Abstract
This study compared the incidence of clinical extensive chronic graft-versus-host disease (GVHD), transplantation-related mortality, survival, and relapse-free survival among recipients randomly assigned to receive a 24-month or a 6-month course of cyclosporine prophylaxis after transplantation of allogeneic marrow from an HLA-identical sibling or alternative donor. Patients who did not have clinical manifestations of chronic GVHD on day 80 after transplantation were eligible for the study if they previously had acute GVHD or if a skin biopsy showed histologic evidence of chronic GVHD. Clinical extensive chronic GVHD developed in 35 of the 89 patients (39%) in the 24-month group and 37 of the 73 patients (51%) in the 6-month group. The hazard of developing chronic GVHD was not significantly different in the 2 groups (hazard ratio = 0.76; 95% confidence interval, 0.48-1.21; P = .25). In addition, there were no significant differences between the 2 groups in transplantation-related mortality, survival, or disease-free survival.
Introduction
Chronic graft-versus-host disease (GVHD) remains the principal cause of late transplantation-related morbidity and mortality after allogeneic marrow transplantation.1Histologic evidence of GVHD in the skin or lip mucosa and a history of acute GVHD have been used to predict development of chronic GVHD in patients without clinical manifestations of the disease by day 80 after transplantation.2 Administration of prednisone did not prevent development of clinical manifestations in patients who had only histologic evidence of GVHD in the skin or lip by day 80.3Likewise, administration of thymic factors or immunoglobulin did not prevent chronic GVHD.4 5
Standard GVHD prophylaxis with methotrexate (MTX) and cyclosporine (CSP) involves a tapering of CSP doses after day 50 and discontinuation of CSP administration by day 180.6 With this regimen, chronic GVHD tends to occur during the CSP tapering and the ensuing 6 months after discontinuation.7 The hypothesis that chronic GVHD might be prevented by extending administration of CSP beyond 6 months7 was supported by results from single-arm studies.8-10 Here, we compared chronic GVHD, transplantation-related mortality, survival, and disease-free survival in patients randomly assigned to receive a 6-month or 24-month course of CSP prophylaxis.
Study design
Patients with aplastic anemia or any other nonmalignant disease, myelodysplasia, chronic myeloid leukemia in chronic phase, or hematologic malignant diseases in remission were eligible for this study between day 80 and day 100 after they had undergone an allogeneic marrow transplantation if they previously had acute GVHD or if a skin biopsy showed histological evidence of subclinical GVHD. Patients were excluded if they had manifestations of clinical extensive chronic GVHD, recurrent malignant disease, serum creatinine levels above 177 μmol/L (2.0 mg/dL) or more than twice the normal values, hypertension uncontrolled by medications, or previous life-threatening toxicity due to CSP. Patients receiving at least 2 mg/kg of body weight of prednisone per day for treatment of acute GVHD were not eligible. Patients receiving lower doses of prednisone were eligible, and treatment was tapered to discontinuation within 6 to 8 weeks after enrollment.
All patients gave written informed consent as approved by our institutional review board before being randomly assigned to continue oral CSP prophylaxis for either 6 or 24 months after transplantation. Randomization was stratified according to patient age below 20 years or 20 years or higher, HLA-identical sibling donor or alternative donor, previous presence or absence of acute GVHD, current presence or absence of chronic GVHD on skin biopsy evaluation, and current treatment with or without prednisone.
All patients were given CSP and MTX for GVHD prophylaxis.6In patients who did not have acute GVHD, the dose of oral CSP was tapered by 5%/week from a baseline dose of 12.5 mg/kg per day, beginning on day 50 after transplantation. In the 6-month treatment arm, CSP prophylaxis ended on day 180. In the 24-month arm, the dose of CSP was maintained at 6.0 mg/kg per day from day 120 through day 540, reduced to 5.5 mg/kg per day from day 541 through 569, and then tapered by 5%/week through day 720.
A total of 169 patients were randomly assigned—90 to the 24-month group and 79 to the 6-month group. An audit disclosed that one patient assigned to the 24-month group was not eligible because of recurrent malignant disease. Informed consent could not be documented for 3 patients assigned to the 6-month group. Three other patients assigned to the 6-month group were not eligible because of clinical extensive chronic GVHD at the time of random assignment (one patient) or because they had received a peripheral blood stem cell transplantation instead of a marrow transplantation (2 patients). Demographic characteristics of the 162 remaining patients were similar in the 2 treatment arms (Table 1).
Patient characteristics
| Characteristic . | Duration of CSP prophylaxis . | |
|---|---|---|
| 6 months (n = 73) . | 24 months (n = 89) . | |
| Patient age | ||
| Less than 20 years | 15 (21) | 12 (13) |
| 20 years or older | 58 (79) | 77 (87) |
| Donor/recipient sex | ||
| Female/female | 14 (19) | 18 (20) |
| Female/male | 14 (19) | 19 (21) |
| Male/female | 22 (30) | 19 (21) |
| Male/male | 23 (32) | 33 (37) |
| Donor type | ||
| HLA-identical sibling | 29 (40) | 39 (44) |
| Other | 44 (60) | 50 (56) |
| History of grades II-III GVHD | ||
| No | 17 (23) | 22 (25) |
| Yes | 56 (77) | 67 (75) |
| Skin biopsy results* | ||
| Negative | 27 (37) | 34 (38) |
| Positive | 46 (63) | 55 (62) |
| Treatment with prednisone* | ||
| No | 8 (11) | 7 (8) |
| Yes | 65 (89) | 82 (92) |
| Characteristic . | Duration of CSP prophylaxis . | |
|---|---|---|
| 6 months (n = 73) . | 24 months (n = 89) . | |
| Patient age | ||
| Less than 20 years | 15 (21) | 12 (13) |
| 20 years or older | 58 (79) | 77 (87) |
| Donor/recipient sex | ||
| Female/female | 14 (19) | 18 (20) |
| Female/male | 14 (19) | 19 (21) |
| Male/female | 22 (30) | 19 (21) |
| Male/male | 23 (32) | 33 (37) |
| Donor type | ||
| HLA-identical sibling | 29 (40) | 39 (44) |
| Other | 44 (60) | 50 (56) |
| History of grades II-III GVHD | ||
| No | 17 (23) | 22 (25) |
| Yes | 56 (77) | 67 (75) |
| Skin biopsy results* | ||
| Negative | 27 (37) | 34 (38) |
| Positive | 46 (63) | 55 (62) |
| Treatment with prednisone* | ||
| No | 8 (11) | 7 (8) |
| Yes | 65 (89) | 82 (92) |
Values are numbers (percentages).
CSP indicates cyclosporine; GVHD, graft-versus-host disease.
At time of enrollment.
The diagnosis and grading of chronic GVHD were established according to clinical and pathological criteria.11 The assigned schedule of CSP administration was abandoned when clinical extensive chronic GVHD developed in a patient, and treatment was prescribed according to available protocols.
The primary end point of this study was the incidence of clinical extensive chronic GVHD, with follow-up to either the time of recurrent malignant disease or date of last contact. Secondary end points were transplantation-related mortality, overall survival, and survival without recurrent malignant disease. A sample size of 80 patients in each treatment arm was planned to provide 90% power for detecting a decrease in the incidence of clinical extensive chronic GVHD from 70% in the 6-month arm2 to 45% in the 24-month arm. Cumulative incidence rates were used to estimate the probabilities of chronic GVHD and transplantation-related mortality.12 The Kaplan-Meier method was used to estimate overall and disease-free survival.13 A log rank test was used to compare hazards, and 2-sided P values below .05 were considered to represent statistical significance. The minimum follow-up period among surviving patients without chronic GVHD at the time of analysis was 2.1 years.
Results and discussion
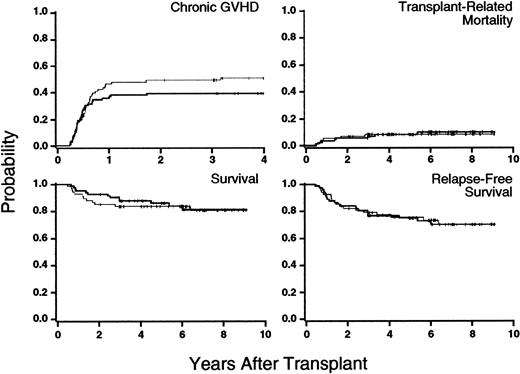
Results are summarized in Figure 1. Clinical extensive chronic GVHD developed in 35 of the 89 patients randomly assigned to the 24-month group, for a cumulative incidence of 39% (95% confidence interval [CI], 29%-49%) at 3 years after transplantation. In the 6-month group, clinical extensive chronic GVHD developed in 37 of the 73 patients, for a cumulative incidence of 51% (95% CI, 39%-62%) at 3 years after transplantation. The hazard ratio (HR) for clinical extensive chronic GVHD in the 24-month group compared with the 6-month group was 0.76 (95% CI, 0.48-1.21;P = .25). In 7 patients in the 24-month group and 6 in the 6-month group, clinical extensive chronic GVHD developed before any differences in administration of CSP began on day 120 after transplantation. The HR for clinical extensive chronic GVHD after day 120 among the 149 remaining patients was 0.72 (95% CI, 0.43-1.20;P = .21). Fifteen patients in the 24-month group discontinued treatment with CSP 2 to 18 months (median, 12 months) earlier than prescribed by the protocol. Chronic GVHD developed in only 2 of these patients.
Results showing that outcomes were similar in patients who received 24 months of cyclosporine prophylaxis (bolded line) and in those given 6 months of such prophylaxis (thin line).
Tic marks indicate the end of follow-up in surviving patients. No new cases of chronic GVHD were diagnosed more than 4 years after transplantation.
Results showing that outcomes were similar in patients who received 24 months of cyclosporine prophylaxis (bolded line) and in those given 6 months of such prophylaxis (thin line).
Tic marks indicate the end of follow-up in surviving patients. No new cases of chronic GVHD were diagnosed more than 4 years after transplantation.
Sixteen patients in the 24-month group and 13 in the 6-month group died. Infections and recurrent malignant disease were the most common causes of death. The cumulative incidence of transplantation-related mortality at 3 years was 7% in the 24-month group and 8% in the 6-month group; the hazard was not significantly different in the 2 groups (HR = 1.1; 95% CI, 0.4-3.2; P = .83). Overall survival at 3 years was 88% in the 24-month group and 84% in the 6-month group, and the risk of death was similar (HR = 1.0; 95% CI, 0.5-2.1; P = .99). The rate of survival without recurrent malignant disease at 3 years was 77% in the 24-month group and 79% in the 6-month group. The risk of failure to survive without recurrent disease was similar in the 2 groups (HR = 1.1; 95% CI, 0.6-2.0;P = .74).
The incidence of chronic GVHD in this study was lower than the 69% incidence among patients with positive results on a skin biopsy assessment or a previous history of acute GVHD described by Loughran et al.2 Among the patients studied by Wagner et al,14 however, we found a 40% incidence of chronic GVHD among those who met the entry criteria for the current study (unpublished data, February 2001). Despite careful analysis, reasons for the decreased incidence of chronic GVHD in this study compared with our earlier experience2 were not readily apparent.
In conclusion, this randomized clinical trial did not show any significant advantage for extended CSP prophylaxis in reducing the incidence of clinical chronic GVHD or improving survival after allogeneic marrow transplantation. These results do not support the suggestion derived from earlier single-arm studies that prolonged administration of CSP might decrease the risk of chronic GVHD.8-10 More effective approaches are needed to prevent chronic GVHD after allogeneic hematopoietic stem cell transplantation.
We thank the physicians and nurses who cared for the patients and Aurora Brandvold, RN, and Judy Campbell, RN, for assistance with data collection and data management.
Supported by Public Health Service grants CA15704, HL36444, CA18221, and CA18029 from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Paul Martin, Fred Hutchinson Cancer Research Center D2-100, PO Box 19024, Seattle, WA 98109-1024; e-mail:pmartin@fhcrc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal