Abstract
Transfected murine cell lines are commonly used to study the function of many human cytokine or receptor mutants. This study reports the inappropriate activation of the human granulocyte-macrophage colony-stimulating factor (hGM-CSF) receptor by the human GM-CSF antagonist, E21R, when the human receptor is introduced into the murine cell line BaF-B03. E21R-induced proliferation of the BaF-B03 cells is dependent on transfection with both hGM-CSF receptor α and βc subunits. Studies on the underlying mechanism revealed constitutive association between human and mouse βc and GM-CSF receptor-α, tyrosine phosphorylation of mouse and human βc, and association of phosphorylated mouse βc into an activated human GM-CSF receptor complex in response to E21R and GM-CSF. This interspecies receptor cross-talk of receptor signaling subunits may produce misleading results and emphasizes the need to use cell lines devoid of the cognate endogenous receptors for functional analysis of ligand and receptor mutants.
Introduction
Human granulocyte-macrophage colony-stimulating factor (hGM-CSF) is a pleiotropic cytokine that stimulates the proliferation, differentiation, and survival of myeloid precursors and induces the effector functions of mature myeloid cells.1,2 These multiple functions are mediated by binding to high-affinity receptors that comprise a GM-CSF–specific α chain (hGMR-α) and an affinity-converting, signal-transducing β subunit (hβc), which is shared with the interleukin-3 (IL-3) and IL-5 receptors.3,4 In the murine system there are 2 β subunits, mβc, which is analogous to hβc, transducing signals induced by mGM-CSF, mIL-3 or mIL-5, and mβIL-3, which is specific for mIL-3.5 6Mouse myeloid cell lines, for instance FDCP-1 and BaF-B03, express both mouse β subunits.
Given the importance of hβc in transducing signals that regulate immune responses, hematologic recovery, and, in some cases, leukemia, a significant amount of work has been devoted to structure-function analysis of these cytokine-receptor systems. Studies have led to the engineering of cytokine analogs with unique properties such as the hGM-CSF mutant E21R that behaves as a specific hGM-CSF antagonist.7 Characterization of the receptors has sought to identify functional regions involved in receptor activation and has identified regions in the cytoplasmic domain of hβc that couple to specific signaling molecules such as JAKs, STATs, and the ras/MAP kinase pathway.8-10 However results from these studies have in some cases been ambiguous or even conflicting.
Central to the analysis of cytokine and receptor mutants is the choice of experimental system. Predominantly, mouse myeloid cell lines, which are readily transfected with human receptor subunits, have been used. A major problem is the expression of endogenous receptors for these cytokines in these cell lines. For example, transfection of hGMR-α alone in murine FDCP-1 cells was initially reported to be sufficient to mediate a proliferative signal despite only displaying low-affinity hGM-CSF binding.11 However, it was later shown that functional reconstitution of hGMR required both hGMR-α and hβc subunits,12 and that the initial observation with hGMR-α alone was confounded by the recruitment of endogenous mβc.13 Likewise, interaction of an extracellular point mutant of hβc with an endogenous mGMR-α has been shown to lead to factor-independent proliferation14 and chimeras between a constitutively active erythropoietin receptor with the cytoplasmic domain of GMR-α promoted proliferation but only in presence of mβc.15 Here we show an abnormal response of the human GM-CSF antagonist E21R in transfected mouse cell lines and the molecular basis for human-mouse GM-CSF receptor cross-talk. These results emphasize the need for caution when interpreting data from experiments using transfectants and the desirability of using homologous systems.
Study design
Cell lines and proliferation assays
The human erythroleukemic cell line TF1.8, the megakaryocytic leukemia human cell line, UT7, and the murine pro-B-cell line, BaF-B03 (transfected with human GMR-α and hβc), were grown as previously described.16,17 Following cytokine starvation overnight, the proliferation of cell lines in response to hGM-CSF or E21R was determined as previously described.18 E21R, a single point mutant of hGM-CSF (Gln21Arg) was donated by Bresagen (Adelaide, Australia).
Antibodies
Murine monoclonal antibodies 1C1 and 8E4, for detection of hβc, and 4H1 for immunoprecipitating hGMR-α were produced as previously described.16 1C1 was biotinylated using a cellular labeling and immunoprecipitation kit (Boehringer Mannheim, Rose Park, SA, Australia) and streptavidin–horseradish peroxidase (HRP) was purchased from Amersham Life Science (Little Chalfont, United Kingdom). Anti-mβc rabbit polyclonal K-17 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). HRP-conjugated antibodies specific for mouse or rabbit immunoglobulins were purchased from Pierce (Rockford, IL) and Dako (Botany, NSW, Australia), respectively. The HRP-conjugated antiphosphotyrosine monoclonal antibodies, PY20 and 4G10, were obtained from Transduction Laboratories (Lexington, KY) and Upstate Biotechnology (Lake Placid, NY), respectively.
Immunoprecipitation, sodium dodecylsulfate–polyacrylamide gel electrophoresis, and immunoblotting
Analysis of ligand-induced receptor complexes and their phosphotyrosine status was determined using starved cells that were stimulated with factors for 5 minutes at room temperature at indicated concentrations. Cells were lysed and immunoprecipitated proteins were run on reducing sodium dodecylsulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and subjected to immunoblotting as previously described.19
Results and discussion
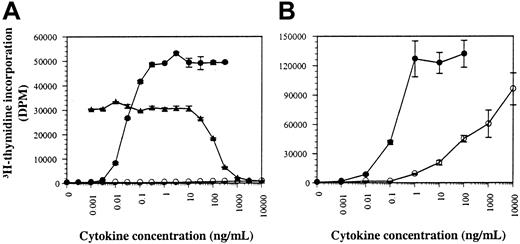
Human GM-CSF supports the proliferation of human cells by activating endogenous hGMR.7 The antagonistic hGM-CSF mutant, E21R, however, cannot mediate a proliferative response in human cells and is unable to bind hGMR with high affinity.7 We observed that the biologic activity of E21R is strikingly different when analyzed in hGMR-transfected murine cell lines. First, the biologic activity of E21R was determined in comparison to wild-type hGM-CSF on the human cell line, TF1.8, and the murine cell line BaF-B03 transfected with both hGMR-α and hβc (hGMR BaF-B03). Human GM-CSF induces proliferation of TF1.8 cells with an effective dose (ED50) of 0.03 ng/mL, whereas E21R is unable to elicit a proliferative response and antagonizes the activity of wild-type GM-CSF (Figure 1A).7Surprisingly, however, E21R induced a proliferative response in the hGMR BaF-B03 cell line at concentrations of E21R above 10 ng/mL (Figure 1B).
The human GM-CSF antagonist E21R behaves as an agonist on mouse cell lines.
Factor-deprived human TF1.8 (A) and mouse hGMR BaF-B03 cells (B) were stimulated with hGM-CSF (●) or E21R (○). Functional antagonism of 0.03 ng/mL GM-CSF with increasing concentrations of E21R on human TF1.8 cells is also shown (▴). Cells were cultured with cytokine for 48 hours and the resulting proliferation was measured by the incorporation of 3H-thymidine. The results are expressed in disintegrations per minute (dpm) and each point represents the mean of triplicate determination with error bars representing 1 SD.
The human GM-CSF antagonist E21R behaves as an agonist on mouse cell lines.
Factor-deprived human TF1.8 (A) and mouse hGMR BaF-B03 cells (B) were stimulated with hGM-CSF (●) or E21R (○). Functional antagonism of 0.03 ng/mL GM-CSF with increasing concentrations of E21R on human TF1.8 cells is also shown (▴). Cells were cultured with cytokine for 48 hours and the resulting proliferation was measured by the incorporation of 3H-thymidine. The results are expressed in disintegrations per minute (dpm) and each point represents the mean of triplicate determination with error bars representing 1 SD.
It has been previously described that hGMR-α can interact with either hβc or mβc to transmit a growth signal in response to hGM-CSF in BaF-B03 cells.13 However, the interaction of hGMR-α with mβc, unlike hβc, does not form a high-affinity receptor. The proliferation induced by E21R in hGMR BaF-B03 cells is also the result of a low-affinity interaction with a dissociation constant of approximately 4 nM (data not shown) as determined by Scatchard analysis of saturation binding,18 identical to the affinity of E21R measured on human neutrophils.7
The abnormal proliferative response of hGMR BaF-B03 cells to E21R differs from the previously described cross-talk phenomenon between hGMR-α and mβc because it is dependent on the coexpression of both hGMR-α and hβc. This is highlighted by the inability of BaF-B03 cells expressing hGMR-α alone to respond to E21R, even at concentrations of 100 μg/mL (data not shown). Because the abnormal agonism was only observed in cell lines where mβc is endogenously expressed, it suggested that the unexpected activity demonstrated by E21R may be the result of a novel cross-species interaction, involving both hβc and mβc.
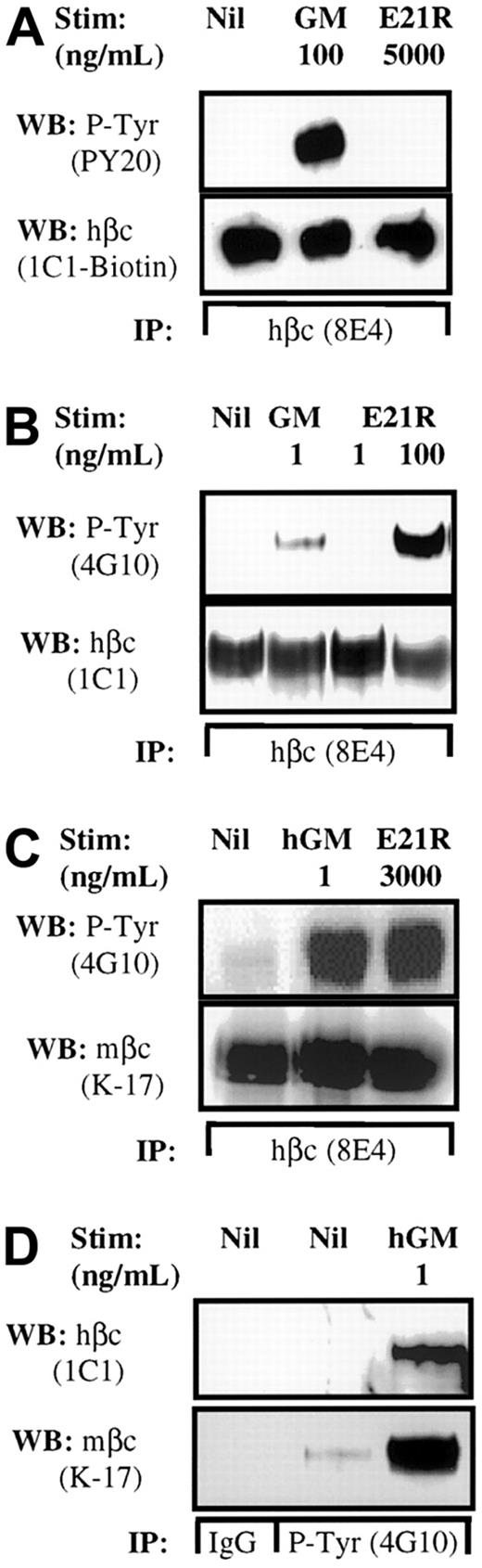
Together with the inability of E21R to mediate a proliferative response in human cells, it cannot stimulate tyrosine phosphorylation of hβc. The tyrosine phosphorylation status of hβc was investigated to determine if E21R can activate hβc in this murine system. Tyrosine phosphorylation of hβc was readily detected in the murine cell line hGMR BaF-B03 after stimulation with E21R (Figure2B), but not the human cell line expressing endogenous hβc, UT7 (Figure 2A), and TF1.8 (data not shown). In comparison, tyrosine phosphorylation of hβc was detected following stimulation with hGM-CSF in both human UT7 cells and murine hGMR BaF-B03 cells (Figure 2A,B). The dose response of E21R-induced tyrosine phosphorylation of hβc is consistent with the proliferation data where responsiveness occurs at a concentration of E21R above 10 ng/mL, with no response at 1 ng/mL (Figure 1B).
Transfected hβc spontaneously associates with endogenous mβc and the βc complex is phosphorylated inappropriately in response to E21R.
Factor-deprived human UT7 cells (A) or mouse hGMR BaF-B03 cells (B-D) were stimulated as indicated for 5 minutes at 25°C. Cells were lysed and hβc was immunoprecipitated with 8E4 anti-hβc antibody (A-C). The presence of tyrosine phosphorylated hβc was detected using an antiphosphotyrosine antibody, PY20 (A) or 4G10 (B,C). Filters were then stripped and reprobed for the presence of hβc with 1C1 anti-hβc antibody (A,B). The presence of mβc following hβc immunoprecipitation was determined on a duplicate filter using anti-mβc antibody, K-17 (C). Tyrosine phosphorylated proteins were immunoprecipitated with the 4G10 antiphosphotyrosine antibody (D) and the presence of hβc detected using 1C1 antibody. The filter was then stripped and reprobed for the presence of mβc with K-17.
Transfected hβc spontaneously associates with endogenous mβc and the βc complex is phosphorylated inappropriately in response to E21R.
Factor-deprived human UT7 cells (A) or mouse hGMR BaF-B03 cells (B-D) were stimulated as indicated for 5 minutes at 25°C. Cells were lysed and hβc was immunoprecipitated with 8E4 anti-hβc antibody (A-C). The presence of tyrosine phosphorylated hβc was detected using an antiphosphotyrosine antibody, PY20 (A) or 4G10 (B,C). Filters were then stripped and reprobed for the presence of hβc with 1C1 anti-hβc antibody (A,B). The presence of mβc following hβc immunoprecipitation was determined on a duplicate filter using anti-mβc antibody, K-17 (C). Tyrosine phosphorylated proteins were immunoprecipitated with the 4G10 antiphosphotyrosine antibody (D) and the presence of hβc detected using 1C1 antibody. The filter was then stripped and reprobed for the presence of mβc with K-17.
Because mβc functions when recruited to a hGM-CSF–hGMR-α complex, we investigated if this molecule plays a role in facilitating the tyrosine phosphorylation of hβcin response to hGM-CSF or E21R in hGMR BaF-B03 cells. Stimulation of hGMR BaF-B03 cells with either GM-CSF or E21R resulted in tyrosine phosphorylation of hβc (Figure 2C). Significantly, mβc was associated with hβc and this interaction appears to occur regardless of stimulation (Figure 2C). It is interesting to note that dimerization of a least 2 hβcsubunits is required for hGMR activation.19 20 The interaction between mouse and human βc may provide the molecular basis to support the agonistic activity of E21R and mediate this perverted receptor response observed in this murine cell line.
Phosphotyrosine immunoprecipitations were performed to address if preassociation between the human and murine βc subunits allows mβc to be associated with an activated hGMR complex. Human βc was immunoprecipitated by an antiphosphotyrosine antibody following stimulation of hGMR BaF-B03 cells with hGM-CSF but not when left unstimulated or with an isotype-matched control antibody (Figure 2D). Mouse βcappears to be weakly phosphorylated in the absence of stimulation, but interestingly was strongly coimmunoprecipitated with antiphosphotyrosine antibody after stimulation with hGM-CSF (Figure2D), suggesting an increase in phosphorylation of mβc on stimulation with the human ligand.
The hGMR subunits GMR-α and hβc have previously been shown to exist as a preformed complex on human cells,20 21and we have now shown that mβc and hβc are also associated prior to ligand stimulation on hGMR BaF-B03 cells. To determine whether mβc is also a component of a preformed complex on hGMR BaF-B03 cells, hGMR-α was immunopreciptated from nonstimulated and GM-CSF–stimulated cells. Immunoblotting revealed that both hβc and mβc were associated with hGMR-α regardless of stimulation (data not shown) suggesting that mβc chain may be a component of a preformed hGMR receptor on hGMR BaF-B03 cells. Therefore activation of the receptor by GM-CSF and E21R may be mediated by a preformed hGMR-α–hβc–mβc complex in these cells.
The ability of human and mouse βc subunits to interact and influence responses of a hGM-CSF variant introduces a new level of complexity in the analysis of ligand-receptor interaction and subsequent signaling capabilities. It also raises the question of the influence this interaction has had on previous hGMR studies that have been performed in murine cell lines. Clearly mβc can become associated within an active hGMR complex, and it is important to consider its contribution to the receptor's biologic response. This is of concern especially when investigating the activation of downstream signaling molecules.22 In light of the interaction between hβc and mβc it will be difficult to discriminate which signaling molecules have emanated from the hβc alone.23 In addition, the associated mβc subunit may facilitate signaling by an otherwise inactive hβc mutant. This may explain the surprising result seen where hβc deficient in intracellular tyrosines is still capable of responding to hGM-CSF.10 In addition, the observation that a cytoplasmically truncated hβc can still respond to hGM-CSF when expressed in BaF-B03 cells may be potentiated via signaling through mβc.24 The contribution this interspecies subunit interaction plays when distinguishing regions in hGMR responsible for differentiation or proliferation in murine myeloid cell lines remains unclear. It has been demonstrated previously that hβc can form homodimers that are activated in response to hGM-CSF.20 21 The association between mβcand hβc must result in a complex that has different properties to hβc homodimers because E21R is unable to activate the latter.
A number of receptors belonging to the cytokine receptor family show cross-species specificity between human and murine components. Because receptor dimerization is a common theme in receptor activation, it cannot be ruled out that interaction of interspecies signaling components may obscure results when studying a wide range of human receptors in murine cells and similar precautions may need to be taken. In addition to our findings that the use of transfected murine cell lines may not be the best approach when studying hGM-CSF ligand variants, other cytokines show similar discrepancies. An analogous situation has been reported for a human IL-4 mutant that can mediate either agonistic or antagonistic responses when studied in either a murine or human cell line, respectively.25 Similarly, mutational analysis of thrombopoietin shows conflicting data in the identification of residues functionally important in interaction with its receptor when screened on Mpl-transfected BaF-B03 cells compared to enzyme-linked immunosorbent assay or Biacore analysis.26 27
The demonstration of an inherent association between mouse and human βc shown here highlights the need for a careful selection of appropriate systems. In the case of the hGMR the availability of βc knockout mice permits the use of cells from these animals for receptor reconstitution experiments as a better background to analyze structural and functional outcomes with the hGMR.
Supported by grants from the National Health and Medical Research Council of Australia. J.W. is a Research Fellow of the Anti-Cancer Foundation of South Australia.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Joanna Woodcock, Cytokine Receptor Laboratory, Hanson Centre for Cancer Research, Institute of Medical and Veterinary Science, Adelaide, SA 5000, Australia; e-mail:joanna.woodcock@imvs.sa.gov.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal