Abstract
The transactivator protein of human T-lymphotropic virus I (HTLV-I), Tax, has been associated with the up-regulation of several host cell genes, including interleukin 2 (IL-2), the IL-2 receptor-α(IL-2Rα) chain (CD25), interferon γ(IFN-γ), and tumor necrosis factor (TNF). It has been proposed that an IL-2/CD25 autocrine loop plays a part in maintaining the very high proviral loads often found in HTLV-I infection. Furthermore, abnormal production of inflammatory cytokines might contribute to the pathogenesis of the inflammatory diseases associated with HTLV-I infection. However, there has been no study of the expression of these genes in freshly isolated peripheral blood mononuclear cells (PBMCs) naturally infected with HTLV-I. In the present study, flow cytometry was used to determine which cytokines are produced by freshly isolated PBMCs that spontaneously express the HTLV-I Tax protein. Surprisingly, the results show that intracellular Tax expression is associated with rapid up-regulation of IFN-γ but not TNF or IL-2. A proportion of HTLV-I–infected cells express both IFN-γ and the surface markers of effector memory cells. Such cells are capable of migration through peripheral tissues and could therefore contribute to the inflammation seen in diseases such as HTLV-I–associated myelopathy/tropical spastic paraparesis.
Introduction
Human T-lymphotropic virus type I (HTLV-I), which belongs to the HTLV-bovine leukemia virus (BLV) subfamily of retroviruses, infects an estimated 10 million people worldwide.1 Unlike human immunodeficiency virus, HTLV-I causes no disease in a majority of infected subjects (asymptomatic carriers; ACs). However, approximately 2% to 3% develop an aggressive T-cell malignancy, adult T-cell leukemia/lymphoma (ATL) and another 2% to 3% develop a disabling chronic inflammatory disease, involving the central nervous system (CNS) (HTLV-I–associated myelopathy/tropical spastic paraparesis; HAM/TSP), the eyes, lungs, or skeletal muscles.2
HTLV-I shares with other replication-competent retroviruses the 3 main genomic regions of gag, pol, and env, but unlike most leukemia viruses it has an additional region calledpX that codes for 2 transcriptional regulatory proteins, the Tax and Rex proteins.2 The Rex protein stabilizes viral messenger RNAs and regulates their splicing and transport. The Tax protein is of central importance in virus dynamics because, as well as transactivating viral transcription, it is thought to up-regulate several cellular genes including interleukin (IL)-2 receptor (CD25) and IL-2.3-8
Several observations suggest that direct or indirect Tax-induced up-regulation of cellular genes involved in lymphocyte proliferation or inflammation plays a role in the pathogenesis of HTLV-I–associated diseases.2,3,9,12 It has been suggested that an autocrine loop involving IL-2 and the IL-2 receptor is an important mechanism by which HTLV-I–infected cells proliferate in vivo.6-8 The same mechanism could also play a part in the development of ATL. Overproduction of interferon γ (IFN-γ) by infected lymphocytes has been hypothesized to contribute to the chronic inflammation observed in the CNS of patients with HAM/TSP.3,9 Furthermore, others have suggested that IFN-γ is also produced by Tax-specific CD8+ T cells in response to Tax-expressing cells that accumulate in the CNS.10-12
The effects of HTLV-I infection on cytokine production have so far been studied either in mixed cell populations or in in vitro– derived T-cell clones, because there has been no sensitive assay to identify HTLV-I protein expression in individual cells. We wished to identify the cytokines produced by T cells naturally infected with HTLV-I, with minimal in vitro manipulation. We therefore used our recently described sensitive flow cytometric assay to detect intracellular Tax protein expression.13
Patients, materials, and methods
Patients and cells
Peripheral blood mononuclear cells (PBMCs) were obtained from 3 patients with HTLV-I infection with a clinical diagnosis of HAM/TSP or other neurological symptoms (patient codes beginning with T) and 3 asymptomatic carriers of the virus (codes beginning with H). All subjects gave informed consent. PBMCs were isolated on a Histopaque-1077 (Sigma, Dorset, United Kingdom) density gradient and washed 3 times with phosphate-buffered saline (PBS). Cells were cultured at 1 × 106/mL in RPMI 1640 medium (Gibco, Paisley, United Kingdom) supplemented with 10% fetal calf serum (FCS) (Sigma), 2 mM glutamine (Gibco), 100 IU/mL penicillin (Gibco), and 100 μg/mL streptomycin (Gibco). To induce cytokine production by PBMCs, 0.1 ng/mL phorbol myristate acetate (PMA) (Sigma) and 0.5 μg/mL A23187 (Sigma) were added to the culture medium. Alternatively, 200 IU/mL streptokinase/streptodornase (SK/SD) was added to the culture medium of PBMCs to stimulate the production of IFN-γ by SK/SD-specific T lymphocytes. In certain experiments, 20 nM concanamycin A (CMA) (Sigma), 2 mM monensin (Sigma), 5 μg/mL anti–major histocompatibility complex (MHC) class I monoclonal antibody (mAb) (W6/32, IgG2a) (Serotec, Oxford, United Kingdom), or 5 μg/mL anti-MHC class II mAb (Tü39, IgG2a) (Becton Dickinson, Oxford, United Kingdom) were also added to the culture medium.
Cell surface staining
After being harvested, cells were fixed in PBS containing 2% paraformaldehyde (Sigma) for 20 minutes and resuspended in PBS at 4°C until use. Fixed cells were washed with PBS containing 7% of normal goat serum (Sigma). Cells were then incubated for 15 minutes at room temperature with various combinations of fluorescence-conjugated mAbs (all from Beckman Coulter, Bedfordshire, United Kingdom, unless otherwise specified) as follows: fluorescein isothiocyanate (FITC)–labeled anti-CD45RO, Cy5-labeled anti-CD45RA (Pharmingen, Oxford, United Kingdom), PC5-labeled anti-CD4, energy-coupled dye (ECD)–labeled anti-CD8, phycoerythrin (PE)–labeled anti-CD25, PE-labeled anti-CD69, PE-labeled anti-CD71, or anti-CCR7 (CCR7.6B3) (see Hasegawa et al24). The anti-CCR7 mAb was detected with an R-phycoerythrin (RPE)–labeled goat F(ab′)2 antimouse IgG1 serum (Southern Biotechnology, Birmingham, AL). For CD62L detection, cells were stained with FITC-labeled anti-CD62L (Beckman Coulter) before fixation and then processed as described above.
Detection of intracellular Tax and cytokines
After cell surface labeling, cells were washed and permeabilized with PBS/7% normal goat serum containing 0.2% saponin (Sigma) (PBS-SAPO) for 10 minutes at room temperature. Permeabilized cells were washed and resuspended in PBS-SAPO containing an anti-HTLV-I Tax Mab (Lt-4; IgG3)15 or an isotype control mAb (Southern Biotechnology) for 20 minutes at room temperature. The cells were then washed twice and resuspended for 20 minutes at room temperature in PBS-SAPO containing FITC-labeled goat F(ab′)2 antimouse IgG3 serum (Southern Biotechnology) and anti–tumor necrosis factor (anti-TNF) (Pharmingen), anti-IFN-γ (Pharmingen), anti-IL-2 (Beckman Coulter), or anti-IL-4 (Pharmingen) mAb. Finally, the cells were washed twice and analyzed by flow cytometry on a Coulter EPICS XL (Beckman Coulter).
Results
Effect of HTLV-I Tax expression on infected PBMCs
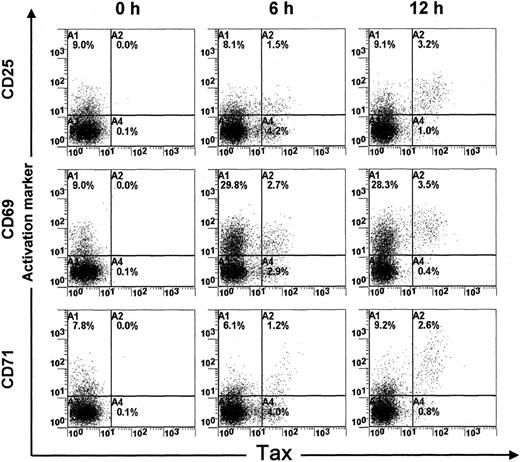
Short-term cultivation of PBMCs obtained from HTLV-I–infected patients is associated with increased Tax protein expression in infected PBMCs.13 We recently described a sensitive flow cytometric assay to detect intracellular Tax protein expression.13 In this study, we used this flow cytometric assay to characterize the biologic effect of Tax expression on naturally infected PBMCs. For this purpose, PBMCs were isolated from 3 ACs of the virus and 3 HAM/TSP patients and harvested directly or after 6 or 12 hours of cultivation in vitro. After being harvested, cell samples were fixed and processed to detect concomitantly Tax and the expression of 3 lymphocyte surface markers of activation (CD25, CD69) or proliferation (CD71). Figure 1 shows the results of one representative experiment of 6. The results show that Tax protein was not detected in freshly isolated PBMCs. In contrast, after 6 hours of cultivation, a significant proportion (about 55%) of PBMCs expressed the Tax protein. Interestingly, more than 50% of Tax-expressing cells were negative for the CD25, CD69, or CD71 markers at 6 hours. In contrast, after 12 hours of cultivation, the majority (> 75%) of Tax-expressing cells became positive for CD25, CD69, and CD71. These results indicate that a significant proportion of HTLV-I–infected PBMCs do not express activation/proliferation markers in peripheral blood. However, once they express the Tax protein for more than 6 to 12 hours, they become activated, which is consistent with previous studies showing the transactivating effect of Tax.3-7
Expression of activation markers (CD25, CD69, and CD71) in HTLV-I Tax+ PBMCs. PBMCs were isolated from an HTLV-I–infected individual (TAZ) and harvested directly or after 6 or 12 hours of cultivation in vitro.
One representative experiment of 16 is shown.
Expression of activation markers (CD25, CD69, and CD71) in HTLV-I Tax+ PBMCs. PBMCs were isolated from an HTLV-I–infected individual (TAZ) and harvested directly or after 6 or 12 hours of cultivation in vitro.
One representative experiment of 16 is shown.
IFN-γ but not IL-2 is associated with Tax expression in PBMCs
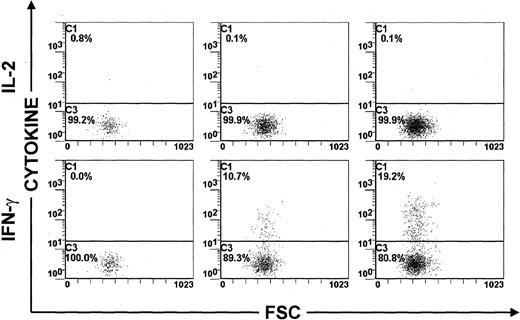
The results shown in Figure 1 raised the important and interesting possibility that cellular activation by intracellular Tax protein is associated with the production of cytokines. Several proinflammatory cytokines, including IFN-γ and TNF, are thought to be involved in the pathogenesis of HAM/TSP.9-12 We therefore investigated whether activation of HTLV-I–infected PBMCs is associated with the production of these proinflammatory cytokines. We also detected the production of IL-2 and IL-4 in Tax-expressing lymphocytes, because of the potential importance of IL-2 in HTLV-I infection6-8and the role of IL-4 in controlling the balance of Th1/Th2 cells in the immune response. PBMCs were isolated from 3 ACs of the virus and 3 HAM/TSP patients and cultivated for 6, 12, and 24 hours in the presence of monensin to induce the accumulation of cytokines within activated cells.16 In addition, CMA, which is an inhibitor of the perforin-dependent cytotoxic pathway,17 was added to the culture medium to prevent the destruction of Tax-expressing PBMCs by HTLV-I–specific cytotoxic T lymphocytes (CTLs).13 18 The results of this time-course study are shown in Table 1 and Figure2. After 6 hours of cultivation, Tax-expressing PBMCs were negative for IFN-γ, TNF, IL-2, or IL-4. After 12 hours, IFN-γ but not IL-2, IL-4, or TNF was detected in a proportion of Tax-expressing PBMCs. At this time point, more than 10% of Tax-expressing PBMCs were capable of producing IFN-γ. After 24 hours of cultivation, IL-2, IL-4, or TNF production remained negative, whereas the frequency of IFN-γ-producing PBMCs continued to increase within Tax+ cells (Figure2). Similar conclusions were made using PBMCs obtained from 3 ACs of the virus and 3 patients with HTLV-I–associated neurological disease (Table 1). Furthermore, the concomitant detection of IFN-γ and another HTLV-I antigen, the p24 Gag protein, gave comparable results. More than 10% of HTLV-I p24+ PBMCs were positive for IFN-γ (data not shown).
Detection of cytokine expression in Tax+peripheral blood mononuclear cells isolated from asymptomatic carriers of human T-cell lymphotropic virus type I and patients with HTLV-I–associated neurological disease
| Patient . | Cytokine-expressing cells (%) in Tax+ PBMCs* . | |||
|---|---|---|---|---|
| TNF-α . | IFN-γ . | IL-2 . | IL-4 . | |
| TAF | 0.0 | 10.7 | 0.1 | 0.0 |
| TAT | 0.3 | 5.4 | 0.3 | 0.0 |
| TXX | 4.7 | 21.4 | 0.0 | 0.0 |
| HH | 0.0 | 3.8 | 0.0 | 0.0 |
| HS | 0.0 | 2.8 | 1.5 | 0.0 |
| HT | 2.4 | 11.6 | 0.0 | 0.0 |
| Patient . | Cytokine-expressing cells (%) in Tax+ PBMCs* . | |||
|---|---|---|---|---|
| TNF-α . | IFN-γ . | IL-2 . | IL-4 . | |
| TAF | 0.0 | 10.7 | 0.1 | 0.0 |
| TAT | 0.3 | 5.4 | 0.3 | 0.0 |
| TXX | 4.7 | 21.4 | 0.0 | 0.0 |
| HH | 0.0 | 3.8 | 0.0 | 0.0 |
| HS | 0.0 | 2.8 | 1.5 | 0.0 |
| HT | 2.4 | 11.6 | 0.0 | 0.0 |
The cells were cultivated for 12 hours with 1 μM monensin.
Time-course study of IL-2 and IFN-γ expression in Tax+ PBMCs. PBMCs were isolated from an HTLV-I–infected individual (TAF), cultivated for 6, 12, or 24 hours in vitro and then harvested.
One representative experiment of 3 is shown.
Time-course study of IL-2 and IFN-γ expression in Tax+ PBMCs. PBMCs were isolated from an HTLV-I–infected individual (TAF), cultivated for 6, 12, or 24 hours in vitro and then harvested.
One representative experiment of 3 is shown.
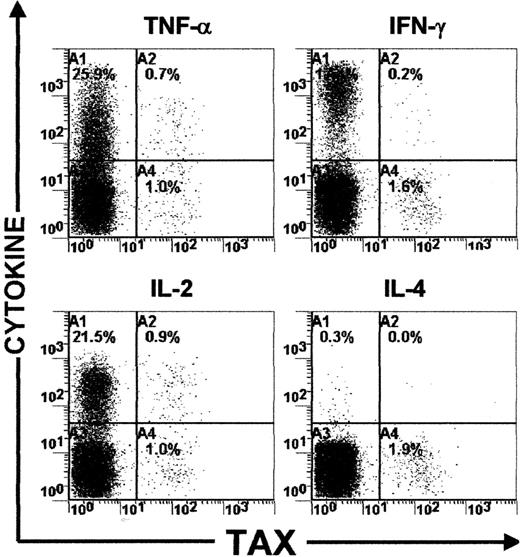
The failure to detect IL-2, IL-4, or TNF could be due to poor sensitivity of the intracellular cytokine detection assay used. To investigate this possibility, PBMCs from the same 6 HTLV-I–infected individuals were activated using a polyclonal activator (a combination of PMA and A23187). After 6 hours of cultivation, cells were harvested and processed to detect IFN-γ, TNF, IL-2, or IL-4 production. Interestingly, IFN-γ, TNF, and IL-2 were detectable in a significant proportion of Tax-expressing PBMCs in the 6 HTLV-I–infected individuals (Figure 3 and Table2). More than 50% of Tax-expressing PBMCs were capable of producing TNF or IL-2 and about 10% could produce IFN-γ (Figure 3 and Table 2). In addition, IL-4 was detected in a proportion of Tax− PBMCs but not in Tax+ cells (Figure 3 and Table 2). These results show that although a high proportion of HTLV-I–infected T cells retain the ability to produce IL-2, TNF, or IL-4, the production of these cytokines is not detectably associated with intracellular Tax expression.
Detection of IFN-γ, TNF, IL-2, and IL-4 expression in mitogen-activated PBMCs.
Dot plots showing both cytokine and HTLV-I Tax expressions in PBMCs isolated from one HTLV-I–infected individual (HS).
Detection of IFN-γ, TNF, IL-2, and IL-4 expression in mitogen-activated PBMCs.
Dot plots showing both cytokine and HTLV-I Tax expressions in PBMCs isolated from one HTLV-I–infected individual (HS).
Detection of cytokine expression in Tax+peripheral blood mononuclear cells isolated from asymptomatic carriers and patients with HTLV-I–associated neurological disease
| Patient . | Cytokine-expressing cells (%) in Tax+ PBMCs activated with mitogen* . | |||
|---|---|---|---|---|
| TNF-α . | IFN-γ . | IL-2 . | IL-4 . | |
| TAF | 58.8 | 28.9 | 73.0 | 2.8 |
| TAT | 40.4 | 23.0 | 50.9 | 12.4 |
| TXX | 56.0 | 14.5 | 55.4 | 2.8 |
| HH | 29.8 | 16.6 | 40.0 | 4.6 |
| HS | 42.8 | 10.2 | 45.5 | 1.0 |
| HT | 43.3 | 19.6 | 55.8 | 5.8 |
| Patient . | Cytokine-expressing cells (%) in Tax+ PBMCs activated with mitogen* . | |||
|---|---|---|---|---|
| TNF-α . | IFN-γ . | IL-2 . | IL-4 . | |
| TAF | 58.8 | 28.9 | 73.0 | 2.8 |
| TAT | 40.4 | 23.0 | 50.9 | 12.4 |
| TXX | 56.0 | 14.5 | 55.4 | 2.8 |
| HH | 29.8 | 16.6 | 40.0 | 4.6 |
| HS | 42.8 | 10.2 | 45.5 | 1.0 |
| HT | 43.3 | 19.6 | 55.8 | 5.8 |
The cells have been cultivated for 6 hours with 1 μM monensin.
Proportion of cells expressing IFN-γ, TNF, IL-2, and IL-4 among Tax+ PBMCs isolated from 3 ACs and 3 patients with neurological disease. Cells were harvested after 6 hours of cultivation in vitro.
The MHC class II antigen presentation pathway is not involved in the induction of IFN-γ production by Tax-expressing PBMCs
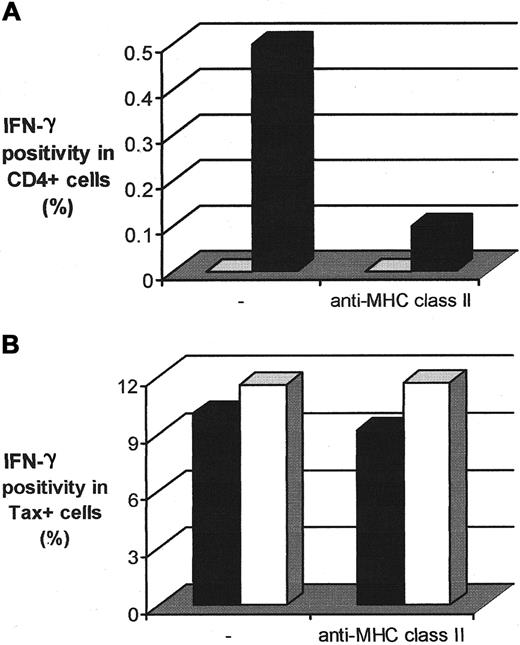
The production of cytokines associated with the observed increase in Tax expression in CD4+ T cells could result either from transactivation of cytokine genes by intracellular Tax protein or from class II MHC–restricted presentation of viral antigens to the CD4+ T cells. To distinguish between these possibilities, we used a mAb directed against MHC class II molecules that is known to inhibit the MHC class II presentation pathway.19 As a control, we used the SK/SD bacterial antigen that contains MHC class II–restricted epitopes and therefore elicits the production of IFN-γ by SK/SD-specific CD4+ T cells.20 PBMCs isolated from one AC and one patient with HAM/TSP were cultivated overnight in the presence or absence of this anti-MHC class II mAb. The cells were then harvested and processed for Tax and intracellular cytokine detection. The results (Figure4B) show that the presence of this anti-MHC class II mAb did not inhibit the production of IFN-γ by Tax-expressing cells. In contrast, a powerful inhibition of IFN-γ production by SK/SD-specific CD4+ T cells was observed in the presence of the anti-MHC class II mAb (Figure 4A), which confirmed the ability of this mAb to inhibit the MHC class II presentation pathway. These observations indicate that the MHC class II antigen presentation pathway was not involved in the induction of IFN-γ production by Tax-expressing PBMCs. The spontaneous IFN-γ production associated with Tax expression in CD4+ T cells is therefore more likely to be due to the direct or indirect transactivating effect of Tax on certain host cell genes.3-7 In addition, IFN-γ might be produced by HTLV-I–specific CD8+ T cells after stimulation via the class I pathway of antigen presentation.
Involvement of the MHC class II presentation pathway in the production of IFN-γ by Tax+ PBMCs.
(A) Inhibiting effect of an anti-MHC class II mAb on the production of IFN-γ by SK/SD-specific CD4+ T lymphocytes. PBMCs were isolated from an uninfected individual, cultivated in vitro for 12 hours with SK/SD, and then harvested. ■ indicates control; ▪, SK/SD. (B) Effect of the anti-MHC class II mAb on the production of IFN-γ by Tax+ PBMCs. Cells were isolated from one AC (HT) of the virus and one HAM/TSP patient (TAF) and harvested after 12 hours of cultivation in vitro. ▪ indicates TAF; ■, HT.
Involvement of the MHC class II presentation pathway in the production of IFN-γ by Tax+ PBMCs.
(A) Inhibiting effect of an anti-MHC class II mAb on the production of IFN-γ by SK/SD-specific CD4+ T lymphocytes. PBMCs were isolated from an uninfected individual, cultivated in vitro for 12 hours with SK/SD, and then harvested. ■ indicates control; ▪, SK/SD. (B) Effect of the anti-MHC class II mAb on the production of IFN-γ by Tax+ PBMCs. Cells were isolated from one AC (HT) of the virus and one HAM/TSP patient (TAF) and harvested after 12 hours of cultivation in vitro. ▪ indicates TAF; ■, HT.
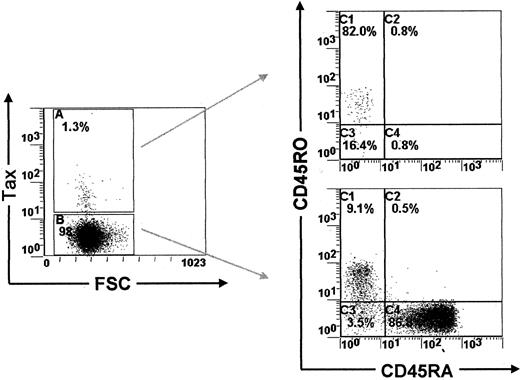
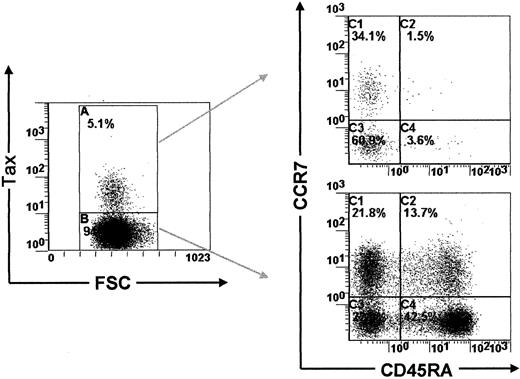
A proportion of Tax-expressing PBMCs are effector-memory T lymphocytes
Previous reports have shown that the majority of HTLV-I–infected PBMCs carry markers of the memory phenotype of T lymphocytes.13,21 In accordance with these reports, we show here using PBMCs isolated from 3 ACs and 3 HAM/TSP patients that 79% to 87% of Tax-expressing PBMCs were indeed CD45RA−CD45RO+ (Figure5 and Table3). In this context, Sallusto and colleagues14 have recently shown that the memory phenotype of T lymphocytes can be divided into 2 functionally different populations, which they name central-memory and effector-memory T lymphocytes, on the basis of CCR7 and CD45RA expression.22 Furthermore, Sallusto and coworkers14 demonstrated that cells of the central-memory phenotype recirculate within the central lymphoid tissues, whereas those with the effector-memory phenotype migrate in peripheral tissues. Therefore, to characterize the recirculation pattern of HTLV-I–infected PBMCs, we investigated the expression of CCR7 and CD45RA in Tax-expressing PBMCs freshly isolated from 3 ACs and 3 HAM/TSP patients. Table 4 shows that Tax-expressing PBMCs contain both central-memory T cells and effector-memory T cells. We then investigated the expression of CD62L, an adhesion molecule involved in the recirculation of T lymphocytes through the central lymphoid tissues.14 Consistent with the results presented above, we observed that a significant proportion of Tax-expressing cells were CD62Llow (data not shown). Together, these results indicate that a fraction of HTLV-I–infected PBMCs expresses the effector-memory phenotype of T lymphocytes. This implies that these effector-memory HTLV-I–infected PBMCs are able to leave the circulation and migrate in the parenchyma of peripheral tissues.
CD45RA and CD45RO expression in PBMCs isolated from HTLV-I–infected patients.
Dot plots showing CD45RA/CD45RO expression in Tax+ and in Tax− PBMCs (HT).
CD45RA and CD45RO expression in PBMCs isolated from HTLV-I–infected patients.
Dot plots showing CD45RA/CD45RO expression in Tax+ and in Tax− PBMCs (HT).
Detection of CD45RA/CD45RO expression in Tax+peripheral blood mononuclear cells isolated from asymptomatic carriers of the virus and patients with HTLV-I–associated neurological disease
| Patient . | CD45RO+CD45RA− cells (%) in Tax+ PBMCs . |
|---|---|
| TAF | 84.1 |
| TAT | 83.6 |
| TXX | 86.3 |
| HH | 78.9 |
| HS | 83.0 |
| HT | 86.8 |
| Patient . | CD45RO+CD45RA− cells (%) in Tax+ PBMCs . |
|---|---|
| TAF | 84.1 |
| TAT | 83.6 |
| TXX | 86.3 |
| HH | 78.9 |
| HS | 83.0 |
| HT | 86.8 |
Proportion of CD45RA/CD45RO expression in Tax+ PBMCs isolated from 3 ACs of the virus and 3 patients with neurological disease. Cells were harvested after 6 hours of cultivation in vitro.
Detection of CD45RA/CCR7 expression in Tax+peripheral blood mononuclear cells isolated from asymptomatic carriers and patients with HTLV-I–associated neurological disease
| Patient . | Tax-expressing PBMCs . | |||
|---|---|---|---|---|
| CD45RA©CR7+ . | CD45RA+ CCR7+ . | CD45RA− CCR7− . | CD45RA+ CCR7− . | |
| TAF | 9.8 | 2.1 | 83.9 | 4.2 |
| TAT | 37.4 | 0.6 | 56.7 | 5.1 |
| TXX | 43.9 | 0.4 | 51.9 | 3.7 |
| HH | 22.2 | 0.0 | 71.4 | 6.3 |
| HS | 10.7 | 0.0 | 88.7 | 0.6 |
| HT | 25.8 | 0.0 | 74.2 | 0.0 |
| Patient . | Tax-expressing PBMCs . | |||
|---|---|---|---|---|
| CD45RA©CR7+ . | CD45RA+ CCR7+ . | CD45RA− CCR7− . | CD45RA+ CCR7− . | |
| TAF | 9.8 | 2.1 | 83.9 | 4.2 |
| TAT | 37.4 | 0.6 | 56.7 | 5.1 |
| TXX | 43.9 | 0.4 | 51.9 | 3.7 |
| HH | 22.2 | 0.0 | 71.4 | 6.3 |
| HS | 10.7 | 0.0 | 88.7 | 0.6 |
| HT | 25.8 | 0.0 | 74.2 | 0.0 |
Proportion of CD45RA/CCR7 expression in Tax+ PBMCs isolated from 3 ACs and 3 patients with neurological disease. Cells were harvested after 6 hours of cultivation in vitro.
Discussion
Tax is thought to up-regulate several cellular genes includingIL-2R (CD25) and IL-2.3-8This abnormal gene expression could play a role in the pathogenesis of the inflammatory and malignant diseases associated with the virus. In this study, we used intracellular staining of HTLV-I Tax protein to investigate the cytokine and surface marker phenotype of cells that express Tax. Using this technique, we confirmed that CD4+ lymphocytes naturally infected with HTLV-I spontaneously up-regulate several cellular genes including markers of activation (CD25, CD69) and proliferation (CD71) (Figure 1). We also demonstrate that HTLV-I Tax expression is associated with the production of IFN-γ in a significant proportion of HTLV-I–infected cells (Table 1 and Figure 2).
Two hypotheses could explain the observed cellular activation of Tax-expressing cells. First, Tax expression might directly or indirectly induce the expression of host genes involved in cellular activation. Several reports support this hypothesis and show the transactivating capability of HTLV-I Tax on cellular genes.3-8 However, there is a second possibility. If HTLV-I–infected cells carry a T-cell receptor (TCR) that is specific for HTLV-I epitopes,18 HTLV-I protein expression in vitro could lead to cellular activation of HTLV-I–infected cells through HTLV-I peptide/MHC class II complex presentation. However, Figure 4B shows that the presence of anti-MHC class II mAb did not inhibit the production of IFN-γ by Tax-expressing cells, although it did inhibit the presentation of another MHC class II-restricted antigen. The cellular activation and the spontaneous IFN-γ production associated with Tax expression are therefore most probably due to the direct or indirect transactivating effect of intracellular Tax protein on several cellular genes.
Despite up-regulating CD25, CD69, CD71, and IFN-γ, Tax-expressing PBMCs did not produce detectable levels of intracellular IL-2 within 24 hours of in vitro culture (Table 1 and Figure 2). Samples from HAM/TSP patients and ACs gave similar results. This was not due to an inherent inability of Tax-expressing PBMCs to produce these cytokines because mitogens were able to stimulate the production of IFN-γ, IL-2, and TNF by the same cells (Figure 3 and Table 2). The absence of IL-2 production by Tax-expressing PBMCs naturally infected with HTLV-I contrasts with previous studies that showed transactivation of theIL-2 gene in cells transfected with HTLV-Itax.6-8 However, a critical examination of the previous studies shows that the increase in IL-2 expression was much smaller and later than the increase in CD25 (IL-2Rα) expression. These observations raise the possibility that IL-2 expression is an indirect consequence of Tax expression rather than the result of direct transactivation. In fact, Inoue and colleagues5 reached a similar conclusion. Therefore, our observations do not support the hypothesis that a Tax-induced IL-2/CD25 autocrine loop plays an important part in the HTLV-I–induced proliferation of infected lymphocytes in vivo.5-8 Indirect up-regulation of IL-2 resulting from antigen-driven activation of T cells could, however, contribute to T-cell proliferation. Moreover, because the CD25 molecule is a subunit of both the IL-2 receptor and the IL-15 receptor, it is possible that an autocrine loop exists between CD25 and IL-15 rather than IL-2. Further studies must therefore investigate the effect of HTLV-I Tax on IL-15 production in naturally infected cells. Finally, in the experiments described here we used cells from HTLV-I–infected subjects without malignant disease. Our results do not exclude the possibility that an IL-2/CD25 autocrine loop operates in the development of ATL.
Cellular activation and proinflammatory cytokine production of HTLV-I–infected cells could contribute to the pathogenesis of HAM/TSP disease. It is well established that HTLV-I infection is associated with a high proviral load in peripheral blood.23 Our results suggest that infected cells are potentially harmful and could induce nonspecific inflammatory responses in peripheral tissues. This conclusion is based on the following observations on freshly isolated HTLV-I infected cells: (1) There is abundant Tax expression in vitro and a persistent activation of anti-Tax CTLs13; we concluded that such Tax expression probably also occurs in vivo. (2) Tax expression is associated with cellular activation, as shown by the up-regulation of surface markers of activation and proliferation (Figure 1). (3) Tax expression is also associated with the expression of IFN-γ (Table 1 and Figure 2), which is a proinflammatory and neurotoxic cytokine. (4) Based on the expression of CCR7/CD45RA and CD62L, we determined that Tax- expressing cells exhibit the effector-memory phenotype of T lymphocytes (Figures 5 and6 and Tables 3 and 4), which suggests that such cells can migrate in peripheral tissues including the CNS.14 In this context, it is interesting to note that the risk of HAM/TSP is positively correlated with the magnitude of the proviral load in the blood.23 The probability of an AC developing HAM/TSP might therefore be related to the frequency of infected cells in the blood that are potentially capable of producing proinflammatory cytokines. Further studies must investigate the expression of specific adhesion molecules on Tax-expressing cells. This approach will help to identify the tissues that are targeted by HTLV-I–infected cells in vivo.
CD45RA and CCR7 expression in PBMCs isolated from HTLV-I–infected subjects.
Dot plots showing CD45RA/CCR7 expression in Tax+ and in Tax− PBMCs (TAT).
CD45RA and CCR7 expression in PBMCs isolated from HTLV-I–infected subjects.
Dot plots showing CD45RA/CCR7 expression in Tax+ and in Tax− PBMCs (TAT).
In conclusion, we characterized here the phenotype of PBMCs that are naturally infected with HTLV-I. Tax expression in those cells was associated with cellular activation and with production of IFN-γ, which is a proinflammatory and neurotoxic cytokine. Our observations suggest that these cells are capable of migration in peripheral tissues, including the CNS, and contribute to the inflammatory response. These results have important implications for the pathogenesis of the inflammatory diseases such as HAM/TSP that are associated with HTLV-I infection.
We thank the staff and patients of St Mary's Hospital Medical Trust.
Supported by the Wellcome Trust (United Kingdom) and the Fonds National Belge de la Recherche Scientifique (FNRS, Belgium). E.H. is a Senior Research Assistant of the FNRS. The HTLV European Research Network supported by the European Community Biomed Programme provided a forum for critical appraisal of this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Charles Bangham, Department of Immunology, Imperial College School of Medicine, St Mary's Campus, Norfolk Place, W2 1PG, London, United Kingdom; e-mail: c.bangham@ic.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal