Abstract
Haptoglobin serves as an antioxidant by virtue of its ability to prevent hemoglobin-driven oxidative tissue damage. It was recently demonstrated that an allelic polymorphism in the haptoglobin gene is predictive of the risk for numerous microvascular and macrovascular diabetic complications. Because these complications are attributed in large part to an increase in oxidative stress, a study was conducted to determine whether the different protein products of the 2 haptoglobin alleles differed in the antioxidant protection they provided. A statistically significant difference was found in the antioxidant capacity of purified haptoglobin protein produced from the 2 different alleles, consistent with the hypothesis that differences in genetically determined antioxidant status may explain differential susceptibility to diabetic vascular complications. These differences may be amplified in the vessel wall because of differences in the sieving capacity of the haptoglobin types. Therefore, an attempt was made to identify the minimal haptoglobin sequences necessary to inhibit oxidation by hemoglobin in vitro, and 2 independent haptoglobin peptides that function in this fashion as efficiently as native haptoglobin were identified. Identification of the biochemical basis for differences among haptoglobin types may lead to the rational development of new pharmacologic agents, such as the mini-haptoglobin described here, to avert the development of diabetic vascular complications.
Introduction
Haptoglobin is a serum protein that functions as an antioxidant by virtue of its ability to bind to hemoglobin1 and thereby to prevent the oxidative tissue damage that may be mediated by free hemoglobin.2 The importance of this protective mechanism has been demonstrated in haptoglobin knockout mice in which a marked increase in oxidative tissue damage develops in response to hemolysis.3 In humans, 2 alleles (denoted 1 and 2) exist for the haptoglobin gene.1,2,4 Biophysical and biochemical properties of the haptoglobin polymeric molecules resulting from the 3 possible combinations (haptoglobin 1-1, 2-1, or 2-2) of these 2 alleles are dramatically different.2
We have recently found in multiple independent studies5-8(and in A.P.L. et al, manuscript submitted for publication) of more than 1000 persons in Israel, Belgium, and the United States that the haptoglobin phenotype is a predictor of the risk for microvascular and macrovascular complications of diabetes. Specifically, diabetic patients with the haptoglobin 1-1 phenotype were shown to be remarkably resistant to the development of diabetic retinopathy, diabetic nephropathy, and cardiovascular disease.5-8 Moreover, we found that a graded effect was evident with regard to risk and to the number of haptoglobin 2 alleles.7 8 For example, in a prospective study of incident cardiovascular disease, we found that participants homozygous for the haptoglobin 2 allele had a 5-fold increase in the risk for cardiovascular disease compared with patients homozygous for the haptoglobin 1 allele, whereas heterozygotes were found to have an intermediate risk (A.P.L. et al, manuscript submitted for publication).
It has been proposed that an increase in oxidative stress plays a crucial role in the development of diabetic vascular complications.9,10 Accordingly, differences in genetically endowed antioxidant status may confer increased or decreased susceptibility to the development of these diabetic vascular complications. We demonstrate here that purified haptoglobin 1-1 is a superior antioxidant to purified haptoglobin 2-2 in vitro. A key site of action of haptoglobin in neutralizing the oxidative capacity of hemoglobin is the extravascular space, particularly after endothelial injury. Haptoglobins 1-1 and 2-2 clearly differ in their ability to sieve into the extravascular compartment across the endothelial cell barrier.2 Because this difference in sieving is almost certainly a reflection of the profound differences in the sizes of haptoglobin 1-1 dimers and haptoglobin 2-2 cyclic polymers, we sought to identify a minimal haptoglobin peptide with preserved antioxidant function that would have an improved ability to penetrate the extravascular space. We describe identification of 2 distinct haptoglobin peptides, each of which can function as efficiently as haptoglobin in preventing oxidation by hemoglobin in vitro.
Materials and methods
Oxidation of linolenic acid by hemoglobin
All reagents were from Sigma Israel (Rehovot) unless otherwise indicated. Fatty acid micelles were prepared by adding 1 μL linolenic acid to 1 mL buffer A (50 mM Tris HCl, pH 6.5) and vortexing vigorously for 10 seconds. Hemoglobin (H-7379; Sigma) was prepared at a concentration of 10 mg/mL in buffer A and used within 4 hours of its preparation. Haptoglobin (1-1 or 2-2) was prepared in buffer A, and the concentration of the solution was determined from the calculated extinction coefficient of haptoglobin (EmM 53.9 for haptoglobin 1-1 and EmM 58.65 for haptoglobin 2-2). The molar concentration of haptoglobin was based on the monomer properties of that particular type of haptoglobin because each haptoglobin monomer (whether in the 1-1 or the 2-2 complex) is thought to be capable of binding a single hemoglobin molecule.2
The standard reaction (720 μL) consisted of the following reagents, all incubated at room temperature: 120 μL micelles (final concentration linolenic acid, 0.55 mM), 3 μL of a 10-mg/mL solution of hemoglobin in 1 mL buffer A (final concentration hemoglobin, 0.62 μM), and haptoglobin diluted in buffer A to the desired concentration. Additional buffer A was added to achieve a final volume of 720 μL. All components except for hemoglobin were added directly to a quartz cuvette and mixed by inverting 6 times. Three microliters hemoglobin solution was then added, and the cuvette was inverted to mix the ingredients. The zero time point was designated as the time at which hemoglobin was added to the solution. The formation of conjugated dienes was monitored by the change in absorption of the solution at 232 nm (A232) at room temperature for 60 minutes using a Lightwave S2000 spectrophotometer (WPA, Cambridge, United Kingdom). Readings were taken every 10 minutes. For all experiments assessing the ability to inhibit diene formation with haptoglobin or vitamins, 6 simultaneous reactions were performed to permit direct comparison of the increase in A232 obtained from the incubation of hemoglobin alone compared with hemoglobin with the antioxidants to be tested at the various concentrations. The change in A232with hemoglobin alone at 60 minutes was taken as the 100% value, and the change in A232 with each of the antioxidants at the different concentrations of haptoglobin at 60 minutes was determined relative to this value and was expressed as a percentage of relative oxidation. For each concentration of each antioxidant, the reaction was performed at least 6 separate times, and the mean ± SEM was determined. P values were determined using the paired Student t test, with P < .05 considered statistically significant.
Oxidation of low-density lipoprotein by hemoglobin.
Low-density lipoprotein (LDL) was isolated from human plasma by sequential ultracentrifugation as previously described.11,12 Oxy-Hb was obtained by chromatography methods, verified spectrophotometrically, and converted to met-Hb as previously described.13 LDL (200 μg/mL) was incubated for 4 hours at 37°C with met-Hb (10 μM) in the presence of H2O2 (20 μM). To this standard assay were added various concentrations of haptoglobin 1-1 or 2-2. Oxidation of LDL lipids was determined using the thiobarbituric reactive substances (TBARS) assay14 using a WPA Lightwave spectrophotometer. All experiments were performed at least 3 times, and the data are presented as mean inhibition by haptoglobin compared with the absence of haptoglobin. Relative inhibition was calculated by integrating the area under the curve of the TBARS assay using the MATLAB program. All values are expressed as mean ± SEM.P values were determined using the paired Studentt test, with P < .05 considered statistically significant.
Determination of the free hemoglobin concentration in the haptoglobin hemoglobin incubation conditions used to assess the antioxidant activity of haptoglobin.
Haptoglobin and met-hemoglobin were used at the concentrations described above for oxidation of LDL or linolenic acid. After a 10-minute incubation at room temperature, the haptoglobin-hemoglobin mixture was placed in a Centricon ultrafiltration apparatus with 100 kd cutoff (YM-100; Millipore, Bedford, MA). The apparatus was then subjected to centrifugation for 30 minutes at 1000gaccording to the manufacturer's instructions, resulting in the retention of complexes larger than 100 kd in the upper chamber and smaller than 100 kd in the lower chamber (filtrate). Because only free hemoglobin (molecular weight of the hemoglobin monomer is 64 kd) could pass into the filtrate, the concentration of hemoglobin in the filtrate, determined spectrophotometrically, was used to determine the concentration of free hemoglobin. For these studies, we used an extinction coefficient for met-hemoglobin of EmM 179 at 405 nm.12
Preparation of recombinant truncated haptoglobin
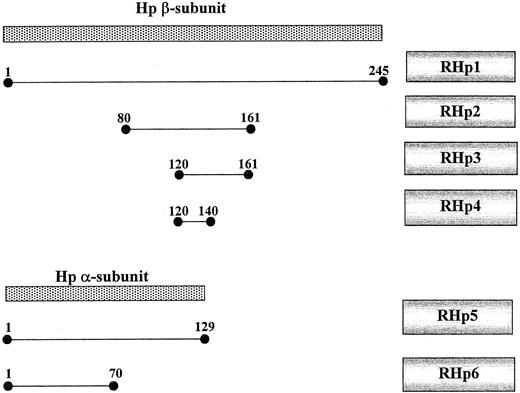
Truncated haptoglobin–glutathione-S-transferase (GST) fusion proteins were prepared as follows. Haptoglobin cDNA was prepared from the human Hep G2 cell line by reverse transcription–polymerase chain reaction (PCR). PCR primers were designed to produce a series of truncated haptoglobin products as noted in Tables1 and 2 and as shown schematically in Figure 1. These PCR products were first cloned into Teasy (Promega, Madison, WI), sequenced, and subcloned into pGEX-2TK (Pharmacia, Piscataway, NJ). Recombinant protein from pGEX was prepared by the induction of logarithmically growing BL21923 cultures with 0.1 mM isopropyl thiogalactopyranoside and purification of the sonicated cell lysate on GST-Sepharose (Bio-Rad, Rishon LeZion, Israel) as previously described.15 The GST-haptoglobin fusion protein was analyzed for purity on sodium dodecyl sulfate–polyacrylamide gel electrophoresis followed by Coomassie blue staining. Concentration of the fusion proteins was determined by using the Bradford reagent.
Primer sequences used for cloning haptoglobin β and α subunit constructs
| No. . | Primer name . | Sequence . |
|---|---|---|
| 1 | F-β-1 | 5′-CGCGGATCCATCCTGGGTGGACACCTGGATGCC-3′ |
| 2 | R-β-245 | 5′-GCGGAATTCTTAGTTCTCAGCTATGGTCTTCTGAAC-3′ |
| 3 | F-β-80 | 5′-CGCGGATCCAACTACTCCCAGGTAGATATTGGGCTC-3′ |
| 4 | R-β-161 | 5′-GCGGAATTCTTACTTCTTTTCGGGGACTGTGCT-3′ |
| 5 | F-β-120 | 5′-CGCGGATTCGTTTCTGGGTGGGGGCGAAATGCC-3′ |
| 6 | R-β-140 | 5′-GCGGAATTCTTACAGCATGACATACTTCAGATG-3′ |
| 7 | F-α-1 | 5′-CGCGGATCCGTAGACTCAGGCAATGATGTCACG-3′ |
| 8 | R-α-70,129 | 5′-GCGGAATTCTTATGCTTCACATTCAGGAAGTTT-3′ |
| No. . | Primer name . | Sequence . |
|---|---|---|
| 1 | F-β-1 | 5′-CGCGGATCCATCCTGGGTGGACACCTGGATGCC-3′ |
| 2 | R-β-245 | 5′-GCGGAATTCTTAGTTCTCAGCTATGGTCTTCTGAAC-3′ |
| 3 | F-β-80 | 5′-CGCGGATCCAACTACTCCCAGGTAGATATTGGGCTC-3′ |
| 4 | R-β-161 | 5′-GCGGAATTCTTACTTCTTTTCGGGGACTGTGCT-3′ |
| 5 | F-β-120 | 5′-CGCGGATTCGTTTCTGGGTGGGGGCGAAATGCC-3′ |
| 6 | R-β-140 | 5′-GCGGAATTCTTACAGCATGACATACTTCAGATG-3′ |
| 7 | F-α-1 | 5′-CGCGGATCCGTAGACTCAGGCAATGATGTCACG-3′ |
| 8 | R-α-70,129 | 5′-GCGGAATTCTTATGCTTCACATTCAGGAAGTTT-3′ |
Primer pairs and deduced product sizes
| RHp no. . | Haptoglobin subunit . | Forward primer . | Reverse primer . | DNA fragment size, bp . | Protein size, amino acids . | Fusion protein size, kd . |
|---|---|---|---|---|---|---|
| RHp1 | β | 1 | 2 | 735 | 245 | 66.0 |
| RHp2 | β | 3 | 4 | 243 | 81 | 39.2 |
| RHp3 | β | 5 | 4 | 123 | 41 | 32.7 |
| RHp4 | β | 5 | 6 | 60 | 20 | 29.3 |
| RHp5 | α | 7 | 8 | 387 | 129 | 42.0 |
| RHp6 | α | 7 | 8 | 210 | 70 | 34.9 |
| RHp no. . | Haptoglobin subunit . | Forward primer . | Reverse primer . | DNA fragment size, bp . | Protein size, amino acids . | Fusion protein size, kd . |
|---|---|---|---|---|---|---|
| RHp1 | β | 1 | 2 | 735 | 245 | 66.0 |
| RHp2 | β | 3 | 4 | 243 | 81 | 39.2 |
| RHp3 | β | 5 | 4 | 123 | 41 | 32.7 |
| RHp4 | β | 5 | 6 | 60 | 20 | 29.3 |
| RHp5 | α | 7 | 8 | 387 | 129 | 42.0 |
| RHp6 | α | 7 | 8 | 210 | 70 | 34.9 |
bp indicates base pair.
Schematic map of the haptoglobin subunits and truncated haptoglobin mutants.
Native haptoglobin (hatched) is made as a single polypeptide and then cleaved into an α- and a β-chain joined by disulfide bonds to form a haptoglobin monomer. The 2 alleles for haptoglobin differ only in their α-subunit. The RHp constructs were made as described in “Materials and methods” and correspond to the amino acids of the β or α chain, as shown. RHp 1 is the entire β-chain. RHp2 to RHp4 are truncated mutants of the β-chain. RHp5 is the α-chain from the 2 allele, and RHp6 is the α-chain from the 1 allele.
Schematic map of the haptoglobin subunits and truncated haptoglobin mutants.
Native haptoglobin (hatched) is made as a single polypeptide and then cleaved into an α- and a β-chain joined by disulfide bonds to form a haptoglobin monomer. The 2 alleles for haptoglobin differ only in their α-subunit. The RHp constructs were made as described in “Materials and methods” and correspond to the amino acids of the β or α chain, as shown. RHp 1 is the entire β-chain. RHp2 to RHp4 are truncated mutants of the β-chain. RHp5 is the α-chain from the 2 allele, and RHp6 is the α-chain from the 1 allele.
Enzyme-linked immunosorbent assay for qualitative determination of binding of truncated haptoglobin to hemoglobin
We determined the relative ability of the truncated haptoglobin fusion proteins to bind to hemoglobin in an enzyme-linked immunosorbent assay (ELISA). A specified amount of purified recombinant haptoglobin (1-250 μg) in 10 mM Tris-buffered saline (TBS), pH 8.0, was incubated in a 96-well plate overnight. The haptoglobin solution was aspirated, the wells were washed 5 times with TBS, and blocking was performed with a 5% dry milk solution in TBS for 1 hour. Twenty micrograms hemoglobin at a concentration of 200 μg/mL in TBS was then added for a 1-hour incubation at room temperature. The hemoglobin was then aspirated, the plate was washed 5 times with TBS, and a monoclonal antihemoglobin antibody was added (rabbit anti–human hemoglobin; DAKO, Glostrup, Denmark) at a 1:2000 dilution for overnight incubation at room temperature. This antihemoglobin antibody was then removed, and the wells were washed 5 times with TBS and incubated with antirabbit alkaline phosphatase–conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at a 1:2000 dilution. After again washing the wells 5 times, p-nitrophenyl phosphate (Sigma, Rehovot, Israel) was added according to the manufacturer's protocol, and the absorbance at 405 nM was recorded over time. GST alone or TBS alone was used as a negative control, and haptoglobin purified from human serum was used as a positive control. In this qualitative assay, binding was categorized as 0 (not significantly different from TBS or GST), 2+ if significant binding was present using less than 10 μg recombinant protein, and 1+ if significant binding was present only when using more than 100 μg recombinant protein in the assay.
Antioxidant activity of truncated haptoglobin
Recombinant GST-fusion proteins were analyzed in the linolenic acid oxidation assay for their ability to inhibit the oxidation of linolenic acid by hemoglobin as described above. GST alone had no effect on the oxidation of linolenic acid by hemoglobin even when used at concentrations 10 times greater than that used for the recombinant GST-haptoglobin fusion proteins.
Results
Inhibition of oxidation of linolenic acid by purified haptoglobin
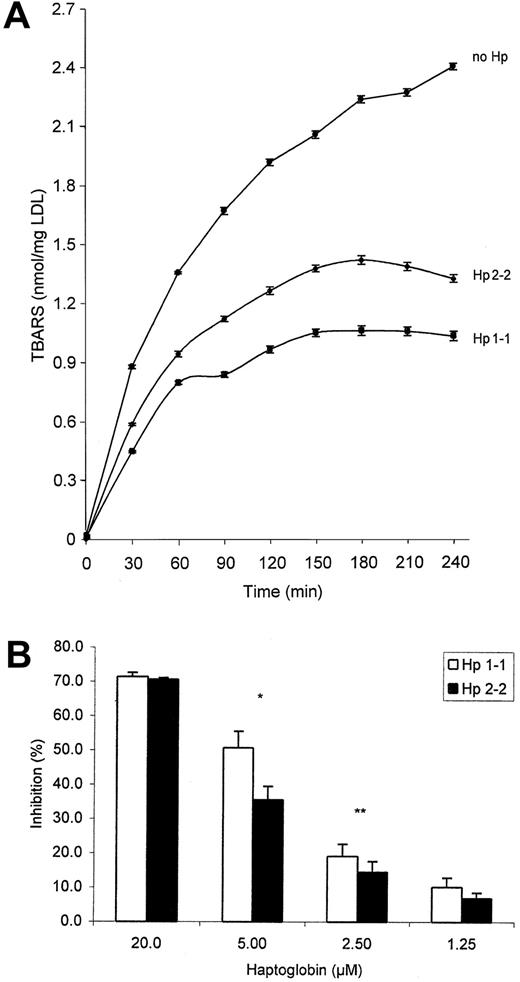
As previously demonstrated, we found that hemoglobin can oxidize linolenic acid in a time-dependent fashion as assessed using conjugated diene (A232) formation (Figure2A). This oxidation of linolenic acid by hemoglobin was shown to be inhibited by stoichiometric amounts of a mixture of the different haptoglobins prepared from pooled human sera.16 17 We sought to determine whether the ability to inhibit the oxidation of linolenic acid by hemoglobin as assessed by diene formation was different between the haptoglobin 1-1 and 2-2 proteins. Figure 2A provides a representative example of the differences in diene formation produced by the oxidation of linolenic acid in the presence of no haptoglobin and in the presence of haptoglobin 1-1 or haptoglobin 2-2, each at a concentration of 0.6 μM haptoglobin. At this concentration, haptoglobin 1-1 provided statistically significant greater protection against linolenic oxidation than haptoglobin 2-2 (Figure 2B). The percentage inhibition of the hemoglobin-induced oxidation of linolenic acid by both haptoglobin type 1-1 and haptoglobin type 2-2 was linearly related to the concentration of haptoglobin in our assay over a range of haptoglobin concentrations, from approximately 0.1 to 0.7 μM. Over this concentration range, haptoglobin 1-1 consistently demonstrated more inhibition of oxidation of linolenic acid than haptoglobin 2-2 (Figure 2B). At haptoglobin concentrations greater than 1.0 μM, there was complete (100%) inhibition of linolenic acid oxidation by both types of haptoglobin, whereas at haptoglobin concentrations less than 0.1 μM, there was no significant inhibition of linolenic acid oxidation by either type of haptoglobin.
Oxidation of linolenic acid by hemoglobin.
(A) Standard reaction demonstrating the time-dependent increase in conjugated diene (A232) formation when linolenic acid is incubated with hemoglobin in the presence of no haptoglobin, haptoglobin 1-1 (0.6 μM), or haptoglobin 2-2 (0.6 μM), as described in “Materials and methods.” Data shown are the mean ± SEM for 9 independent experiments. (B) Comparison of the percentage inhibition of hemoglobin-induced oxidation of linolenic acid by haptoglobin 1-1 or 2-2. Hemoglobin was used at a concentration of 0.62 μM, and haptoglobin was used at the concentrations shown. Data for the 2 types of haptoglobin are expressed as the percentage inhibition of oxidation that occurred in a reaction performed in parallel in the absence of any haptoglobin at the 60-minute time point. Data shown are the mean ± SEM of 9 independent experiments for each concentration of haptoglobin. *Difference in the mean percentage inhibition between haptoglobin 1-1 and haptoglobin 2-2 at that concentration was statistically significant (P < .05).
Oxidation of linolenic acid by hemoglobin.
(A) Standard reaction demonstrating the time-dependent increase in conjugated diene (A232) formation when linolenic acid is incubated with hemoglobin in the presence of no haptoglobin, haptoglobin 1-1 (0.6 μM), or haptoglobin 2-2 (0.6 μM), as described in “Materials and methods.” Data shown are the mean ± SEM for 9 independent experiments. (B) Comparison of the percentage inhibition of hemoglobin-induced oxidation of linolenic acid by haptoglobin 1-1 or 2-2. Hemoglobin was used at a concentration of 0.62 μM, and haptoglobin was used at the concentrations shown. Data for the 2 types of haptoglobin are expressed as the percentage inhibition of oxidation that occurred in a reaction performed in parallel in the absence of any haptoglobin at the 60-minute time point. Data shown are the mean ± SEM of 9 independent experiments for each concentration of haptoglobin. *Difference in the mean percentage inhibition between haptoglobin 1-1 and haptoglobin 2-2 at that concentration was statistically significant (P < .05).
Inhibition of low-density lipoprotein oxidation by haptoglobin
As previously demonstrated,13 we found that hemoglobin can oxidize LDL in a time-dependent fashion as assessed by measuring TBARS (Figure 3A). This oxidation of LDL by hemoglobin was previously shown to be inhibited by stoichiometric amounts of a mixture of haptoglobin proteins prepared from pooled human sera.13 We sought to determine whether the ability to inhibit the oxidation of LDL by hemoglobin was different between haptoglobin 1-1 and 2-2 proteins. Figure 3A provides a representative example of the differences in TBARS formation produced by the oxidation of LDL in the presence of no haptoglobin and in the presence of haptoglobin 1-1 or haptoglobin 2-2, each at a concentration of 5 μM haptoglobin. At this haptoglobin concentration, haptoglobin 1-1 provided statistically significant greater protection against LDL oxidation than haptoglobin 2-2 (Figure 3B). The percentage inhibition of the hemoglobin-induced oxidation of LDL by haptoglobin 1-1 or 2-2 was linearly related to the concentration of haptoglobin in the assay over a range of haptoglobin concentrations, from 1 to 20 μM. Over this concentration range, haptoglobin 1-1 consistently demonstrated greater inhibition of oxidation of LDL than haptoglobin 2-2 (Figure3B). Outside this concentration range for haptoglobin, where either haptoglobin was present in extreme molar excess or hemoglobin was present in extreme molar excess, the 2 types of haptoglobin were not different in protecting against hemoglobin-induced oxidation of LDL, analogous to what was described for linolenic acid above.
Oxidation of LDL by hemoglobin.
(A) Standard reaction demonstrating the time-dependent increase in TBARS formation when LDL is incubated with hemoglobin (10 μM) in the presence of no haptoglobin, haptoglobin 1-1 (5 μM), or haptoglobin 2-2 (5 μM) as described in “Materials and methods.” Data shown are the mean ± SEM for 4 independent experiments. (B) Comparison of the ability to inhibit the hemoglobin-induced oxidation of LDL by haptoglobin 1-1 or 2-2. Hemoglobin was used at a concentration of 10 μM, and haptoglobin was used at the concentrations shown. Data are expressed as a percentage of inhibition of the amount of TBARS obtained in the absence of any haptoglobin over the entire incubation period by integrating the area under the TBARS versus the time curve using MATLAB, as described in “Materials and methods.” Data shown are the mean ± SEM of 4 independent experiments for each haptoglobin concentration. *Difference in the mean percentage inhibition between haptoglobin 1-1 and haptoglobin 2-2 was statistically significant (P < .004).
Oxidation of LDL by hemoglobin.
(A) Standard reaction demonstrating the time-dependent increase in TBARS formation when LDL is incubated with hemoglobin (10 μM) in the presence of no haptoglobin, haptoglobin 1-1 (5 μM), or haptoglobin 2-2 (5 μM) as described in “Materials and methods.” Data shown are the mean ± SEM for 4 independent experiments. (B) Comparison of the ability to inhibit the hemoglobin-induced oxidation of LDL by haptoglobin 1-1 or 2-2. Hemoglobin was used at a concentration of 10 μM, and haptoglobin was used at the concentrations shown. Data are expressed as a percentage of inhibition of the amount of TBARS obtained in the absence of any haptoglobin over the entire incubation period by integrating the area under the TBARS versus the time curve using MATLAB, as described in “Materials and methods.” Data shown are the mean ± SEM of 4 independent experiments for each haptoglobin concentration. *Difference in the mean percentage inhibition between haptoglobin 1-1 and haptoglobin 2-2 was statistically significant (P < .004).
Effective hemoglobin-binding capacity of haptoglobin 1-1 and haptoglobin 2-2
Differences in the antioxidant protection provided by our haptoglobin preparations could be a result of a systematic error made in estimating haptoglobin hemoglobin-binding capacity. We used haptoglobin monomer molar concentrations in these studies because it has been established that every haptoglobin monomer can bind 1 hemoglobin molecule (alpha-beta dimer) (stoichiometry of 1-1).1 This stoichiometry of the binding reaction is thought to be identical for all forms of haptoglobin.1,18,19 However, it has been proposed that not every haptoglobin monomer in the larger cyclic polymers found in haptoglobin type 2-2 is capable of binding hemoglobin because of steric considerations.20 If every haptoglobin monomer in a cyclic 2-2 polymer cannot bind hemoglobin, then over the concentration range of haptoglobin used to inhibit the oxidation of LDL or linolenic acid we predicted there would be a greater amount of free hemoglobin in reactions using haptoglobin 2-2 than in those using haptoglobin 1-1. The excess free hemoglobin in the reaction using haptoglobin 2-2 would be expected to result in more oxidation of LDL or linolenic acid than what was observed with haptoglobin 1-1.
To investigate the effective hemoglobin-binding capacity of our haptoglobin 1-1 and 2-2 preparations, we developed a filtration assay designed to monitor the amount of unbound hemoglobin, as described in “Materials and methods.” The filtration assay used the Centricon microfiltration system by way of a membrane with a 100-kd cutoff. Haptoglobin and hemoglobin were mixed together at the same molar ratios used in the oxidation studies described above and then were subjected to Centricon ultrafiltration. The amount of free or unbound hemoglobin was determined spectrophotometrically in the ultrafiltrate. If the greater amount of oxidation in the reactions involving haptoglobin 2-2 was attributed to less hemoglobin-binding capacity than those involving haptoglobin 1-1, we would have expected to see more free hemoglobin in the ultrafiltrate when using haptoglobin 2-2. This was not the case. There was no significant difference in the free hemoglobin concentrations in the ultrafiltrate of haptoglobin 1-1–hemoglobin solutions than in haptoglobin 2-2–hemoglobin solutions. At a concentration of 10 μM hemoglobin and 5 μM haptoglobin (concentrations at which we found haptoglobin 1-1 was significantly superior to haptoglobin 2-2 in protecting against hemoglobin-induced LDL oxidation), we found that the molar ratio of free hemoglobin in the ultrafiltrate for haptoglobin 1-1–hemoglobin solutions compared to haptoglobin 2-2–hemoglobin solutions was 1.09 ± −0.15 (P = .835; n = 6). Therefore, the inferior antioxidant capacity of our preparation of haptoglobin 2-2 could not be explained by a lower effective hemoglobin-binding capacity than our preparation of haptoglobin 1-1.
Identification of putative hemoglobin-binding sites on haptoglobin by ELISA using truncated haptoglobin
The hemoglobin-haptoglobin complex has not as yet been crystallized; thus, the residues involved in binding are not definitively known. Findings from gel permeation studies with purified haptoglobin have suggested that the β-chain of haptoglobin is responsible for binding to hemoglobin.1 The importance of several residues in the β-chain has been suggested by the use of proteolytic peptides of haptoglobin and the ascertainment of their ability to bind to hemoglobin in native polyacrylamide gels.21 Further assessment of the putative residues on haptoglobin capable of binding to hemoglobin using recombinant haptoglobin-truncated mutants or haptoglobin peptides has not been performed. Therefore, we developed a simple ELISA capable of differentiating qualitative differences in the binding of haptoglobin to hemoglobin. A battery of α- and β-chain recombinant fusion proteins was made and is shown schematically in Figure 1. We were able to identify binding not only in the β-chain but, surprisingly, also in the α-chain (qualitatively denoted by 0-2+ binding in Table3). This assay was only used to detect the presence or absence of binding of specific haptoglobin mutants before ascertaining whether they had activity as antioxidants and could not be used to demonstrate quantitative differences in binding affinity between the different mutants.
Binding of truncated haptoglobin to hemoglobin
| Substrate . | Binding . |
|---|---|
| TBS | − |
| GST | − |
| RHp1 | ++ |
| RHp2 | ++ |
| RHp3 | + |
| RHp4 | − |
| RHp5 | + |
| RHp6 | ++ |
| Substrate . | Binding . |
|---|---|
| TBS | − |
| GST | − |
| RHp1 | ++ |
| RHp2 | ++ |
| RHp3 | + |
| RHp4 | − |
| RHp5 | + |
| RHp6 | ++ |
RHp indicates recombinant haptoglobin; GST, glutathione-S-transferase; and TBS, Tris-buffered saline.
Truncated haptoglobin can prevent the oxidation of linolenic acid by hemoglobin. We tested the ability of the truncated haptoglobin fusion proteins that were shown to bind to hemoglobin in the ELISA for their ability to inhibit the oxidation of linolenic acid by hemoglobin as described in “Materials and methods.” Using progressive deletion analysis, we were able to identify in the β-chain an 81–amino acid fragment (construct recombinant haptoglobin 2 [RHp2], Table 2) that could completely prevent the oxidation of linolenic acid by hemoglobin at concentrations of 0.18 μM based on Bradford protein assay (Figure 4A), suggesting that this truncated construct is at least as potent in inhibiting the oxidation of linolenic acid by haptoglobin as native haptoglobins. A 40–amino acid fragment within this 81–amino acid fragment could not inhibit the oxidation of linolenic acid, even though it demonstrated specific binding to hemoglobin (Figure 4B, Table 3). We were also able to demonstrate that the α-chain of haptoglobin (RHp 5 or 6, corresponding to the α-chain from allele 1 or 2) can inhibit oxidation by hemoglobin as efficiently as haptoglobin (data not shown).
Truncated haptoglobin inhibits the oxidation of linolenic acid by hemoglobin.
Recombinant haptoglobin was produced as described in “Materials and methods.” (A) Lack of inhibition of oxidation of linolenic acid by a 40–amino acid construct RHp3 (derived from RHp2). (B) Concentration-dependent inhibition of oxidation of linolenic acid by an 81–amino acid construct derived from the haptoglobin β-chain (RHp2).
Truncated haptoglobin inhibits the oxidation of linolenic acid by hemoglobin.
Recombinant haptoglobin was produced as described in “Materials and methods.” (A) Lack of inhibition of oxidation of linolenic acid by a 40–amino acid construct RHp3 (derived from RHp2). (B) Concentration-dependent inhibition of oxidation of linolenic acid by an 81–amino acid construct derived from the haptoglobin β-chain (RHp2).
Discussion
We have demonstrated that there are functional differences in the antioxidant capacity of the different haptoglobin proteins toward hemoglobin, suggesting that those with haptoglobin 1-1 protein may have superior antioxidant protection than those with haptoglobin 2-2 protein. These data are consistent with earlier findings showing that the consumption of vitamin C in the plasma in vitro of persons with haptoglobin 2-2 was more rapid than in the plasma of those with haptoglobin 1-1 and that vitamin C levels are significantly lower in those with haptoglobin 2-2.22
The stoichiometries of haptoglobin 1-1 and 2-2 binding to hemoglobin are identical.1,18 19 Our results showing a difference between the amount of hemoglobin-inducible oxidation between our 2 different preparations was not caused by a systematic error in the determination of the hemoglobin-binding capacity of our preparations of the 2 haptoglobin types. Using concentrations of haptoglobin and hemoglobin identical to what was used in the oxidation reactions described in this study, we found that there was no significant difference between the amount of free hemoglobin using our haptoglobin 1-1 preparations and our haptoglobin 2-2 preparations. This was important to demonstrate because if our haptoglobin 2-2 had lower hemoglobin-binding capacity than our haptoglobin 1-1, the amount of free hemoglobin available to oxidize linolenic acid or LDL in the oxidation reactions using haptoglobin 2-2 would have been greater. Therefore, the inferior antioxidant capacity of our haptoglobin 2-2 could not be attributed to lower hemoglobin-binding capacity.
The antioxidant activity of haptoglobin results, at least in part, from the binding of haptoglobin to hemoglobin, thereby preventing the dissociation of ferric heme from globin.23 Demonstration that iron is one of the species directly responsible for the oxidation of linolenic acid has been demonstrated by 2 groups using the iron chelator, desferrioxamine.16,17 However, Gutteridge16 has also shown that hemoglobin is capable of stimulating lipid peroxidation for a short period of time by a reaction independent of free heme (ie, inhibited by haptoglobin but not inhibited by desferrioxamine) and that heme release only occurs after the products of lipid peroxidation damage the hemoglobin molecule, causing it to release iron. The difference between haptoglobin 1-1 and haptoglobin 2-2 in inhibiting the oxidation of linolenic acid, according to these mechanistic schemes, may be the result of differences in the ability of the different types of haptoglobin to prevent the release of heme. We have not observed that the differences between the 2 types of haptoglobin are more exaggerated at earlier time points in the oxidation reaction, which might have suggested that the alternative mechanism proposed by Gutteridge16 is a point of difference between the 2 types of haptoglobin. The haptoglobin-hemoglobin complex has been demonstrated to have a potent peroxidase activity24 that could also serve as a point of difference between the 2 haptoglobin types and could provide an explanation for their differences in apparent antioxidant activity.
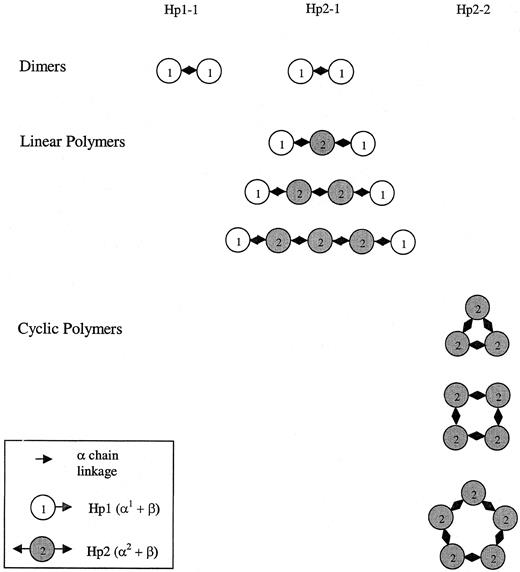
The statistically significant but relatively modest differences we have described here in antioxidant capacity between the different haptoglobin types may be dramatically amplified in vivo because of differences in the ability of the different haptoglobin types to gain access to the vessel wall. A schematic drawing of the different haptoglobin polymers1,25 in persons with haptoglobin 1-1 or 2-2, as shown in Figure 5, demonstrates the large differences in size between the different haptoglobin types likely to account for these differences in sieving capacity. At sites of blood vessel injury (ie, after coronary angioplasty), there is a sudden release of free hemoglobin into the blood vessel wall. Haptoglobin is not normally found in appreciable concentrations in the normal vessel wall. Therefore, the ability of haptoglobin to sieve into the vessel wall to neutralize hemoglobin is likely to be of great importance. In the patient with diabetes, already burdened with increased oxidative stress from hyperglycemia,9 10 differences in genetically determined endogenous antioxidant protection may have exaggerated importance.
Schematic map of the different shapes of the haptoglobin polymers as determined by phenotype.
These shapes have been confirmed by electron microscopic analysis of haptoglobin purified from patients with haptoglobin 1-1, 2-1, or 2-2.20 Critical disulfide linkages necessary for covalent cross-linking of haptoglobin monomers (circles) to form polymers are found on exons 3 and 4 (α-chain of haptoglobin). The haptoglobin 2 allele has a duplication of exons 3 and 4. The haptoglobin 1 monomer is monovalent (note single arrow) and thus can only associate with one other haptoglobin molecule to create dimers. The haptoglobin 2 monomer is bivalent (note 2 arrows) and can associate with 2 different haptoglobin monomers. Consequently, the haptoglobin in persons homozygous for the 2 allele will be cyclic polymers.
Schematic map of the different shapes of the haptoglobin polymers as determined by phenotype.
These shapes have been confirmed by electron microscopic analysis of haptoglobin purified from patients with haptoglobin 1-1, 2-1, or 2-2.20 Critical disulfide linkages necessary for covalent cross-linking of haptoglobin monomers (circles) to form polymers are found on exons 3 and 4 (α-chain of haptoglobin). The haptoglobin 2 allele has a duplication of exons 3 and 4. The haptoglobin 1 monomer is monovalent (note single arrow) and thus can only associate with one other haptoglobin molecule to create dimers. The haptoglobin 2 monomer is bivalent (note 2 arrows) and can associate with 2 different haptoglobin monomers. Consequently, the haptoglobin in persons homozygous for the 2 allele will be cyclic polymers.
We have identified 2 peptides derived from haptoglobin that can independently bind to hemoglobin and prevent it from oxidizing substrate, analogous to the full-length haptoglobin molecule. We have not determined the affinity of these constructs for hemoglobin, nor have we demonstrated that the stoichiometry of binding of these constructs is identical to native haptoglobin. Our studies suggest that these truncated haptoglobins are similar in their potency to native haptoglobin in terms of their ability to inhibit the oxidation of linolenic acid by hemoglobin. Such a mini-haptoglobin may be expected to have improved access to the extravascular space and thus may be proposed as a candidate drug in animal models of diabetic vascular complications. We are performing progressive truncation of these mini-haptoglobins to define the absolute minimal haptoglobin that can inhibit oxidation by hemoglobin.
We have learned from epidemiologic studies that haptoglobin type is fundamentally important in the development of diabetic vascular disease. Elucidation of the biochemical basis for differences between the haptoglobin types is the first step necessary for the development of new drugs and strategies to limit diabetic vascular complications.
Supported by National Heart Lung and Blood Institute grants RO1 HL-58510 and RO1 HL66195 (A.P.L.), the Israel Cancer Research Fund (A.P.L.), the Israel Cancer Association (A.P.L.), the Israel Science Foundation (A.P.L.), and the Bruce Rappaport Fund for Biochemical Research (A.P.L.).
M.M.-F., O.L., and B.I.E. contributed equally to the preparation of this manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Andrew P. Levy, Technion Faculty of Medicine, POB 9649, Haifa 31096, Israel; e-mail:alevy@tx.technion.ac.il.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal