 days for patients with cGVHD and 274 days for those without cGVHD. Each bar indicates the mean ± SD in each patient group. (B) Surface phenotype of T cells of a representative case with cGVHD. CD3+ T cells were gated and analyzed for expression of OX40, CD25, CD69, and HLA-DR. (C) Serial changes in the percentage of OX40+CD4+ T cells at 4 different time points around the onset of cGVHD. The first point is the day when patients visited the outpatient clinic 2 months before the onset of GVHD. The second point is the latest visit about 1 month before the onset. The third point is the time point at the onset of cGVHD just before immunosuppressive therapy. The fourth point is the next visit 1 to 2 weeks after the immunosuppressive therapy. The changes between the first 2 points and the comparison between the nonresponders (n = 5; closed point) and the responders (n = 5; open point) at the onset are depicted separately.
In the 11 patients who developed cGVHD, we could serially follow the expression kinetics of OX40+ T cells before and after the onset of cGVHD and introduction of immunosuppressive therapy (Figure 1C). OX40+CD4+ T cells gradually increased before the appearance of clinical manifestations of cGVHD. In addition, the percentages of OX40+ cells in CD4+ T cells were significantly higher 1 month before the onset than 2 months before (23.7% ± 6.9% versus 12.3% ± 3.9%,P = .0007; Figure 1C). The percentage of OX40+cells just before the immunosuppressive therapy was closely correlated with the therapeutic response. These patients who did not respond to first line therapy for cGVHD tended to show a more highly elevated value than responders (nonresponders versus responders, 59.8% ± 17.9% versus 36.9% ± 10.2%, P = 0.037; Figure 1C). When the percentage of OX40+CD4+ T cells was elevated above 50%, the conventional immunosuppressive therapy was not effective, and more intensive salvage regimens for cGVHD, such as mycophenolate mofetil, were required. In contrast to OX40+CD4+ T cells, the percentage of OX40+CD8+ T cells did not correlate with the clinical response, although it elevated coincidentally with the onset of cGVHD (data not shown).
Our findings that the expression of CD25, CD69, and HLA-DR were hardly or partially expressed on OX40+CD3+ T cells implies that OX40+CD4+ T cells in patients with cGVHD might be distinct from recently activated T cells. Furthermore, we detected OX40+CD8+ T cells in almost all cGVHD patients. In mice ex vivo OX40 staining on tumor infiltrating CD8+ T cells was described.16 As far as we know, the present study first established that the expression of OX40 is inducible on human CD8+ T cells. The relationship between this subset and the effector cells of cGVHD is to be determined.
Our study for the first time showed a strong correlation between the expression of OX40 on T cells and cGVHD. In contrast, previous studies showed that monitoring OX40+ T cells was not useful for the prediction of aGVHD.17 We assume that the pathogenesis of aGVHD and cGVHD may not be identical2 and infections that are more common within 100 days after allo-HSCT18 might modify the expression of OX40.
Because accumulating evidence has indicated that OX40+ T cells play crucial roles in the development of several autoimmune diseases,9,10,13-15 the immune cell activation by the OX40/gp34 system might underlie more closely the pathogenesis of cGVHD. Considering that administration of anti-CD134L mAb significantly ameliorated aGVHD in mice,6 the selective blockade of OX40/gp34 system might be effective for GVHD in humans.
In conclusion, expression of OX40 in peripheral blood T cells can be used as a sensitive indicator of cGVHD after allo-HSCT and might be important for which chemotherapeutic drugs could be used. Modification of the OX40/gp34 system may enable us to better control cGVHD.
We thank Dr M. Sasada, Dr T. Okazaki, Dr A. Takahashi, Dr K. Imada, Dr N. Kadowaki, and Dr C. Ueda for their cooperation in collecting the blood samples, and we thank Ms. K. Fukunaga for expert technical assistance.
Supported by grants-in-aid from the Ministry of Education, Science, Sports and Culture of Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Toshiyuki Hori, Department of Hematology/Oncology, Graduate School of Medicine, Kyoto University, Kyoto 606-8507, Japan; e-mail thori@kuhp.kyoto-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal