In myelodysplastic syndrome (MDS), the expression of the cyclin-dependent kinase inhibitor p15ink4B (p15) is frequently decreased because of the aberrant methylation of the gene promoter; p15 is normally up-regulated during megakaryocytic differentiation. It was hypothesized that p15 methylation and deregulation of gene expression contribute to defective megakaryocytopoiesis in patients with MDS. Here it is shown that the increasing autocrine production of TGF-β1 stimulates megakaryocytic differentiation in normal CD34+ cells and that p15 mediates, at least in part, this effect. This TGF-β1–dependent pathway is altered in MDS CD34+progenitors because of p15 methylation. The demethylating agent 2-deoxyAZAcytidin can restore the normal demethylated state of thep15 gene and increase its expression. Nevertheless, MDS CD34+ cells only poorly differentiate to the megakaryocytic lineage. These findings suggest that p15 methylation occurs in a neoplastic clone with a profound defect of cell proliferation, survival, and differentiation that cannot be overcome by using a demethylating drug.
Introduction
We have recently shown that expression of the cyclin-dependent kinase inhibitor (CDKI) p15ink4B (p15) is up-regulated during in vitro granulocytic and megakaryocytic differentiation of normal CD34+ hematopoietic progenitors.1,2 An aberrant methylation of CpG islands in the p15 promoter region commonly occurs in myelodysplastic syndromes (MDS), such as refractory anemia with excess of blasts (RAEB) or RAEB in transformation (RAEB-t), and is associated with the loss of p15 expression.3 4
An important function of p15 is to mediate extracellular antimitotic signals, and p15 has been identified as the effector of the G1-arrest induced by transforming growth factor β1 (TGF-β1) in a keratinocyte cell line.5 TGF-β1 is a multifunctional hematopoietic regulator.6 With respect to megakaryocytopoiesis, in vitro studies suggest that TGF-β1 exerts an inhibitory effect on early and late stages of megakaryocytopoiesis, affecting both megakaryocytic differentiation and endomitosis.7-9
Megakaryocytic dysplasia and thrombocytopenia are frequent findings in patients with RAEB and RAEB-t. We hypothesize that the loss of p15 expression contributes to the impaired megakaryocytic differentiation in these patients.
The aims of the present study were to clarify the association between TGF-β1 and p15 in normal megakaryocytic differentiation and to address the role of p15 methylation in the altered megakaryocytopoiesis of MDS.
Study design
Patients
CD34+ cells were isolated from bone marrow samples of 5 RAEB-free donors undergoing marrow harvest for allogeneic transplantation and from 11 patients affected by RAEB. Diagnosis was established according to the French-American-British classification.10 The proportion of blast in the bone marrow ranged between 15% and 20%, and all patients had platelet counts less than 60 × 109/L. Cytogenetic analysis was performed in 7 of 11 patients: 6 patients had normal karyotype, and one patient showed del(5)(q14q34). All samples were obtained after informed consent.
CD34+ cell isolation and megakaryocytic differentiation
Normal and myelodysplastic CD34+ cells were isolated using the CD34+ Multisort kit (Miltenyi Biotec, Auburn, CA) according to the instructions of the manufacturer. In all the experiments, more than 95% of the collected cells were CD34+. Isolated cells were seeded in serum-free medium supplemented with 20 ng/mL IL-6 and 10 ng/mL thrombopoietin (both purchased from PeproTech EC, London, United Kingdom), as previously described.2 In some experiments neutralizing anti–human TGF-β1 monoclonal antibody (35 μg/mL; R&D Systems, Minneapolis, MN) was added to the culture medium. In addition, CD34+ cell cultures of patients with MDS and control subjects were grown with and without the demethylating agent 2-deoxyAZAcytidin (AZA; 10−6 M) (Sigma, St Louis, MO). On days 0, 7, and 14, CD41 expression was evaluated using flow cytometry (anti-CD41 fluorescein isothiocyanate-conjugated monoclonal antibody from Caltag Laboratories, Burlingame, CA). On days 7 and 14, CD41+ cells were isolated by immunomagnetic sorting, as previously described,2 for analysis of DNA methylation status and gene expression using reverse transcription–polymerase chain reaction (RT-PCR).
DNA methylation assay
DNA methylation patterns in the p15 promoter were determined by methylation-specific PCR, after bisulphite treatment of DNA samples, according to the method of Herman et al.11 We used the same primers and PCR conditions described by Uchida et al.3 PCR reaction product (25 μL) was directly loaded onto a 3% agarose gel, stained with ethidium bromide, and visualized under UV light. Amplified products of unmethylated and methylated reactions consisted of 154 base pairs (bp) and 148 bp, respectively.
RT-PCR analysis
All samples were analyzed for p15 and TGF-β1 expression by semiquantitative RT-PCR. Total RNA extraction and semiquantitative RT-PCR were performed using β-actin as an internal control.2 A 30-cycle PCR for p15 was performed using the primers 5′-TGG GGG CGG CAG CGA TGA G-3′ and 5′-AGG TGG GTG GGG GTG GGA AAT-3′ and an annealing temperature of 56°C. The MgCl2concentration in the PCR mixture was 1.5 mM. RT-PCR for TGF-β1 was performed using the commercial kit purchased from R&D Systems, according to the manufacturer's instructions. Bone marrow mononuclear cells were used as positive control.
TGF-β1 enzyme-linked immunosorbent assay
TGF-β1 levels were measured in culture supernatant (previously subjected to sequential acid activation and neutralization) using the Quantikine Human TGF-β1 immunoassay (R&D Systems), as previously described.12 All samples were evaluated in duplicate.
Statistical methods
Data on cell proliferation and CD41 expression were analyzed by Mann-Whitney U and paired t tests, as appropriate.
Results and discussion
Normal CD34+ cells
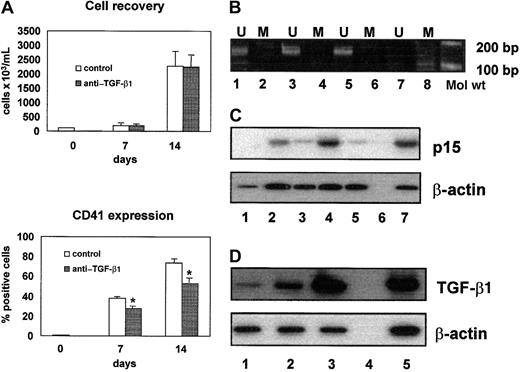
In the presence of IL-6 and thrombopoietin, CD34+hematopoietic progenitor cells can be expanded and differentiated into CD41+ megakaryocytic cells. On 7 and 14 days of culture, 38% ± 3% and 75% ± 3% (mean values ± SEM of 5 experiments; range, 28% to 45% and 62% to 85%, respectively) of recovered cells were CD41+ (Figure1A). As expected, the p15 gene remained persistently unmethylated (Figure 1B) and p15, undetectable in CD34+ cells, was clearly expressed in CD41+cells after 7 and 14 days of culture (Figure 1C, lanes 2 and 4). The up-regulation of p15 expression was dependent on the autocrine production of TGF-β1 from CD41+ cells, as demonstrated by the finding that a neutralizing anti–TGF-β1 antibody inhibited the expression of p15 (Figure 1C, lanes 3 and 5). Accordingly, TGF-β1 was detected in the culture supernatants (133 ± 108 and 1078 ± 297 pg/mL on days 7 and 14, respectively; mean values ± SEM of 3 experiments) and TGF-β1 messenger RNA (mRNA) levels progressively increased in differentiating cells (Figure 1D). The neutralization of autocrine TGF-β1 was associated with a significant decrease in the output of CD41+ cells both on days 7 and 14 (38% ± 3% versus 28% ± 3% positive cells on day 7, P < .01; 75% ± 3% versus 51% ± 3% positive cells on day 14,P < .01), whereas no significant differences were found in total cell recovery (Figure 1A) with and without anti–TGF-β1 antibody. No differences were found in total cell recovery and CD41+ cell recovery between cultures with or without AZA (data not shown). These observations suggest that the autocrine production of TGF-β1 favors megakaryocytic differentiation mediated, at least in part, by the up-regulation of p15.
Role of p15 and TGF-β1 in normal megakaryocytic differentiation.
(A) Neutralizing anti–TGF-β1 antibody induced a significant decrease of CD41+ cell number (*P < .001), without affecting total cell recovery. (B) p15 Amplification bands were detectable only in the presence of unmethylated sequence-specific primer pairs. Lanes 1 and 2, CD34+ cells; lanes 3 and 4, CD41+ cells on day 7; lanes 5 and 6, CD41+cells on day 14; lanes 7 and 8, negative control. U indicates unmethylated; M, methylated. (C) Semiquantitative RT-PCR analysis of p15 mRNA expression showed that p15 was not expressed in CD34+ cells (lane 1) and progressively increased in CD41+ cells on day 7 (lane 2) and day 14 (lane 4). The p15/β-actin ratios were 0.34 and 0.82 on days 7 and 14, respectively. Neutralizing anti–TGF-β1 antibody induced a significant reduction of p15 mRNA expression on day 7 (lane 3; p15/β-actin ratio, 0.14) and day 14 (lane 5; p15/β-actin ratio, 0.12). Negative and positive controls are shown in lanes 6 and 7, respectively. (D) Semiquantitative RT-PCR analysis of TGF-β1 mRNA expression demonstrated that TGF-β1 mRNA increases during megakaryocytic differentiation. TGF-β1/β-actin ratios were 0.25 in CD34+ cells (lane 1), 0.96 in CD41+ cells on day 7 (lane 2), and 2.63 in CD41+ cells on day 14 (lane 3). Negative and positive controls are shown in lanes 4 and 5, respectively.
Role of p15 and TGF-β1 in normal megakaryocytic differentiation.
(A) Neutralizing anti–TGF-β1 antibody induced a significant decrease of CD41+ cell number (*P < .001), without affecting total cell recovery. (B) p15 Amplification bands were detectable only in the presence of unmethylated sequence-specific primer pairs. Lanes 1 and 2, CD34+ cells; lanes 3 and 4, CD41+ cells on day 7; lanes 5 and 6, CD41+cells on day 14; lanes 7 and 8, negative control. U indicates unmethylated; M, methylated. (C) Semiquantitative RT-PCR analysis of p15 mRNA expression showed that p15 was not expressed in CD34+ cells (lane 1) and progressively increased in CD41+ cells on day 7 (lane 2) and day 14 (lane 4). The p15/β-actin ratios were 0.34 and 0.82 on days 7 and 14, respectively. Neutralizing anti–TGF-β1 antibody induced a significant reduction of p15 mRNA expression on day 7 (lane 3; p15/β-actin ratio, 0.14) and day 14 (lane 5; p15/β-actin ratio, 0.12). Negative and positive controls are shown in lanes 6 and 7, respectively. (D) Semiquantitative RT-PCR analysis of TGF-β1 mRNA expression demonstrated that TGF-β1 mRNA increases during megakaryocytic differentiation. TGF-β1/β-actin ratios were 0.25 in CD34+ cells (lane 1), 0.96 in CD41+ cells on day 7 (lane 2), and 2.63 in CD41+ cells on day 14 (lane 3). Negative and positive controls are shown in lanes 4 and 5, respectively.
Myelodysplastic CD34+ cells
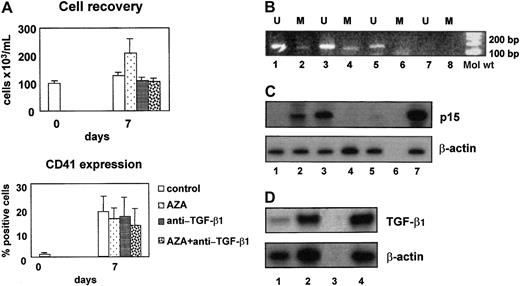
Proliferation and megakaryocytic differentiation of CD34+ cells isolated from patients with RAEB significantly differed from their normal counterpart. On day 7 the cell output in the MDS cultures was still comparable to the cell output in the normal CD34+ cultures (Figure 2A). Thereafter, however, in 9 of 11 patients no viable cells could be collected on day 14. The proportion of CD41-expressing cells was significantly lower in cultures of MDS CD34+ cells than in cultures of normal CD34+ cells (19% ± 6% versus 38% ± 3% positive cells, mean values ± SEM,P = .02). In 8 (72%) of 11 evaluated samples, in CD34+ cells, and in CD41+ cells, p15 amplification bands were detectable in the presence of unmethylated and methylated sequence-specific primer pairs (Figure 2B, lanes 1 and 2 and 3 and 4, respectively). In contrast, in cells treated with AZA, only unmethylated bands were appreciable, showing that AZA treatment effectively removed p15 gene methylation (Figure 2B, lanes 5 and 6). Despite partial methylation of the p15 gene promoter, p15 was up-regulated in CD41+ cells at 7 days of culture, and AZA treatment increased its expression (Figure 2C, lanes 2 and 3). Nevertheless, AZA treatment did not enhance the proportion of CD41+ cells (Figure 2A). MDS CD41+ cells produced TGF-β1, as demonstrated by the presence of TGF-β1 mRNA in these cells (Figure 2D) and by the presence of TGF-β1 in the culture supernatants (58 ± 42 and 28 ± 12 pg/mL, mean values ± SEM of 3 experiments, in cultures with and without AZA, respectively). Furthermore, the neutralization of TGF-β1 significantly reduced the expression of p15 also in the presence of AZA (Figure 2C, lanes 4 and 5).
Role of p15 and TGF-β1 in myelodysplastic megakaryocytic differentiation.
(A) On day 7 the cell output in the MDS cultures was comparable to the cell output in the normal CD34+ cultures. In contrast, the proportion of CD41-expressing cells was significantly lower. These findings were not modified by AZA or by anti–TGF-β1 antibody. (B) p15 Amplification bands were detectable in the presence of unmethylated and methylated sequence-specific primer pairs in CD34+cells (lanes 1 and 2) and in day 7 CD41+ cells (lanes 3 and 4). On the contrary, in CD41+ cells cultured in the presence of AZA, p15 amplification bands were detectable only in the presence of unmethylated sequence-specific primer pairs (lanes 5 and 6). Lanes 7 and 8 show negative controls. U indicates unmethylated; M, methylated. (C) Semiquantitative RT-PCR analysis of p15 mRNA expression showed that p15 was not expressed in CD34+ cells (lane 1), was expressed after 7 day culture in CD41+ cells (lane 2, p15/β-actin ratio 0.4), and was strongly up-regulated in CD41+ cells treated with AZA (lane 3, p15/β-actin ratio 1). Neutralizing anti–TGF-β1 antibody abrogated p15 expression both in control (lane 4) and in demethylated (lane 5) CD41+cells. Negative and positive controls are shown in lanes 6 and 7, respectively. (D) Semiquantitative RT-PCR analysis of TGF-β1 mRNA expression demonstrated the increase of TGF-β1 during megakaryocytic differentiation. TGF-β1/β-actin ratios are 0.4 in CD34+cells (lane 1) and 0.95 in CD41+ cells on day 7 (lane 2). Negative and positive controls are shown in lanes 3 and 4, respectively.
Role of p15 and TGF-β1 in myelodysplastic megakaryocytic differentiation.
(A) On day 7 the cell output in the MDS cultures was comparable to the cell output in the normal CD34+ cultures. In contrast, the proportion of CD41-expressing cells was significantly lower. These findings were not modified by AZA or by anti–TGF-β1 antibody. (B) p15 Amplification bands were detectable in the presence of unmethylated and methylated sequence-specific primer pairs in CD34+cells (lanes 1 and 2) and in day 7 CD41+ cells (lanes 3 and 4). On the contrary, in CD41+ cells cultured in the presence of AZA, p15 amplification bands were detectable only in the presence of unmethylated sequence-specific primer pairs (lanes 5 and 6). Lanes 7 and 8 show negative controls. U indicates unmethylated; M, methylated. (C) Semiquantitative RT-PCR analysis of p15 mRNA expression showed that p15 was not expressed in CD34+ cells (lane 1), was expressed after 7 day culture in CD41+ cells (lane 2, p15/β-actin ratio 0.4), and was strongly up-regulated in CD41+ cells treated with AZA (lane 3, p15/β-actin ratio 1). Neutralizing anti–TGF-β1 antibody abrogated p15 expression both in control (lane 4) and in demethylated (lane 5) CD41+cells. Negative and positive controls are shown in lanes 6 and 7, respectively. (D) Semiquantitative RT-PCR analysis of TGF-β1 mRNA expression demonstrated the increase of TGF-β1 during megakaryocytic differentiation. TGF-β1/β-actin ratios are 0.4 in CD34+cells (lane 1) and 0.95 in CD41+ cells on day 7 (lane 2). Negative and positive controls are shown in lanes 3 and 4, respectively.
Here we show that normal megakaryocytic differentiation requires autocrine TGF-β1 production, which induces the up-regulation of p15. This mechanism is deeply perturbed in MDS CD34+ cells and is associated with p15 gene methylation. Nevertheless,p15 gene demethylation does not improve the differentiation capacity of MDS progenitors toward the megakaryocytic lineage. Moreover, CD34+ cells from patients with MDS, showing unmethylated p15 gene and normal p15 expression, share the same low cell output as methylated samples, with a low generation of CD41+ cells.
Several recent studies pointed to accelerated apoptosis as an attractive explanation for the ineffective hematopoiesis and bone marrow failure in myelodysplasia.13,14 Leukemic transformation may be the result of raising the apoptotic threshold in the aberrant and highly proliferative CD34+cells.15 Thus, we can hypothesize that p15 methylation occurs in a neoplastic clone with a profound defect of cell proliferation, survival, and differentiation. Consequently, p15 demethylation is not sufficient to restore the normal differentiation potential.
In conclusion, though the detection of p15 gene methylation can be useful for evaluating the prognosis and the progression of the disease,4 our observations point to potential limitations of the efficacy of demethylating drugs in MDS.
We thank Dr Stefan Hohaus for helpful comments.
Supported by Associazione Italiana per la Ricerca sul Cancro, Milan, and by Ministero della Università e Ricerca Scientifica, Rome, Italy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Luigi Maria Larocca, Istituto di Anatomia Patologica, Università Cattolica del Sacro Cuore, Largo F. Vito 1, 00168, Rome, Italy; e-mail: llarocca@rm.unicatt.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal