To determine whether cytokine-induced signals generate unique responses in multipotential hemopoietic progenitor cells, the signaling domains of 3 different growth factor receptors (Mpl, granulocyte–colony-stimulating factor [G-CSF] receptor, and Flt-3) were inserted into mouse primary bone marrow cells. To circumvent the activation of endogenous receptors, each signaling domain was incorporated into an FK506 binding protein (FKBP) fusion to allow for its specific activation using synthetic FKBP ligands. Each signaling domain supported the growth of Ba/F3 cells; however, only Mpl supported the sustained growth of transduced marrow cells, with a dramatic expansion of multipotential progenitors and megakaryocytes. These findings demonstrate that the self-renewal and differentiation of multipotential progenitor cells can be influenced through distinct, receptor-initiated signaling pathways.
Introduction
Hemopoietic stem cells (HSCs) are defined by their ability to self-renew and to contribute to all lineages of mature blood cells.1 Additionally, HSCs appear capable of contributing to nonhemopoietic tissues including skeletal muscle,2liver,3 and brain.4,5 Multipotential hemopoietic progenitor cells (MHPCs), positioned hierarchically downstream of HSCs, have a more limited capacity for self-renewal and a more restricted potential for differentiation. Among the earliest identifiable MHPCs are the common lymphoid6 and myeloid7 progenitors. HSCs and MHPCs stand poised (or primed) either to self-renew or to commit themselves to a pathway of differentiation.8 Multiplex reverse transcription–polymerase chain reaction (RT-PCR) analysis of single isolated CD34+, lineage− murine bone marrow cells demonstrates that erythroid-, myeloid-, and megakaryocyte-affiliated genes are all transcribed before exclusive commitment to a single lineage.9 Commitment is accompanied by augmented expression of genes affiliated with the chosen lineage and quenching of genes belonging to nonchosen lineages.
Capitalizing on the considerable therapeutic potential of HSCs and MHPCs requires the ability to manipulate their self-renewal and differentiation. Transcription factors stand at the center of these processes.10-12 For example, myb-ets–transformed multipotent chicken progenitor cells can be induced to differentiate into either megakaryocytes or eosinophils by modulating GATA-1 concentrations,13 whereas differentiation to myeloid cells can be induced by overexpressing PU.1.14 Self-renewal can also be controlled, as evidenced by the ability of GATA-2 to inhibit the self-renewal of multipotent FDCP-mix cells and primary mouse bone marrow clonogenic progenitor cells.15 16
In contrast to the well-characterized ability of transforming growth factor-β family members to specify cell fate during embryogenesis,17,18 controversy exists as to whether the fate of MHPCs or HSCs can be modulated by exogenous growth factors. A strict stochastic view holds that self-renewal or lineage specification ensues on the attainment of a critical threshold of one or more transcription factor complexes, assembled with predetermined probabilities that are impervious to influence by growth factors.19,20 In this model, growth factor receptors function merely as ligand-responsive purveyors of cell survival signals. A competing instructive view postulates that the probability of attaining the necessary threshold of transcription factor complexes to permit self-renewal or lineage choice is subject, at least partially, to the directive influence of growth factors.21In this model, signaling by different growth factor receptors is expected to engender different responses in HSCs and MHPCs.
That HSC–MHPC self-renewal and differentiation may be subject to external modulation is suggested by the expression of receptors for a wide range of growth factors, including interleukin-3 (IL-3), IL-1α, granulocyte–colony-stimulating factor (G-CSF), IL-6, stem cell factor (SCF),22 thrombopoietin,23 and flt-3 ligand.24 An effect of growth factors on self-renewal is suggested by the ability of IL-3 to induce self-renewal in multipotent FDCP-mix cells25 and by the ability of SCF to induce a 3-fold expansion of long-term repopulating cells on in vivo administration in mice.26 However, an alternative interpretation of these observations is that IL-3 and SCF merely allow for the survival of cells that have already taken internally programmed self-renewal decisions. Similar arguments pertain to lineage specification, where a bcl-2–mediated survival signal suffices to fulfill many of the functions attributed to growth factors.27-30 Further bolstering this view are demonstrations of redundancy among ectopically expressed growth factor receptors in their abilities to support terminal differentiation of committed hemopoietic progenitor cells.31-34
We have previously shown that signaling by the thrombopoietin receptor Mpl can support the expansion of retrovirally transduced marrow cells for longer than a month of culture, generating megakaryocytes and cells with the ability to form colonies in semisolid media.35 Contrasting simple receptor overexpression experiments, which fail to preclude ligand-activated signaling by endogenous receptors,36,37 we incorporated the Mpl signaling domain into an FKBP fusion protein, allowing for its specific activation using FK1012, a chemical inducer of dimerization (CID).38,39 Here we test whether Mpl specifically induces these responses or whether other receptors known to be expressed in HSCs induce similar responses. Like Mpl, the G-CSF receptor and Flt-3 are both reported to be expressed in HSCs.22-24 Here we show that Mpl signaling can induce MHPC self-renewal and that this ability is not provided for by signals emanating from the G-CSF receptor or Flt-3. Furthermore, we show that though Mpl signaling allows granulocytes to be generated from committed myeloid progenitors, signals other than those provided by Mpl are required for granulocytes to develop from MHPCs.
Materials and methods
Retroviral constructs
The F1Mpl construct has been previously described.35 The F1Flt-3 construct was cloned by inserting a PCR-generated, SalI-linked fragment encoding the intracellular domain of murine Flt-3 into a SalI-digested F1 construct.39 The F36V construct was generated by site-directed mutagenesis of F1 to introduce a phenylalanine for valine substitution at position 36 of the FKBP12 motif. F36VMpl was constructed by inserting a SalI-linked PCR product encoding the intracellular domain of murine Mpl into a SalI- digested F36V construct, and F36VGCSFR was constructed by insertion of aSalI-linked fragment encoding the intracellular portion of the murine GCSF receptor. All constructs were inserted into MSCV-based retroviral vectors,40 which also contained aneo gene. In the case of murine Mpl, a second vector was constructed which contained, rather than neo, a gene encoding green fluorescence protein (GFP).41
Retroviral producer lines
Retroviral producer lines were generated by transfection of the amphotropic packaging line PA317 with vector plasmid, followed by supernatant collection 2 days later that was subsequently used for transduction of the ecotropic packaging line GpE+86. Producer clones were isolated in the presence of G418 and screened for virus titer based on end-point titration using NIH 3T3 cells. Producer clones were isolated for each vector and yielded titers of at least 1 × 105 colony-forming U/mL. Genetic stability was confirmed by Southern blot analysis of clones and pools of producer cells through the use of the restriction enzymes KpnI orEcoRV, which cut once in each long terminal repeat, and a probe directed against the gene encoding eitherneo or GFP.
Cell proliferation assays
Ba/F3 cells were washed twice with phosphate-buffered saline and cultured overnight in IL-3–deficient medium. Then 1 × 104 cells per well were plated in 96-well plates and medium containing either IL-3 or CID to a final volume of 100 μL. Plates were incubated at 37°C in 5% CO2 for 40 hours, and 25 μL of a 5 mg/mL solution of MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) was added. Cells were incubated for an additional 4 hours at 37°C, and 100 μL lysis buffer (20% sodium dodecyl sulfate–40% dimethylformamide–2% glacial acetic acid, pH 4.7) was added. Plates were incubated at 37°C for 2 hours before assay. The OD570–630 value was determined using an enzyme-linked immunosorbent assay plate reader.
Retroviral transduction of primary murine bone marrow cells
Female B6D2F1 mice were injected intraperitoneally with 150 mg/kg 5-fluorouracil. Forty-eight hours later, bone marrow cells were harvested and cultured for 48 hours in Dulbecco minimum essential medium containing 16% fetal calf serum (FCS) 5% IL-3–conditioned medium, 100 ng/mL recombinant human IL-6, and 50 ng/mL recombinant murine SCF in a 37°C, 5% CO2 incubator. After 48 hours of prestimulation, cells were transferred onto irradiated (1500 cGy) producer cells and cocultivated using identical growth conditions except for the addition of polybrene (8 μg/mL). Marrow cells were harvested after 48 hours of cocultivation. Transduction efficiency was assessed using G418 at an active concentration determined to be sufficient to kill all nontransduced cells (800 μg/mL) for colonies containing the neo- vector or GFP expression for colonies containing the GFP vector. Cells were plated at a concentration of 1 × 104 cells/mL in cultures containing 30% FCS, 1% bovine serum albumin, 5 × 10−4 M 2-mercaptoethanol, 10% WEHI-3 cell-conditioned medium, and 1% methylcellulose. Cultures were plated in triplicate and incubated in a highly humidified 37°C, 5% CO2 incubator. Colonies were scored on day 8.
Semisolid progenitor assay
Transduced cells were plated at a concentration of 3 × 104 cells/mL in cultures containing 30% FCS, 1% bovine serum albumin, 5 × 10−4 M 2-mercaptoethanol, and 1% methylcellulose with or without 100 nM AP20187. Cultures were plated in duplicate and incubated in a highly humidified 37°C, 5% CO2 incubator. Colonies were scored on day 8 using an inverted microscope and standard morphologic criteria.
Suspension cultures
After retroviral transduction, marrow cells were cultured in Iscoves modified Dulbecco medium containing 10% FCS, 50 U/mL penicillin, and 50 μg/mL streptomycin either in the presence or the absence of FK1012 (100 nM) or AP20187 (100 nM) in a 37°C 5% CO2 incubator. Cells were counted on the days indicated. Cell morphology was analyzed by light microscopy after Wright-Giemsa staining.
Clonal assays
Marrow cells transduced using the F36Vmpl vector were plated into 96-well plates—at densities of 10, 25, 50, and 100 cells per well—in Iscoves modified Dulbecco medium containing 10% FCS, 50 U/mL penicillin, and 50 μg/mL streptomycin either in the presence or the absence of AP20187 (100 nM) in a 37°C, 5% CO2incubator. GFP-expressing clones were identified using an inverted fluorescence microscope. A fraction of wells demonstrating clonal growth was humanely killed for Turks staining, and cell morphology was evaluated using a 40× inverted light microscope.
Differentiation assays
Cells in suspension cultures were washed with phosphate-buffered saline 3 times and cultured in Iscoves modified Dulbecco medium containing 10% FCS, 50 U/mL penicillin, 50 μg/mL streptomycin, and 50 ng/mL SCF plus 3 U/mL erythropoietin for erythroid differentiation or IL-3 (0.1% conditioned media) plus 100 ng/mL GM-CSF for myeloid differentiation. Cells were analyzed by fluorescence-activated cell sorter for expression of the monocytic marker CD11b, the granulocytic marker Gr-1, and the erythroid marker Ter119.
Megakaryocyte colony-forming unit assay
Megakaryocyte colony-forming unit (CFU-MK) assays were performed using a plasma clot assay. CFU-MK colonies were identified by staining cells with a biotin-labeled anti-CD41 antibody and then by streptavidin-labeled alkaline phosphatase.
Results
Mpl, Flt-3, and G-CSF receptor signaling domains stimulate growth in a factor-dependent cell line
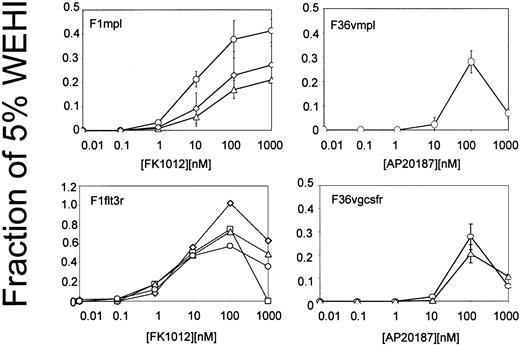
Signaling domains from Mpl, Flt-3, and the G-CSF receptor were incorporated into fusion proteins containing CID-binding sites (Figure1). CID-binding sites consisted of FKBP12, which binds FK1012,38 or of a FKBP12 derivative harboring a single amino acid substitution (F36V) that accommodates binding to a new class of CIDs, including AP20187.42Membrane targeting was achieved using a 14-amino acid myristylation peptide from c-Src.43 Each construct was incorporated into an MSCV-based retroviral vector and tested for the ability to induce CID-dependent growth in the IL-3–dependent cell line Ba/F3.44 Cell proliferation assays demonstrated that each construct was capable of inducing CID-dependent growth (Figure2). The reduced cell growth observed at higher CID concentrations is consistent with excessive occupancy of the CID-binding sites, thereby preventing dimerization. CID concentrations that were optimal for growth of Ba/F3 cells were carried forward for studies using transduced primary murine bone marrow cells.
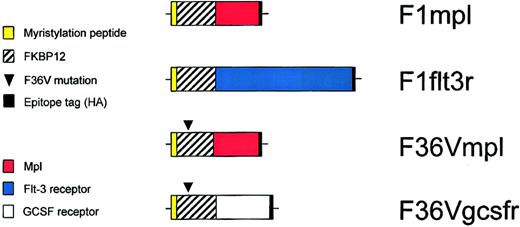
Schematic diagram of constructs.
F1mpl and F1flt3r constructs encode fusion proteins containing 14 amino acids from the amino terminus of c-Src (to allow for myristylation and membrane association), FKBP12 (to allow for binding to the CID FK1012), the intracellular portion of either murine mpl or murine Flt-3, respectively, and a hemagglutinin (HA) epitope tag (to permit detection using the monoclonal antibody HA.11). The construct F36Vmpl is identical to F1mpl except for a phenylalanine to valine substitution at the 36th amino acid of the FKBP12 domain, which allows for binding to the CID AP20187. The F36Vgcsfr construct contains the intracellular portion of the murine GCSF receptor but is otherwise identical to F36Vmpl. The membrane distal F36V domain is “codon wobbled” to reduce repetitive sequences, preventing recombination.
Schematic diagram of constructs.
F1mpl and F1flt3r constructs encode fusion proteins containing 14 amino acids from the amino terminus of c-Src (to allow for myristylation and membrane association), FKBP12 (to allow for binding to the CID FK1012), the intracellular portion of either murine mpl or murine Flt-3, respectively, and a hemagglutinin (HA) epitope tag (to permit detection using the monoclonal antibody HA.11). The construct F36Vmpl is identical to F1mpl except for a phenylalanine to valine substitution at the 36th amino acid of the FKBP12 domain, which allows for binding to the CID AP20187. The F36Vgcsfr construct contains the intracellular portion of the murine GCSF receptor but is otherwise identical to F36Vmpl. The membrane distal F36V domain is “codon wobbled” to reduce repetitive sequences, preventing recombination.
Cell proliferation (MTT) assays of clones of Ba/F3 cells expressing the constructs shown in Figure 1.
Each line depicts results from an individual clone and represents the average of 3 independent experiments. Error bars indicate standard deviations.
Cell proliferation (MTT) assays of clones of Ba/F3 cells expressing the constructs shown in Figure 1.
Each line depicts results from an individual clone and represents the average of 3 independent experiments. Error bars indicate standard deviations.
Only Mpl stimulates growth in primary murine bone marrow cells
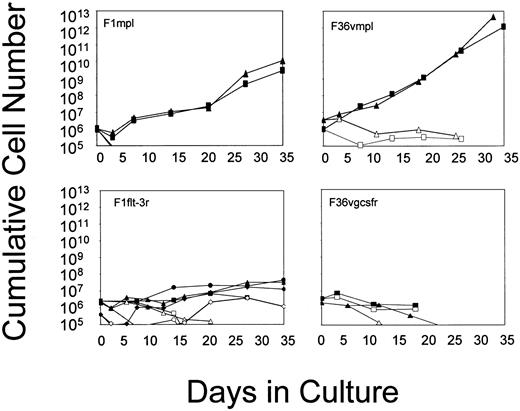
Primary murine bone marrow cells were transduced using the retroviral vectors that had been demonstrated to be functional in Ba/F3 cells. After transduction, marrow cells were cultured in suspension, in the absence of added cytokines, and in the presence or absence of the appropriate CID (Figure 3). Cell growth failed to occur in the absence of CID, whereas cell numbers were consistently higher in the presence of CID. G-CSF receptor signaling through the addition of AP20187 resulted in a delay in cell loss over time, likely reflecting some effect on cell survival. Signaling through Flt-3 produced slight increases in cell numbers over time, indicating a modest ability of this receptor to induce cell growth. In contrast, Mpl consistently induced a sustained growth of transduced marrow cells. Differences between Mpl and the other receptors were not related to the relative efficiency of gene transfer into progenitors (Figure 3, legend). The Mpl effect was highly consistent, allowing for the sustained growth of transduced marrow cells for periods ranging from 98 days to nearly 1 year (Table 1). Cell growth consistently remained strictly CID-dependent (data not shown). These findings indicate that Mpl is able to induce expansion in a subset of primary marrow cells that have an extensive proliferative capacity, whereas the G-CSF receptor and Flt-3 lack this ability.
Growth curves of murine bone marrow cells transduced with the vectors shown in Figure 1 and cultured in suspension in the absence of added cytokines and in the presence (closed symbols) or absence (open symbols) of CID.
Each line represents a single experiment. For F1mpl and F1Flt-3, cells were cultured in FK1012 (100 nM); for F36Vmpl and F36Vgcsfr, cells were cultured in AP20187 (100 nM). Transduction efficiencies as assessed by G418-resistant progenitors are as follows: F1mpl, 100%; F36Vmpl, 36% to 78%; F1Flt-3, 23% to 56%; F36Vgcsfr, 35% to 52%.
Growth curves of murine bone marrow cells transduced with the vectors shown in Figure 1 and cultured in suspension in the absence of added cytokines and in the presence (closed symbols) or absence (open symbols) of CID.
Each line represents a single experiment. For F1mpl and F1Flt-3, cells were cultured in FK1012 (100 nM); for F36Vmpl and F36Vgcsfr, cells were cultured in AP20187 (100 nM). Transduction efficiencies as assessed by G418-resistant progenitors are as follows: F1mpl, 100%; F36Vmpl, 36% to 78%; F1Flt-3, 23% to 56%; F36Vgcsfr, 35% to 52%.
Summary of experiments performed using the F1Mpl and F36VMpl vectors
| Experiment . | Duration of culture (wk) . | CFU-C . | CFU-S . |
|---|---|---|---|
| F1mpl | |||
| 1 | 38 (s) | +(220) | +(NQ) |
| 2 | 14 (d) | +(206) | +(NQ) |
| 3 | 19 (d) | +(76) | — |
| 4 | 37 (s) | +(NQ) | +(NQ) |
| 5 | 21 (d) | +(5) | +(NQ) |
| 6 | 16 (s) | +(17) | ND |
| 7 | 40 (d) | +(35) | ND |
| F36Vmpl | |||
| 1 | 9 (s) | — | — |
| 2 | 14 (s) | +(1) | ND |
| 3 | 16 (s) | ND | ND |
| 4 | 49 (s) | +(157) | ND |
| 5 | 32 (s) | +(87) | +(15.3) |
| Experiment . | Duration of culture (wk) . | CFU-C . | CFU-S . |
|---|---|---|---|
| F1mpl | |||
| 1 | 38 (s) | +(220) | +(NQ) |
| 2 | 14 (d) | +(206) | +(NQ) |
| 3 | 19 (d) | +(76) | — |
| 4 | 37 (s) | +(NQ) | +(NQ) |
| 5 | 21 (d) | +(5) | +(NQ) |
| 6 | 16 (s) | +(17) | ND |
| 7 | 40 (d) | +(35) | ND |
| F36Vmpl | |||
| 1 | 9 (s) | — | — |
| 2 | 14 (s) | +(1) | ND |
| 3 | 16 (s) | ND | ND |
| 4 | 49 (s) | +(157) | ND |
| 5 | 32 (s) | +(87) | +(15.3) |
(d) indicates cells died at the indicated time-point of culture; (s), cultures were stopped at the time-point indicated. (d) cultures died at the time point indicated. Plus signs indicate that cells were capable of generating colonies in semisolid media in the presence of IL-3 (CFU-C) and in the spleens of irradiated mice (CFU-S). Numbers in parentheses indicate clonogenic cells per 104 (for CFU-C) or 107 (for CFU-S) cells plated. Minus signs indicate cells were unable to generate either CFU-C or CFU-S. NQ, not quantified; ND, not done.
Receptor-specific differences in cell morphology
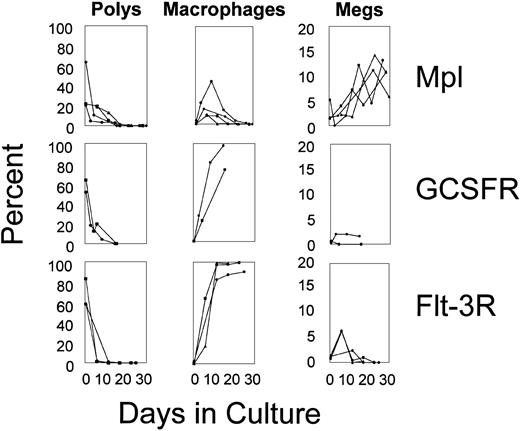
The types of cells emerging in response to CID varied in accordance with receptor type and time in culture (Figure4). In all experiments, granulocytes were abundant at early time points but fell to less than 1% by 21 days of culture. For Flt-3 and the G-CSF receptor, the decline in granulocytes was mirrored by a time-dependent rise in the percentage of macrophages. In contrast, macrophages increased only transiently in response to Mpl signaling. Megakaryocytes, present at low levels immediately after transduction, remained at low levels in response to Flt-3 and G-CSF receptor signaling, whereas megakaryocytes rose in response to signaling by Mpl. In particular, Mpl signaling resulted in a dramatic rise in megakaryocytes as assessed not only morphologically but also by staining using an antibody directed against the megakaryocyte-specific antigen, CD41, which was consistently positive in more than 30% of cells (data not shown). These findings demonstrate that lineage output can be modulated through distinct, receptor-initiated signaling pathways.
Differentials of transduced marrow cells.
Cytospins were performed at various time-points of culture, and differentials were assessed by Wright Giemsa staining.
Differentials of transduced marrow cells.
Cytospins were performed at various time-points of culture, and differentials were assessed by Wright Giemsa staining.
Mpl induces MHPC self-renewal
Marrow cells transduced with the F1mpl or the F36Vmpl vectors were maintained in continuous culture from 3 to 11 months, with cell doubling times ranging from 36 to 72 hours (Table 1). In contrast to the temporal variation in cell types generated during the first 3 weeks, the phenotypes of cells generally stabilized beyond 3 weeks of culture. CD41 were consistently expressed in a significant fraction of cells (30%-80%). Markers of primitive cells (CD34, Sca-1), erythroid cells (Ter119), and myeloid-monocytic cells (Gr-1 and CD11b) were consistently expressed at low levels (less than 5%), whereas c-Kit expression was variable among different experiments (expressed in 5%-90% of cells).
In 11 of 13 experiments, pools of transduced marrow cells expanded in response to Mpl signaling retained the potential for multilineage differentiation (Table 1 and data not shown). Multipotency was retained in subclones derived by single-cell plating from pools of Mpl-transduced marrow cells. Erythroid differentiation was observed in response to SCF plus erythropoietin, and myeloid differentiation occurred in response to IL-3 plus GM-CSF (Figure5). Mpl-expanded cells also retained the capacity to generate colonies in the spleens of irradiated mice (day 12 CFU-S) (Table 1 and data not shown). These functional attributes persisted even after cells had been expanded after more than 100 days of culture and by a factor of more than 1019-fold. In contrast, Mpl-expanded cells were incapable of long-term repopulation in lethally irradiated mice and showed no potential for B- or T-lymphoid differentiation (data not shown). These findings are consistent with an ability of Mpl to induce the self-renewal of MHPC, possibly the functional equivalents of the recently described c-kit+ FcRlo CD34+Sca1− common myeloid progenitors.7 The display of a CD34+, Sca-1− phenotype in approximately 1% of Mpl-expanded cells further supports this possibility (data not shown).
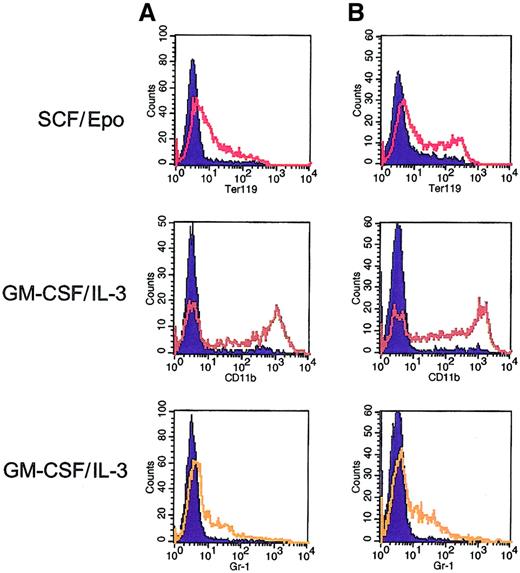
Multilineage differentiation potential of Mpl-expanded bone marrow cells.
Flow cytometric analysis was performed on 2 subclones (A and B) derived from single-cell plating from a pool of F1Mpl-transduced bone marrow cells. Before derivation of the subclones, the pool had been maintained in culture in the presence of FK1012 for more than 3 months. Flow cytometry was performed using antibodies directed against erythroid (Ter119), monocytic (CD11b), and granulocytic (Gr-1) cells in the presence of FK1012 alone (controls) or after 6 days of culture using conditions designed to elicit erythroid (SCF/Epo) or granulocyte–macrophage differentiation (IL-3–GM-CSF). Cells cultured in the presence of FK1012 alone (blue-shaded curves) provided controls for antibody staining. Culture in SCF/Epo produced 35% to 40% Ter119-positive cells, whereas culture in IL-3–GM-CSF resulted in 60% of cells expressing CD11b and 25% to 30% of cells expressing Gr-1.
Multilineage differentiation potential of Mpl-expanded bone marrow cells.
Flow cytometric analysis was performed on 2 subclones (A and B) derived from single-cell plating from a pool of F1Mpl-transduced bone marrow cells. Before derivation of the subclones, the pool had been maintained in culture in the presence of FK1012 for more than 3 months. Flow cytometry was performed using antibodies directed against erythroid (Ter119), monocytic (CD11b), and granulocytic (Gr-1) cells in the presence of FK1012 alone (controls) or after 6 days of culture using conditions designed to elicit erythroid (SCF/Epo) or granulocyte–macrophage differentiation (IL-3–GM-CSF). Cells cultured in the presence of FK1012 alone (blue-shaded curves) provided controls for antibody staining. Culture in SCF/Epo produced 35% to 40% Ter119-positive cells, whereas culture in IL-3–GM-CSF resulted in 60% of cells expressing CD11b and 25% to 30% of cells expressing Gr-1.
Mpl signaling is permissive for differentiation of committed progenitors
To investigate the basis for the temporal variation in cell types emerging in response to Mpl signaling, we performed limiting-dilution assays. Marrow cells transduced using the F36Vmpl vector were plated into 96-well plates at densities of 10, 25, 50, and 100 cells per well, without added cytokines and in the presence or absence of AP20187. To facilitate the detection of clones, the F36Vmpl vector incorporated a GFP reporter that allowed viable cells to be readily identified using an inverted fluorescence microscope. After 7 days of culture, clonogenic cells were detected at frequencies from 1 in 59 to 1 in 89 cells plated. Each clone contained between 8 and 1500 cells, with most clones containing between 50 and 500 cells. Approximately 75% of these clones contained either granulocytes alone or a combination of granulocytes and macrophages (Table 2). The remaining clones contained a mixture of granulocytes and megakaryocytes or were unidentified. The unidentified group might have included nonhemoglobinized erythroid cells that were difficult to classify definitively based on morphologic criteria alone. As seen in our bulk cultures, cell growth failed to occur in limiting-dilution cultures performed in the absence of CID (data not shown).
Frequency and type of clonogenic cells arising in response to Mpl signaling
| Experiment . | Day . | Frequency of clonogenic cells . | G . | GM . | Macro . | Mix . | Meg . | Other . |
|---|---|---|---|---|---|---|---|---|
| 1 | 7 | 59 | 58 | 15 | 4 | 11.5 | 0 | 11.5 |
| 23 | 6 250 | 0 | 0 | 0 | 0 | 100 | 0 | |
| 35 | 7 083 | 0 | 0 | 0 | 0 | 100 | 0 | |
| 77 | 42 500 | 0 | 0 | 0 | 0 | 100 | 0 | |
| 102 | 42 500 | 0 | 0 | 0 | 0 | 100 | 0 | |
| 2 | 7 | 89 | 53 | 20 | 0 | 13 | 7 | 7 |
| 32 | 16 958 | 0 | 0 | 0 | 0 | 100 | 0 | |
| 87 | 50 875 | 0 | 0 | 0 | 0 | 100 | 0 |
| Experiment . | Day . | Frequency of clonogenic cells . | G . | GM . | Macro . | Mix . | Meg . | Other . |
|---|---|---|---|---|---|---|---|---|
| 1 | 7 | 59 | 58 | 15 | 4 | 11.5 | 0 | 11.5 |
| 23 | 6 250 | 0 | 0 | 0 | 0 | 100 | 0 | |
| 35 | 7 083 | 0 | 0 | 0 | 0 | 100 | 0 | |
| 77 | 42 500 | 0 | 0 | 0 | 0 | 100 | 0 | |
| 102 | 42 500 | 0 | 0 | 0 | 0 | 100 | 0 | |
| 2 | 7 | 89 | 53 | 20 | 0 | 13 | 7 | 7 |
| 32 | 16 958 | 0 | 0 | 0 | 0 | 100 | 0 | |
| 87 | 50 875 | 0 | 0 | 0 | 0 | 100 | 0 |
Marrow cells were transduced using the F36VMpl vector, then cultured in suspension at densities of 10, 25, 50, and 100 cells per well in 96-well plates without added cytokines and in the presence of AP20187 (100 nM). The frequency and type of clones emerging in response to AP20187-triggered Mpl signaling were determined on the days indicated. Percentages of clones containing granulocytes (G), granulocyte–macrophages (GM), macrophages (Macro), mixed cells (mix), megakaryocytes (Meg), and unclassified (other) are shown. Clonogenic cell frequency indicates the number of cells plated per clonogenic cell.
To further demonstrate the ability of Mpl to support the differentiation of committed progenitors, marrow cells transduced using the F36Vmpl vector were cultured in semisolid media containing 100 nM AP20187 in the absence of added cytokines. Colonies obtained after 8 days included not only colony-forming unit–granulocyte macrophage (CFU-GM) but also erythroid burst-forming unit (BFU-E) and CFU-MK (Table 3). These findings confirm that Mpl can support the terminal differentiation of committed myeloid, erythroid, and megakaryocytic progenitor cells.
Mpl signaling allows for the differentiation of committed myeloid, erythroid, and megakaryocytic progenitor cells
| Experiment . | CFU-GM . | BFU-E . | CFU-MK . | CFU-Mix . |
|---|---|---|---|---|
| 1 | 176 | 7 | 11 | 6 |
| 2 | 188 | 11 | 6 | 4 |
| Experiment . | CFU-GM . | BFU-E . | CFU-MK . | CFU-Mix . |
|---|---|---|---|---|
| 1 | 176 | 7 | 11 | 6 |
| 2 | 188 | 11 | 6 | 4 |
Marrow cells were transduced with the F36VMpl vector, then plated at a concentration of 3 × 104 cells/mL in methylcellulose containing AP20187 (100 nM) without added cytokines. Results show the number of colonies per 6 × 104transduced marrow cells plated.
Mpl supports the sustained proliferation of committed megakaryocyte progenitor cells
In the limiting-dilution assays, fewer than 1 in 6000 cells were capable of sustaining growth beyond 3 weeks of culture (Table 2). These rare clones contained both morphologically nondescript mononuclear cells and megakaryocytes, whereas granulocytes were consistently absent. These cells were capable of forming megakaryocytic colonies in semisolid media in the presence of thrombopoietin but were incapable of generating myeloid cells in response to IL-3 and GM-CSF or erythroid cells in response to SCF and erythropoietin. They failed to generate colonies when cultured in semisolid media in the presence of IL-3 and were incapable of forming spleen colonies when injected into lethally irradiated mice. The longest lived among these clones was sustained in culture for slightly longer than 100 days. We conclude that these clones represented committed megakaryocyte progenitor cells.
Discussion
Recent reports demonstrate that the stem cells of normal adults retain enormous plasticity. Hemopoietic stem cells can differentiate into muscle,2 liver,3 and brain,4,5 neural stem cells can differentiate into blood,45 gut, liver, lung, or muscle,46 and muscle stem cells can differentiate into blood.2,47Furthermore human embryonic stem (ES) cells, capable of contributing to all human tissues, have recently been generated.48 Having firmly established the vast differentiation potential of stem cells, focus now shifts to determining whether, among the many available options, desired fates can be specifically elicited. In practice, this restates the question of whether stem cell fate is intrinsically determined or whether it is subject to extrinsic influence.
Our results using chemically activated growth factor receptors suggest that MHPCs can be instructed to adopt specific fates. Mpl supports MHPC self-renewal, yet Flt-3 and the G-CSF receptor both failed to support MHPC self-renewal. The ability of Mpl to support MHPC self-renewal is consistent with its established role in maintaining normal numbers of progenitors and stem cells in vivo.49 50 Our inability to generate MHPCs in clonal assays contrasted with our relatively consistent ability to generate MHPCs from pools of Mpl-transduced marrow cells. In the clonal assays, 50 000 transduced cells were analyzed, whereas pooled cultures routinely allowed between 2 and 5 million transduced cells to be evaluated. We conclude that MHPCs capable of self-renewal in response to Mpl signaling are rare (fewer than 1 in 50 000), thus providing the likely basis for the discrepancy between our results using clones and pools of transduced cells.
Mpl preferentially enabled MHPCs to differentiate toward the megakaryocytic lineage. Mpl, the receptor for thrombopoietin, is a primary regulator of platelet production.51 The preferential production of megakaryocytes might have resulted from megakaryocytic progenitors being relatively more responsive to signaling by Mpl than were progenitors committed to other cell lineages. This interpretation is supported by the Mpl-supported sustained growth of megakaryocytic progenitors in our limiting-dilution assays (Table 1). Alternatively, signaling by Mpl might alter the probability with which MHPCs commit to the megakaryocytic pathway.
Mpl's specificity in the present study stands in apparent contrast to that in a previous report using knock-in mice. Substitution of the Mpl signaling domain with the corresponding portion of the G-CSF receptor reduced megakaryocyte progenitors by only 30%.32 Although these findings were interpreted as arguing against an instructive role of Mpl in hematopoietic cell fate decisions, we suggest that the absence of an Mpl signal might have been compensated for by alternative signaling pathways that remained available in vivo, such as those activated by IL-6 or IL-11. Alternatively it is possible in our own studies that even though the G-CSF receptor fusion was functional in Ba/F3 cells, it might have lacked properties necessary to generate an “authentic” G-CSF signal in primary MHPCs.
Finally, our results suggest that though Mpl is permissive for the terminal differentiation of committed progenitors, signals other than those provided by Mpl are required for MHPCs to generate erythroid or granulocyte progeny. Mpl's ability to support the terminal differentiation of committed progenitor cells is reminiscent of various findings in which ectopically expressed receptors are able to support the terminal differentiation of progenitors in which they are not normally expressed.31-34 These findings suggest that though receptor swapping is permissible in committed progenitor cells, constraints are placed on the interchangeability of receptors within MHPCs.
Our results open the possibility that HSCs/MHPCs might be manipulated to perform various functions, depending on the particular receptor signaling domain activated. An especially attractive feature of the CID-based approach derives from the possibility of inducing a population of genetically modified MHPCs to adopt a desired fate in vivo.41 Mpl may induce qualitative or quantitative changes in signaling that are required for MHPC self-renewal and that are not provided for by G-CSF receptor and Flt-3. Alternatively, the G-CSF receptor and Flt-3 may activate pathways that are inhibitory to MHPC self-renewal. Understanding these mechanisms may not be only biologically important, it may have therapeutic implications.
We thank Tim Clackson (Ariad Pharmaceuticals) for AP20187, Christine Halbert and James Yan for technical assistance, and Ken Kaushansky, Belinda Avalos, and Ihor Lemischka for cDNAs.
Supported by National Institutes of Health grants 5R01DK52997, 1R01DK57525, 2P01HL53750, 1P01DK55820, and 2P01DK47754; by an American Society of Hematology Junior Faculty Scholar Award; and by an award from the Fanconi Anemia Research Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
C. Anthony Blau, Dept of Medicine, Division of Hematology, University of Washington, Mailstop 357710, Health Sciences Bldg, Seattle, WA 98195; e-mail:tblau@u.washington.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal