Abstract
Leukemic CD34+ immature acute myeloid leukemia (AML) cells express Fas receptor but are frequently resistant to Fas agonistic reagents. Fas plays an important role in T-cell–mediated cytotoxicity, and recently it has been suggested that altered Fas signaling may contribute to drug resistance. Therefore, Fas resistance could be one of the mechanisms by which AML progenitors escape chemotherapy or T-cell–based immune intervention. However, the molecular mechanism of Fas resistance in AML cells has not been identified. Fas signaling can be interrupted at 3 mains levels: Fas clustering, alteration of death-inducing-signaling-complex (DISC) formation, and effector caspase inhibition of downstream caspase-8. This study shows that in the Fas-resistant CD34+CD38− KG1a cells, Fas agonists resulted in Fas aggregation but not in caspase-8 activation, related to a defect in DISC formation. However, pretreatment with chelerythrin, but not with calphostin C, resulted in the restoration of Fas-induced caspase-8 activation and cytotoxicity, suggesting that some atypical protein kinase C (PKC) isoforms contributed to the lack of DISC formation. Indeed, treatment with antisense oligonucleotides directed against PKCζ and enforced expression of Par-4, a negative regulator of PKCζ activity, restored Fas-induced caspase-8 activity and apoptosis. Moreover, it was found that PKCζ interacts with FADD and that PKCζ immunoextracts prepared from KG1a cells are able to phosphorylate FADD in vitro, whereas this phosphorylation is dramatically reduced in Par-4 transfectant cells. In conclusion, it is suggested that in AML cells, PKCζ plays an important role in Fas resistance by inhibiting DISC formation, possibly by phosphorylating FADD.
Introduction
Fas (APO-1/CD95) is a 45-kd membrane protein that belongs to the tumor necrosis factor (TNF)–nerve growth factor receptor family, a group of type 1 transmembrane receptors.1 Mutational analysis of Fas and the human TNF receptor (TNFR-1) proteins demonstrates that the cytoplasmic domains share a homologous region necessary to transduce the apoptotic signal. This conserved region of approximately 70 amino acids was, therefore, designated as the death domain (DD). The only known physiological ligand of Fas, Fas-L (CD95L), belongs to the family of TNF-related cytokines.2 Fas-L is synthesized as a transmembrane molecule, and soluble Fas-L trimers can be generated through processing by a metalloprotease.3,4 Engagement of Fas by agonistic anti-Fas antibodies or by Fas-L triggers apoptosis in a variety of cell types. However, only membrane-bound or multimerized Fas-L induces cell death.3,4 Moreover, ligand-dependent activation of Fas death pathway requires the oligomerization of Fas receptor, but ligand-independent activation can occur on Fas aggregation induced by Fas overexpression or treatment with anticancer drugs or radiation.5-9 Clustering of Fas recruits Fas-associated death domain (FADD)–containing protein, which is a bipartite molecule with a death effector domain (DED) at the amino terminus and a DD at the carboxyl terminus. FADD binds to Fas through a DD–DD interaction and recruits the DED-containing procaspase-8 through a DED–DED interaction. The formation of this death-inducing signaling complex (DISC) results in caspase-8 activation, believed to be the first step of a proteolytic cascade that triggers the activation of other caspases such as caspase-3, -7, and -6.10,11 Although other cell death pathways could be initiated from Fas activation,12-14 analysis of lymphocytes from FADD−/− mice has demonstrated the prominent role of the FADD/caspase-8 pathway in Fas-mediated cell death.15
Normal CD34+ hematopoietic cells, including the most immature CD34+CD38− subset, express Fas at a low level and are resistant to Fas-induced apoptosis unless they are treated with TNF-α or interferon (IFN)-γ.16-19 These studies have shown also that these cytokines enhanced Fas expression in CD34+ cells; however, they did not provide direct evidence that TNF-α– or IFN-γ–induced sensitization to Fas-induced apoptosis was attributed to increased Fas expression. Moreover, when cultured in the presence of hematopoietic growth factors, CD34+ cells expressed functional Fas; indeed, the CD34+ Fas+ cell population gradually lost CD34 expression and shifted to a CD34− Fas+ and Fas-sensitive cell population.18 These results suggest that Fas is expressed as part of a differentiation program of hematopoietic cells; in fact, functional Fas is expressed in terminally differentiated myeloid cells, including neutrophils, eosinophils, and monocytes.20,21 Fas distribution and function appear to be not very different in leukemic myelopoiesis. Indeed, most fresh CD34+ acute myeloid leukemia (AML) cells express Fas, whereas they are frequently resistant to Fas-induced apoptosis.22,23 These results suggest that in AML cells, as in early normal progenitors, potent negative regulators interfere either upstream or downstream of DISC formation. This may have important physiopathologic and therapeutic implications in AML. First, based on a recent study suggesting that the Fas–Fas-L system exerts a negative regulatory effect on committed progenitor expansion,24 it may be that Fas resistance contributes to leukemic clone expansion. Second, because the Fas–Fas-L system plays an important role in T-cell–mediated cytotoxicity, it is conceivable that resistance to Fas decreases the efficiency of graft-versus-leukemic reaction after allogeneic bone marrow transplantation or donor lymphocyte infusion. Third, because it has been reported that the ligand-dependent and the ligand-independent Fas–caspase-8 death pathways may play a role in drug-induced apoptosis,9,25-29 one can also speculate that the alteration of Fas–caspase-8 signaling may contribute to the natural drug resistance of AML progenitors and subsequently to the high rate of relapse. For these reasons, it could be important to determine the negative regulators of Fas signaling in Fas-expressing AML cells. Among different parameters and based on the influence of protein kinase C (PKC) activity on Fas-induced apoptosis,30-33 we hypothesized that, in AML cells, some isoforms of PKC exert a protective function against Fas death pathway.
CD34+CD38− KG1a AML cells represent an interesting model for investigating Fas resistance in immature AML cells. Because of their immature phenotype, they could be considered representative of early leukemic myelopoiesis. KG1a cells exhibit a high level of Fas expression, whereas they are totally resistant to agonistic anti-Fas antibody.22 In addition, KG1a cells are highly resistant to TNFα, a cytokine that shares common signaling pathways with Fas.34 The goal of this study was to investigate in KG1a cells the early steps of Fas signaling, including Fas clustering, DISC formation, and caspase-8 activation, and to evaluate the role of PKC in KG1a cell Fas resistance.
Materials and methods
Cell cultures
KG1a (promyeloblastic), U937 (monocytic), and Jurkat (T-lymphoid) cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD). Cells were maintained in Iscoves modified Dulbecco medium supplemented with 20% fetal calf serum (KG1a) or RPMI-1640 medium supplemented with 10% fetal calf serum (U937, Jurkat). All media were supplemented with 2 mM L-glutamine, 200 U/mL penicillin, and 100 μg/mL streptomycin (Life-Technology, Cergy-Pontoise, France). Cells were maintained at 37°C in a fully humidified 5% CO2 incubator.
Reagents
Anti-Fas (clone CH11), neutralizing anti-Fas (clone ZB4), and secondary monoclonal antibodies were from Beckman/Coulter (Villepinte, France). Fas-L-FLAG was purchased from Alexis (Coger, France). Recombinant Fas-L was produced by transfected Neuro-2A35(a kind gift from A. Fontana, Lausanne, Switzerland). Anti-PKCζ, anti-actin monoclonal antibodies, and FADD-agarose were purchased from Euromedex (Souffelweyersheim, France); anti-FADD monoclonal antibodies were purchased from Becton Dickinson (Le Pont de Claix, France); anti–caspase-8 and -3 and anti–Par-4 were purchased from Santa Cruz Biotechnology (TEBU, Le Perray-en-Yvelines, France). Sense and antisense oligonucleotides directed against PKCζ were designed and manufactured by Biognostik (Göttingen, Germany; product number 01669). Other products were purchased from Sigma (Saint-Quentin-Fallavier, France).
Fas clustering and confocal analysis
For the detection of Fas monomer aggregation, KG1a and U937 cells were treated or not treated with 0.5 μg/mL Fas-L for 4 hours, fixed for 10 minutes in 3% paraformaldehyde, and washed twice with phosphate-buffered saline (PBS) for 10 minutes. After 15-minute pre-incubation with 2% bovine serum albumin, cells were incubated for 2 hours at room temperature with or without anti-Fas monoclonal antibody (IgG1 clone ZB4, 1/100) diluted in PBS containing 1% bovine serum albumin. Nonimmune mouse IgG1 was used as a negative control. Samples were then washed in PBS and incubated for 45 minutes with FITC-conjugated goat anti-mouse monoclonal antibody. Subsequently, cells were fixed in paraffin on slides and were examined with a confocal imaging system (Zeiss, Oberkochen, Germany) scanning assembly incorporating argon and helium–neon lasers coupled to a Zeiss Axiovert 100 fluorescence microscope.
Western blot analysis
Exponentially growing cells were pre-incubated in the presence or absence of inhibitors and then treated by CH11 monoclonal antibody for different time periods. Cells were washed twice in serum-free medium, centrifuged, and lysed in RIPA buffer (50 mM Tris, pH 8, 150 mM NaCl, 1% Triton X-100, 1% NP-40, 0.1% sodium dodecyl sulfate [SDS], 5 mM EDTA, 1 mM dithiothreitol [DTT], 2 μg/mL leupeptin, 2 μg/mL aprotinin, and 0.1 mM phenylmethylsulfonyl fluoride [PMSF]) for 20 minutes on ice, followed by centrifugation at 10 000g for 15 minutes. Protein concentration in the supernatants was determined as previously described.36 For each lysate, 40 μg total protein was boiled for 5 minutes at 95°C in the presence of 3% β-mercaptoethanol. Proteins were separated on 12.5% (wt/vol) SDS–polyacrylamide gel electrophoresis (PAGE) and were transferred electrophoretically onto nylon membranes (Hybond-C extra; Amersham Life Science, Cergy-Pontoise, France). Nonspecific binding sites were blocked in 10 mM Tris-buffered saline containing 0.1% Tween-20 and 10% nonfat milk. Membranes were then incubated overnight at 4°C with specific primary monoclonal antibody diluted at an appropriate concentration in 10 mM Tris-buffered saline containing 0.1% Tween-20 and 1% nonfat milk. Membranes were then washed 5 times at room temperature, and bound immunoglobulin was detected with anti-isotype monoclonal antibody coupled to horseradish peroxidase (Beckman-Coulter). The signal was visualized by enhanced chemiluminescence (Amersham, Buckinghamshire, United Kingdom) and autoradiography.
DISC formation analysis
Exponentially growing cells (100 × 106) were incubated with 1 μg/mL Fas-L–FLAG (Alexis, San Diego, CA) and 1 μg anti-FLAG monoclonal antibody (Sigma, Saint-Quentin-Fallavier, France) for 15 minutes or 1 hour. Cells were then centrifuged and lysed in lysis buffer (0.2% NP-40, 20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 2 mM sodium vanadate, 10% glycerol, 2 μg/mL leupeptin, 2 μg/mL aprotinin, 0.1 mM PMSF) before protein A–Sepharose was added. Immunoprecipitates were washed 3 times in lysis buffer without protease inhibitors before SDS-PAGE and Western blot analysis.
Caspase-8 activity assay
Caspase-8 colorimetric activity assay (R&D Systems, Abingdon, United Kingdom) was performed according to the manufacturer's recommendations. Briefly, exponentially growing cells treated by CH11 monoclonal antibody (2 μg/mL) for 4 hours were collected by centrifugation. Lysis buffer was added on the cell pellet, incubated on ice for 10 minutes, and centrifuged at 10 000g for 1 minute. For each lysate, 100 μg total protein was incubated with caspase-8 colorimetric substrate for 2 hours at 37°C. Cleavage of the substrate by caspase-8 was quantified spectrophotometrically at a wavelength of 405 nm.
PKCζ antisense experiments
Blocking experiments were performed with antisense or sense phosphorothioate oligonucleotides (10 μM) directed against PKCζ. Briefly, exponentially growing cells were cultured with sense or antisense oligonucleotides 48 hours before CH11 monoclonal antibody (2 μg/mL) was added. After treatment, viability assay (Trypan blue exclusion) and Western blot analysis or caspase-8 activity assay were performed as described above.
Cytochemical staining
Changes in cellular nuclear chromatin were evaluated by DAPI staining. Briefly, after CH11 treatment (2 μg/mL for 5 hours), cells were cytocentrifuged and fixed in 4% paraformaldehyde. Slides were then stained with 1 μg/mL DAPI and analyzed by fluorescence microscopy.
Immunoprecipitation
Cell lysates (5 × 106) were prepared in RIPA lysis buffer for 30 minutes on ice, sonicated, and centrifuged (15 minutes, 10 000g at 4°C). Supernatants were normalized for protein concentration, and each sample (1 mg protein) was immunoprecipitated with anti-PKCζ monoclonal antibody (4 μg) or anti-FADD monoclonal antibody (2 μg) and collected by absorption to protein G–Sepharose. Immunoprecipitates were washed 3 times in RIPA buffer without protease inhibitors before analysis by SDS-PAGE and Western blotting.
In vitro PKCζ kinase assay
Cell lysates (10 × 106) were prepared in lysis buffer (20 mM HEPES, 2 mM EDTA, 125 mM NaCl, 0.1% NP40, 2 μg/mL aprotinin, 2 μg/mL leupeptin, 0.5 mg/mL benzamidine, 1 mM PMSF, 1 mM DTT) for 30 minutes on ice followed by centrifugation (3 minutes, 14 000g). Supernatants were normalized for protein concentration, and each sample (1 mg protein) was immunoprecipitated with anti-PKCζ monoclonal antibody and collected by absorption to protein G–Sepharose. Immunocomplexes bound to protein G–Sepharose were washed in lysis buffer without PMSF and subsequently were resuspended in reaction buffer (20 mM HEPES, 1 mM DTT, 10 mM MgCl2, 4 μg/mL phosphatidylserine, 20 μM cold ATP). For each sample, 10 μCi [γ-32P] ATP (6000 Ci/mmol [222 TBq/mmol]; ICN, Orsay, France) and 1 μg FADD agarose or 3 μg histone H1 were added. Samples were then incubated for 5 minutes at 32°C. The reaction was terminated by the addition of protein loading buffer. Proteins were separated on 10% SDS-PAGE, and the gel was subjected to autoradiography. In parallel, an aliquot of each sample was analyzed by Western blot using anti-PKCζ monoclonal antibody to quantify immunoprecipitated proteins.
Par-4 transfection in KG1a cells
Exponentially growing cells were transfected by a plasmid containing full-length Par-4 cDNA sequence (kindly gift from M. T. Diaz-Meco, Madrid, Spain) using Effectene transfection reagent (Qiagen, Courtaboeuf, France) according to the manufacturer's instructions. Clones were further selected for Par-4, CD34+, CD38− expression by Western blot analysis or flow cytometry.
Statistics
Quantitative experiments were analyzed using Studentt test. All P values resulted from the use of 2-sided tests.
Results
Fas expression and function in KG1a cells
KG1a, U937, and Jurkat cells were treated with increasing concentrations of CH11 monoclonal antibody for 30 minutes in cold medium, then were stained by an indirect immunofluorescence technique using phycoerythrin-labeled goat–anti-mouse immunoglobulin (Ig)M. Fluorescence was evaluated by flow cytometry for each cell line. Saturating concentrations ranged between 0.5 and 2 μg/mL, depending on the cellular model. At the saturating dose of 2 μg/mL, KG1a cells displayed mean fluorescence intensity similar to, if not higher than, that of Fas-sensitive Jurkat or U937 cells (data not shown).
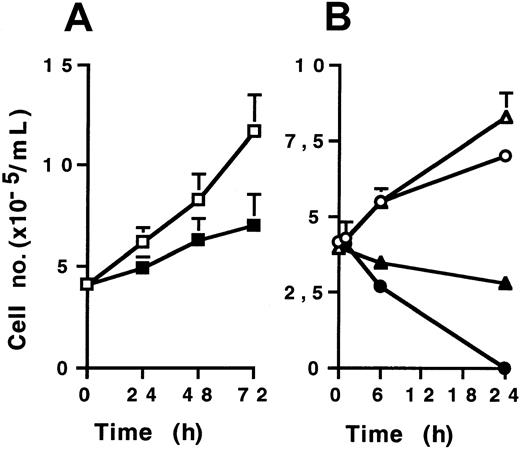
KG1a cells were then treated with various doses of CH11 (2-10 μg/mL) in supplemented Iscoves modified Dulbecco medium culture for 24, 48, and 72 hours. Cell viability was measured by Trypan blue dye exclusion assay. CH11 monoclonal antibody, at a 2 μg/mL dose, induced only a modest, though significant, growth inhibitory effect on KG1a cells compared to IgM-isotypic control-treated cells (Figure1A). Higher doses (up to 10 μg/mL) were also inefficient for inducing KG1a cell death (data not shown). However, CH11-treated Jurkat and U937 cells rapidly died, the former more sensitive than the latter (Figure 1B). Morphologic examination after DAPI staining showed typical features of apoptosis in CH11-treated Jurkat and U937 cells, whereas CH11-treated KG1a cells displayed no morphologic changes (data not shown). Similar results were obtained with recombinant human Fas-L used at various doses (0.2-2 μg/mL) (data not shown). These results confirmed that despite high level of Fas expression, KG1a cells were resistant to Fas-induced apoptosis.
CH11 effect on cell viability.
Cells (4 × 105/mL) were treated by CH11 (2 μg/mL for KG1a and U937; 0.5 μg/mL for Jurkat) or IgM isotypic control for different time periods. Cell viability was evaluated by Trypan blue exclusion assay. (A) KG1a cells treated by CH11 (▪) or IgM (■). (B) Jurkat cells treated by CH11 (●) or IgM (○), U937 cells treated by CH11 (▴) or IgM (▵). Results are the mean ± standard deviation of 3 independent experiments.
CH11 effect on cell viability.
Cells (4 × 105/mL) were treated by CH11 (2 μg/mL for KG1a and U937; 0.5 μg/mL for Jurkat) or IgM isotypic control for different time periods. Cell viability was evaluated by Trypan blue exclusion assay. (A) KG1a cells treated by CH11 (▪) or IgM (■). (B) Jurkat cells treated by CH11 (●) or IgM (○), U937 cells treated by CH11 (▴) or IgM (▵). Results are the mean ± standard deviation of 3 independent experiments.
Fas clustering in KG1a cells
We addressed whether Fas ligation could induce Fas receptor aggregation in KG1a cells. To resolve this question, we used an immunofluorescence technique coupled to confocal microscopy analysis as reported elsewhere.8 In these experiments, KG1a cells were or were not stimulated with Fas-L (0.5 μg/mL) for 4 hours, fixed with 4% paraformaldehyde, incubated with ZB4 murine anti-Fas monoclonal antibody or nonimmune mouse IgG1 (data not shown), and stained by FITC-labeled goat–anti-mouse IgG. For this study, ZB4 monoclonal antibody was preferred to CH11 because, unlike CH11, this antibody recognized a Fas epitope distinct from the Fas-L binding site. Jurkat (data not shown) and U937 cells were used as controls. Confocal laser microscopy showed that though untreated KG1a cells exhibited diffuse staining of Fas (Figure 2A), stimulation of cells with Fas-L resulted in Fas aggregation, enabling a dense, patchy staining that was primarily membrane localized (Figure 2C). Similar findings were found in the Fas-sensitive cell lines, as shown in Figure 2B and D, for U937 cells. Because Fas oligomerization appeared to be functional in Fas-activated KG1a cells, we hypothesized that the interruption of Fas signaling was situated immediately downstream of the Fas receptor and that in these cells, for example, some regulators interfered with DISC formation and caspase-8 activation.
Fas clusters in KG1a and U937 cell lines.
KG1a cells (A, C) and U937 (B, D) were treated (C, D) or not (A, B) with 0.5 μg/mL Fas-L for 4 hours. After washing, cells were incubated for 2 hours with ZB4 monoclonal antibody and then with FITC-conjugated goat–anti-mouse monoclonal antibody. Confocal analysis was performed as described in “Materials and methods.” Fas aggregation was shown as dense patchy staining (see arrows).
Fas clusters in KG1a and U937 cell lines.
KG1a cells (A, C) and U937 (B, D) were treated (C, D) or not (A, B) with 0.5 μg/mL Fas-L for 4 hours. After washing, cells were incubated for 2 hours with ZB4 monoclonal antibody and then with FITC-conjugated goat–anti-mouse monoclonal antibody. Confocal analysis was performed as described in “Materials and methods.” Fas aggregation was shown as dense patchy staining (see arrows).
Caspase-8 activation in KG1a cells
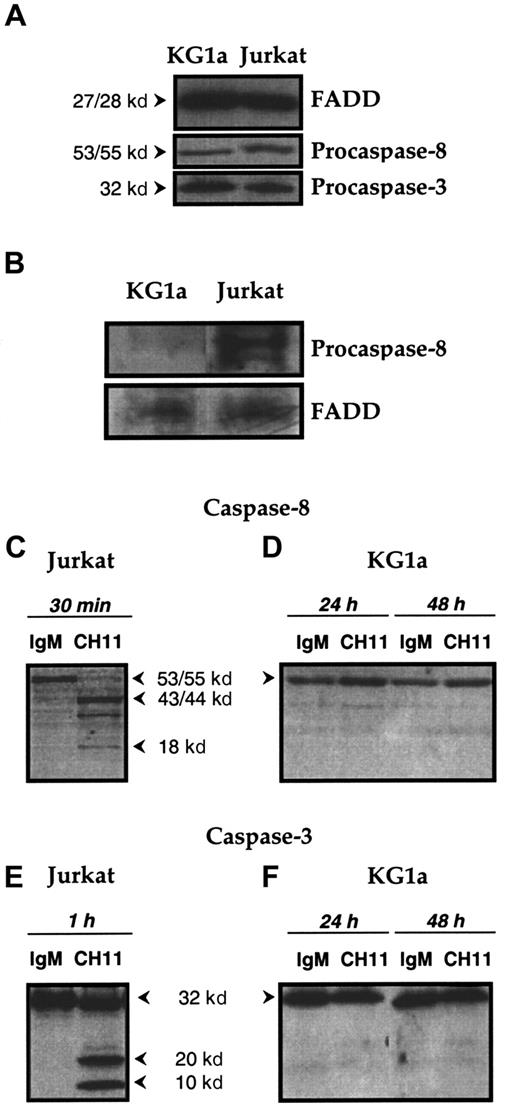
In preliminary experiments, whole-cell lysates were examined by immunoblot analysis and demonstrated that the adapter protein FADD (27-28 kd), procaspase-8 (53-55 kd), and procaspase-3 (32 kd) were present in KG1a cells at a level similar to that of Jurkat cells (Figure 3A). These results showed that the proteins that potentially constitute DISC—ie, Fas, FADD, and caspase-8—are present in KG1a cells.
Functional DISC formation in KG1a and Jurkat cells lines.
(A) Expression of FADD and of procaspase-8 and -3 in KG1a and Jurkat cell lines. FADD, procaspase-8, and procaspase-3 expression in KG1a and Jurkat cells was detected by Western blot as described in “Materials and methods.” (B) DISC formation. KG1a or Jurkat cells were treated, respectively, for 1 hour or 15 minutes by Fas-L–FLAG and anti-FLAG monoclonal antibody before immunoprecipitation. FADD and procaspase-8 were detected by Western blot, as described in “Materials and methods.” (C-F) Fas-induced caspase-8 and -3 activation in KG1a and Jurkat cell lines. Jurkat cells (C, E) were treated for 1 hour with 0.5 μg/mL CH11 or IgM isotypic control. KG1a cells (D, F) were treated by 2 μg/mL CH11 or IgM isotypic control for 24 or 48 hours. Cellular extracts were analyzed by Western blot for caspase-8 (C, D) or caspase-3 (E, F) activation, as described in “Materials and methods.”
Functional DISC formation in KG1a and Jurkat cells lines.
(A) Expression of FADD and of procaspase-8 and -3 in KG1a and Jurkat cell lines. FADD, procaspase-8, and procaspase-3 expression in KG1a and Jurkat cells was detected by Western blot as described in “Materials and methods.” (B) DISC formation. KG1a or Jurkat cells were treated, respectively, for 1 hour or 15 minutes by Fas-L–FLAG and anti-FLAG monoclonal antibody before immunoprecipitation. FADD and procaspase-8 were detected by Western blot, as described in “Materials and methods.” (C-F) Fas-induced caspase-8 and -3 activation in KG1a and Jurkat cell lines. Jurkat cells (C, E) were treated for 1 hour with 0.5 μg/mL CH11 or IgM isotypic control. KG1a cells (D, F) were treated by 2 μg/mL CH11 or IgM isotypic control for 24 or 48 hours. Cellular extracts were analyzed by Western blot for caspase-8 (C, D) or caspase-3 (E, F) activation, as described in “Materials and methods.”
We next determined whether Fas ligation could lead to the formation of a functional DISC. As shown on Figure 3B, DISC formation was well detected in Fas-L–treated Jurkat cells at 15 minutes. In contrast, incomplete DISC formation was detected in Fas-L–treated KG1a cells because only FADD was observed in the complex. This result suggested that in Fas-L–treated KG1a cells, the defect of DISC formation was caused by the absence of procaspase-8 recruitment. Finally, we examined the generation of procaspase-8 cleavage products in KG1a cells treated with CH11 monoclonal antibody. Therefore, KG1a cells were treated with CH11 (2 μg/mL) for 24 and 48 hours, after which whole-cell lysates were subjected to immunoblotting with a mixture of antibodies directed against procaspase-8 and its p20 and p10 cleavage products. As shown in Figure 3, whereas exposure to CH11 monoclonal antibody resulted in procaspase-8 proteolysis in Fas-sensitive Jurkat cells (Figure 3C), there was no generation of cleavage products in CH11-treated KG1a cells (Figure 3D). The lack of caspase-8 activation may explain why in KG1a cells, Fas ligation was unable to generate caspase-3 cleavage intermediates (Figure 3F). Together these results suggested that in KG1a cells, the lack of Fas-induced apoptosis was related to the presence of negative regulators that interfere with DISC formation and subsequent inhibition of caspase-8 activation. Among different parameters, we speculated that in these cells, PKC activity might play an important role in regulating the formation of functional DISC and Fas-mediated cell death. This was investigated by evaluating the capacity of chelerythrin or calphostin C, 2 known PKC inhibitors, to restore Fas-induced cytotoxicity.
Effect of PKC inhibitors on Fas-mediated cytotoxicity in KG1a cells
KG1a cells were pretreated with either chelerythrin (20 μM) or calphostin C (50 nM) for 1 hour, then were incubated in the presence of CH11 monoclonal antibody for 4 hours. Cell viability was measured by Trypan blue exclusion assay. Under these conditions, neither chelerythrin, calphostin C, nor CH11 monoclonal antibody used alone influenced KG1a cell viability (data not shown). As shown in Figure4A, CH11 monoclonal antibody induced a rapid loss of viability with 50% of residual viable cells at 4 hours in the chelerythrin-pretreated population. In addition, in chelerythrin-pretreated cells, CH11 treatment restored caspase 8 activity (Figure 4B). However, cotreatment with calphostin C and CH11 for 4 hours did not affect KG1a cell viability (Figure 4A) or caspase-8 activity (Figure 4B). Chelerythrin and calphostin C are known to target distinct sites of PKC. Indeed, the former interferes with the catalytic site, which is present in all PKC isoforms, whereas the latter acts at the regulatory site of classical and novel PKC isoforms.37,38 Therefore, the fact that chelerytherin, but not calphostin C, could overcome Fas resistance in KG1a cells suggested that atypical ζ or ι PKC isoforms, but not classical (α, β, γ), or novel (δ, ε, τ, ν, μ) isozymes, interfere with Fas signaling. Based on previous studies that have extensively documented the role of PKCζ as a potent negative regulator of apoptosis, including TNFα-induced apoptosis, in different cellular models,39 40 we have speculated that this PKC isoform might play an important role in Fas resistance of KG1a cells.
Effect of PKC inhibitors on Fas-mediated cytotoxicity and caspase-8 activation in KG1a cells.
KG1a cells (4 × 105/mL) were pre-incubated for 1 hour with chelerythrin (20 μM) or calphostin (50 nM). Then cells were treated with 2 μg/mL CH11 or IgM for 4 hours. (A) Fas-induced cell cytotoxicity was evaluated by Trypan blue exclusion assay. Results are the mean ± SD of 3 independent experiments. *P < .05. (B) Caspase-8 activity was evaluated as described in “Materials and methods.” Results are expressed as percentage increase in CH11 against IgM-treated cells and are the mean ± SD of 3 independent experiments. *P < .05
Effect of PKC inhibitors on Fas-mediated cytotoxicity and caspase-8 activation in KG1a cells.
KG1a cells (4 × 105/mL) were pre-incubated for 1 hour with chelerythrin (20 μM) or calphostin (50 nM). Then cells were treated with 2 μg/mL CH11 or IgM for 4 hours. (A) Fas-induced cell cytotoxicity was evaluated by Trypan blue exclusion assay. Results are the mean ± SD of 3 independent experiments. *P < .05. (B) Caspase-8 activity was evaluated as described in “Materials and methods.” Results are expressed as percentage increase in CH11 against IgM-treated cells and are the mean ± SD of 3 independent experiments. *P < .05
Effect of PKCζ inhibition on DISC formation and Fas-induced cytotoxicity in KG1a cells
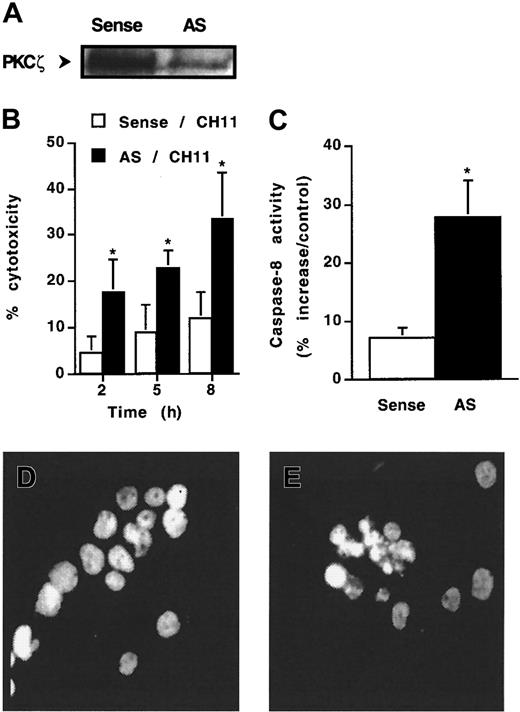
To ascertain the role of PKCζ in Fas resistance, KG1a cells were exposed to the action of antisense oligonucleotides directed against PKCζ and then were treated or not treated by CH11 monoclonal antibody. At first, 48-hour oligonucleotide pretreatment had no effect on cell viability as observed by Trypan blue exclusion assay (data not shown). However, antisense, but not sense, oligonucleotide dramatically decreased PKCζ expression (Figure 5A) and, in parallel, facilitated CH11-induced cytotoxicity. Indeed, though CH11 was unable to induce significant cytotoxicity in KG1a cells treated with sense oligonucleotide, this antibody induced a cytotoxic effect in PKCζ antisense oligonucleotide-treated cells (Figure 5B). Moreover, in these conditions, CH11 induced caspase-8 activity (Figure5C) and apoptosis (Figure 5E). These findings suggested that decreased PKCζ expression resulted in the restoration of functional DISC and activation of downstream Fas death pathway.
Effect of antisense oligonucleotides directed against PKCζ.
KG1a cells were pre-incubated with 10 μM antisense (AS) PKCζ or sense (control) oligonucleotides for 48 hours and then were treated or not treated with CH11 (2 μg/mL). (A) PKCζ expression analyzed by Western blot. (B) Fas-induced cell cytotoxicity was evaluated by Trypan blue exclusion assay. Results are the mean ± SD of 3 independent experiments. *P < .05. (C) Caspase-8 activity was evaluated at 4 hours after CH11 treatment, as described in “Materials and methods.” Results are expressed as percentage increase in CH11 against IgM-treated cells and are the mean ± SD of 3 independent experiments. *P < .05. (D, E) Morphology of CH11-treated cells was analyzed by fluorescence microscopy after DAPI staining. (D) Sense oligonucleotide-treated KG1a cells. (E) Antisense oligonucleotide-treated KG1a cells.
Effect of antisense oligonucleotides directed against PKCζ.
KG1a cells were pre-incubated with 10 μM antisense (AS) PKCζ or sense (control) oligonucleotides for 48 hours and then were treated or not treated with CH11 (2 μg/mL). (A) PKCζ expression analyzed by Western blot. (B) Fas-induced cell cytotoxicity was evaluated by Trypan blue exclusion assay. Results are the mean ± SD of 3 independent experiments. *P < .05. (C) Caspase-8 activity was evaluated at 4 hours after CH11 treatment, as described in “Materials and methods.” Results are expressed as percentage increase in CH11 against IgM-treated cells and are the mean ± SD of 3 independent experiments. *P < .05. (D, E) Morphology of CH11-treated cells was analyzed by fluorescence microscopy after DAPI staining. (D) Sense oligonucleotide-treated KG1a cells. (E) Antisense oligonucleotide-treated KG1a cells.
PKCζ regulation by Par-4 protein
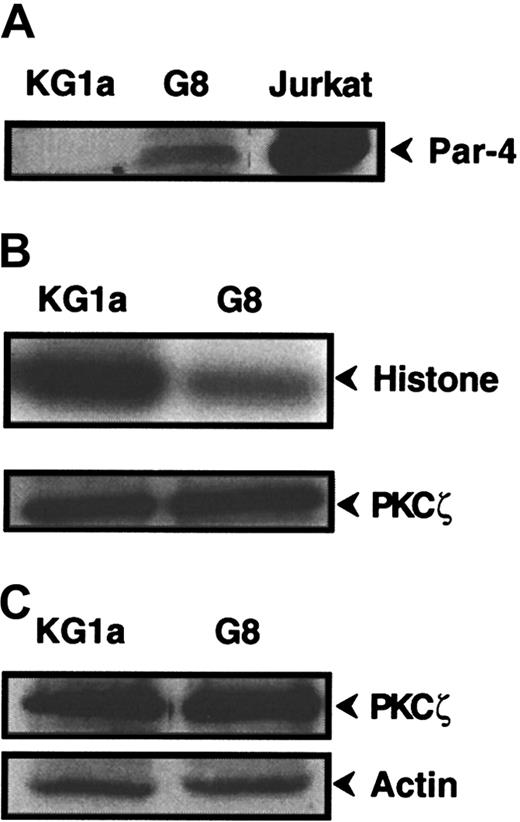
To further explore the role of PKCζ in Fas-resistance of KG1a cells, we investigated the influence of Par-4 (prostate apoptosis response-4), a known specific regulator of PKCζ.41 42Western blot analysis revealed that KG1a cells expressed no detectable Par-4 protein, whereas Jurkat cells displayed high Par-4 level (Figure6A). Hence, KG1a cells were stably transfected by a plasmid containing the full-length Par-4 cDNA sequence. Ten clones were obtained, for which only 2 (clones KG1a/G8 and KG1a/G9) had an immature phenotype (CD34+, CD38−) such as the parental KG1a cell line. Par-4 overexpression in the KG1a/G8 subclone (Figure 6A) resulted in a noticeable reduction of PKCζ activity compared to KG1a cells (Figure6B), whereas it did not influence PKCζ expression (Figure 6C). Moreover, in KG1a/G8 cells, CH11 induced the activation and the cleavage of caspase-8 and, thus, cytotoxicity and apoptosis (Figure7). These results suggested that in KG1a cells, low Par-4 expression level and subsequent PKCζ overactivity played an important role in the lack of DISC formation.
Par-4 and PKCζ expression and PKCζ activity in KG1a/G8 cells.
(A) Par-4 expression was analyzed by Western blot in KG1a, KG1a/G8, and Jurkat cells. (B) KG1a or KG1a/G8 cellular extracts were immunoprecipitated with anti-PKCζ monoclonal antibody. PKCζ activity was determined, as described in “Materials and methods,” using histone H1 as substrate. In parallel, an aliquot of each sample was analyzed by Western blot using anti-PKCζ monoclonal antibody to quantify immunoprecipitated proteins. (C) PKCζ expression was analyzed by Western blot in KG1a and KG1a/G8 cells.
Par-4 and PKCζ expression and PKCζ activity in KG1a/G8 cells.
(A) Par-4 expression was analyzed by Western blot in KG1a, KG1a/G8, and Jurkat cells. (B) KG1a or KG1a/G8 cellular extracts were immunoprecipitated with anti-PKCζ monoclonal antibody. PKCζ activity was determined, as described in “Materials and methods,” using histone H1 as substrate. In parallel, an aliquot of each sample was analyzed by Western blot using anti-PKCζ monoclonal antibody to quantify immunoprecipitated proteins. (C) PKCζ expression was analyzed by Western blot in KG1a and KG1a/G8 cells.
Fas sensitivity of KG1a/G8 cells.
(A) Caspase-8 activity was evaluated as described in “Materials and methods.” Results are expressed as percentage increase in CH11 against IgM-treated cells and are the mean ± SD of 3 independent experiments. *P < .05. (B) Caspase-8 cleavage in KG1a/G8 cells treated by CH11 or IgM (2 μg/mL for 4 hours). (C) After treatment by 2 μg/mL CH11 (■) or IgM (□), KG1a/G8 cell viability was evaluated by Trypan blue exclusion assay. Results are the mean ± SD of 3 independent experiments. *P < .05. (D, E) Morphology of CH11-treated cells was analyzed by fluorescence microscopy after DAPI staining. (D) KG1a cells. (E) KG1a/G8 cells.
Fas sensitivity of KG1a/G8 cells.
(A) Caspase-8 activity was evaluated as described in “Materials and methods.” Results are expressed as percentage increase in CH11 against IgM-treated cells and are the mean ± SD of 3 independent experiments. *P < .05. (B) Caspase-8 cleavage in KG1a/G8 cells treated by CH11 or IgM (2 μg/mL for 4 hours). (C) After treatment by 2 μg/mL CH11 (■) or IgM (□), KG1a/G8 cell viability was evaluated by Trypan blue exclusion assay. Results are the mean ± SD of 3 independent experiments. *P < .05. (D, E) Morphology of CH11-treated cells was analyzed by fluorescence microscopy after DAPI staining. (D) KG1a cells. (E) KG1a/G8 cells.
Interaction between PKCζ and DISC components
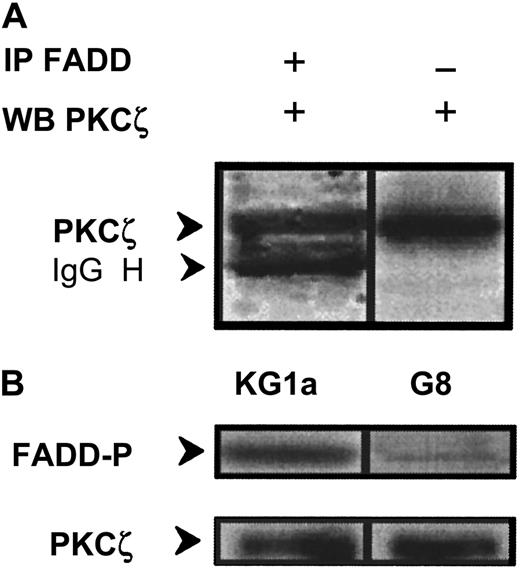
The fact that PKCζ inhibition restored Fas-induced caspase-8 activation in KG1a cells suggested that PKCζ might interact with DISC components. To test this hypothesis, whole-cell extracts were subjected to immunoprecipitation with anti-FADD antibody, and immunoprecipitates were blotted with anti-PKCζ monoclonal antibody. PKCζ was found to interact with FADD (Figure 8A); in parallel, PKCζ immunoextracts prepared from wild-type KG1a cells were able to phosphorylate FADD–agarose complexes (Figure 8B). Furthermore, this phosphorylation is dramatically reduced in Par-4 overexpressed KG1a–G8 cellular extracts (Figure 8B).
Interaction and phosphorylation of FADD by PKCζ.
(A) KG1a cellular extract was immunoprecipitated by anti-FADD monoclonal antibody, and immunocomplexes were analyzed by Western blot using anti-PKCζ monoclonal antibody. In parallel, cellular extract was analyzed by Western blot using anti-PKCζ monoclonal antibody. (B) FADD phosphorylation by PKCζ in KG1a and KG1a/G8 cells. After cell lysis, cellular extracts were immunoprecipitated by anti-PKCζ monoclonal antibody. Immunocomplexes were resuspended in buffer reaction with γ32P-ATP and FADD-agarose as substrate. FADD phosphorylation was analyzed as described in “Materials and methods.” In parallel, an aliquot of each sample was analyzed by Western blot using anti-PKCζ monoclonal antibody to quantify immunoprecipitated proteins.
Interaction and phosphorylation of FADD by PKCζ.
(A) KG1a cellular extract was immunoprecipitated by anti-FADD monoclonal antibody, and immunocomplexes were analyzed by Western blot using anti-PKCζ monoclonal antibody. In parallel, cellular extract was analyzed by Western blot using anti-PKCζ monoclonal antibody. (B) FADD phosphorylation by PKCζ in KG1a and KG1a/G8 cells. After cell lysis, cellular extracts were immunoprecipitated by anti-PKCζ monoclonal antibody. Immunocomplexes were resuspended in buffer reaction with γ32P-ATP and FADD-agarose as substrate. FADD phosphorylation was analyzed as described in “Materials and methods.” In parallel, an aliquot of each sample was analyzed by Western blot using anti-PKCζ monoclonal antibody to quantify immunoprecipitated proteins.
Discussion
Our study shows that in KG1a cells, Fas activation results in Fas aggregation, incomplete DISC formation leading to a defect in caspase-8 activation. However, pretreatment with chelerythrin, an inhibitor of all types of PKC isozymes, and, more specifically PKCζ depletion by antisense oligonucleotides or PKCζ inactivation by Par-4 overexpression, restored Fas-induced caspase-8 activity and cytotoxicity. These results suggest that in KG1a cells, PKCζ plays a critical role in altered DISC formation. To the best of our knowledge, the influence of the atypical PKCζ isozyme on Fas signaling has never been reported. However, the role of other PKC isozymes on Fas-induced apoptosis has already been investigated in lymphoid cells. In these studies, it has been shown that Go 6976, a proposed classical PKC isozyme inhibitor, facilitated Fas cytotoxicity in Jurkat cells whereas treatment with phorbol esters significantly reduced Fas-induced caspase-3, PARP cleavage, and apoptosis in T cells.31-33,43-46 Moreover, in the latter studies, it has been described that in the Jurkat T-cell model, phorbol ester-induced PKC stimulation resulted in decreased Fas aggregation33whereas in other T-cells, it has been shown that phorbol esters or diacylglycerol reduced Fas expression.44 Because of the specificity of these reagents, which selectively target classical or novel PKC isozymes, it can be assumed that in lymphoid cells, nonatypical PKC isoforms can also efficiently regulate Fas-induced apoptosis. Therefore, it appears that depending on the cellular models, PKC may interfere with Fas signaling through distinct PKC isozymes and mechanisms. However, we observed that, in Jurkat cells, PKCζ overexpression partially inhibited Fas-induced apoptosis (A.d.T., unpublished results, 2001), suggesting that PKCζ may also regulate Fas signaling in nonmyeloid leukemic cells.
The mechanism by which PKCζ inhibited DISC formation in KG1a cells was investigated. Fas, FADD, and caspase-8 expression levels were similar in KG1a/G8 cells, compared to KG1a cells (data not shown). This result suggests that PKCζ does not act by decreasing the expression of DISC protein components. The role of FLIP, a potent negative regulator of DISC formation,10 is also unlikely. Indeed, FLIP expression in KG1a cells was similar to that of Fas-sensitive U937 and Jurkat cells, and Par-4 overexpression had no influence on FLIP levels in KG1a cells (data not shown). These results suggest that FLIP plays a minor role in KG1a cell resistance and that PKCζ does not act on FLIP expression. Based on previous studies that described serine phosphorylation sites on FADD protein,47 48 we hypothesized that PKCζ regulates DISC formation by influencing FADD phosphorylation status. Indeed, our study shows that PKCζ interacts with FADD in vivo and that PKCζ may directly phosphorylate FADD in vitro. Moreover, we found a correlation between FADD phosphorylation status and caspase-8 activation. These results strongly suggest that PKCζ-mediated FADD phosphorylation contributes to caspase-8 inhibition and subsequent Fas resistance in KG1a cells.
Oncogenic Ras and growth factors including platelet-derived growth factor or nerve growth factor (TrkA/NGF) may enhance PKCζ activity.49-51 These signaling pathways are potentially stimulated in AML cells. Therefore, it is possible that these stimuli may reduce Fas-sensitivity of AML cells through a PKCζ-dependent mechanism. It has been documented that PKCζ is a target for phosphoinositide-3 kinase (PI3K) lipid products.52,53Interestingly, it has been reported recently that tyrosine kinase receptor-driven PI3K stimulation resulted in the abrogation of FADD–caspase-8 interaction and Fas-induced apoptosis.54Whether PKCζ plays a role in PI3K-induced Fas resistance should be investigated.
In this study we also showed that in KG1a cells, the lack of Par-4 expression plays an important role in Fas resistance. Par-4 interacts with the regulatory domain of PKCζ through its leucine zipper domain, and this interaction inhibits the kinase activity.41 It is generally believed that the negative regulation of PKCζ is the principle mechanism by which Par-4 exerts its pro-apoptotic function though it could also act by modulating Bcl-2 expression and transcription function of WT-1.55-57 Whereas Par-4 has emerged as a pivotal player in neuronal apoptosis,42 so far it has received little attention in leukemia. The fact that the monocytic U937 cells, but not KG1a cells, displayed substantial Par-4 expression level suggests that the Par-4 gene is regulated in AML cells. The mechanism by which Par-4 is regulated remains largely unknown. However, it has been reported that oncogenic Ras causes down-regulation of Par-4 in fibroblasts.58 Therefore, it should be investigated whether Ras, by regulating Par-4 expression, plays an important role in regulating apoptosis induced by Fas, and perhaps by other stress, in AML cells.
To conclude, our study shows that in myeloid leukemic cells, PKCζ and Par-4 are coupled regulators of Fas cell death signaling by interfering with DISC formation. Moreover, because PKCζ is expressed in normal CD34+ cells,59 60 it should be important to investigate whether this kinase also plays a role in Fas resistance of hematopoietic progenitors.
We thank Dr M. T. Diaz-Meco and Dr J. Moscat (Madrid, Spain) for the kind gift of Par-4 cDNA, and Dr C. Bezombes-Cagnac for helpful discussions.
Supported by the Association pour la Recherche contre le Cancer (grants 5526 and 5968). A.d.T. is the recipient of a grant from the Ministère de l'Education Nationale, de l'Enseignement Supérieur, et de la Recherche.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Anne Quillet-Mary, INSERM E9910, Institut Claudius Regaud, 20 rue du Pont St Pierre, 31052 Toulouse, France; e-mail:quillet_mary@icr.fnclcc.fr.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal