Platelet integrin αIIbβ3 (GPIIb/IIIa) plays a central role in the initiation of arterial thrombosis, but its contribution to disseminated microvascular thrombosis is less well defined. Therefore, wild-type mice (β3+/+), β3-integrin–deficient mice (β3−/−), and wild-type mice treated with a hamster monoclonal antibody (1B5) that blocks murine αIIbβ3 function were tested in models of large-vessel and microvascular thrombosis. In the large-vessel model, ferric chloride was used to injure the carotid artery, and the time to thrombosis was measured. In β3+/+mice, the median time to occlusion was 6.7 minutes, whereas occlusion did not occur in any of the β3−/− mice tested (P < .001). Fab and F(ab')2 fragments of 1B5 increased the median time to occlusion. To initiate systemic intravascular thrombosis, prothrombotic agents were administered intravenously, and platelet thrombus formation was monitored by the decrease in circulating platelet count. Three minutes after the injection of adenosine diphosphate (ADP), collagen + epinephrine, or tissue factor, the platelet counts in β3+/+ mice decreased by 289, 424, and 429 × 103/μL, respectively. β3−/− mice and wild-type mice pretreated with 1B5 Fab (1 mg/kg, IP) were nearly completely protected from the effects of ADP. In contrast, β3−/− mice were only partially protected from the effects of collagen + epinephrine and minimally protected from the effects of tissue factor. In all cases, less fibrin became deposited in the lungs of β3−/− mice than in wild-type mice. These results suggest that though αIIbβ3 plays a dominant role in large-vessel thrombosis, it plays a variable role in systemic intravascular thrombosis.
Introduction
Thrombosis, the leading cause of death worldwide, is marked by heterogeneity in inciting causes, contributing elements, locations, and pathologic consequences. As early as the mid-1800s, Virchow recognized that changes in the blood, the blood vessel wall, and blood flow could all contribute to initiating thrombosis. Many experimental and clinical observations have led to the conclusion that blood platelets play a primary role in the initiation of arterial thrombosis,1,2 but the contribution of platelets to systemic intravascular thrombosis is less well defined.3Advances in understanding platelet physiology have led to the identification of a number of surface receptors that contribute to platelet adhesion, aggregation, or both, and the contributions of these receptors to platelet-mediated thrombosis are being defined.4 Inhibitors of the platelet αIIbβ3 (GPIIb/IIIa) receptor are among the most effective antithrombotic agents in animal models of large-vessel arterial thrombosis.5,6 Three αIIbβ3 antagonists, the Fab fragment of the mouse/human chimeric antibody 7E3 (abciximab), the peptide eptifibatide, and the peptidomimetic tirofiban, have demonstrated efficacy in preventing and treating coronary artery thrombosis in humans.7 Less is known, however, about the contribution of αIIbβ3 to systemic intravascular thrombosis.3
We recently reported that β3-integrin–deficient mice, which lack both αIIbβ3 and αVβ3, have bleeding diatheses and platelet function abnormalities essentially identical to those of patients with Glanzmann thrombasthenia, who lack αIIbβ3, and sometimes aVβ3, receptor function on an inherited basis.8 In the present study, we tested these animals in a number of different models of large-vessel and systemic intravascular thrombosis initiated by different stimuli. Because animals deficient in cell receptors on an inherited basis sometimes display responses that differ from those observed by blocking the receptor with monoclonal antibodies (mAbs) or other antagonists,9,10 we also analyzed the effect of hamster mAbs we previously developed to mouse αIIbβ311 12 in some of the same systems.
Materials and methods
Antibodies
Rabbit anti–mouse thrombocyte polyclonal antibody was from Inter-Cell Technologies (Hopewell, NJ); rabbit anti–human fibrinogen polyclonal antisera was from Behring (Marburg, Germany); mAb 350 specific for the heptapeptide sequence exposed at the new N-terminus of the β chain of fibrin after thrombin cleavage of fibrinopeptide B was from American Diagnostica (Greenwich, CT). Hamster mAb 1B5 and 9C2 against mouse platelet αIIbβ3 were prepared as described.11 Purification of the antibodies was performed by subjecting culture supernatant to 50% (NH4)2SO4 precipitation, followed by chromatography of the dialyzed precipitates on an nProtein A AvidGel F column (Unisyn, Tustin, CA). Bound proteins were eluted by lowering the pH in a stepwise fashion. Fab or F(ab′)2 fragments were prepared by incubating mAb with immobilized papain or pepsin, respectively, and separating Fab and F(ab′)2 fragments from IgG and Fc fragments by chromatography on protein A using either an Fab or an F(ab′)2 ImmunoPure preparation kit (Pierce, Rockford, IL). Fab and F(ab′)2 fragments were dialyzed against sterile, endotoxin-free, 0.9% normal saline before injection into mice. 1B5 IgG was conjugated with Alexa 488 (Molecular Probes, Eugene, OR) according to the manufacturer's instructions.
Fibrinogen and fibrin formation
Mouse fibrinogen was from Sigma Chemical (St Louis, MO). Mouse fibrin was prepared by incubating 0.5 mL mouse fibrinogen (0.25 mg/mL) in 10 mM Tris/HCl, 150 mM NaCl, pH 7.4, with 2 U human α-thrombin (ERL, South Bend, IN) at 37°C for 15 minutes, and then it was dissolved in sodium dodecyl sulfate (SDS)–sample buffer. In some experiments, CaCl2 was included in the reaction to promote factor XIII–mediated cross-linking of fibrin, and the reaction was terminated after 1 hour.
Mice
C57Bl/6 and 129Sv mice were purchased from Jackson Labs (Bar Harbor, ME). The generation of integrin β3−/− mice by homologous recombination in embryonic stem cells has been previously described.8 Wild-type (β3+/+), heterozygous (β3+/−), and β3-null (β3−/−) mice were descendants of F2 intercrosses and were on a mixed C57Bl/6–129Sv background. Mice were weaned at 3 weeks, maintained on a 12-hour light–12-hour dark cycle, and fed water and standard rodent chow (5001; Purina Mills, Richmond, IN) ad libitum. All procedures conformed to the recommendations of the Guide for the Care and Use of Laboratory Animals (Department of Health, Education, and Welfare publication number NIH 78-23, 1996) and were approved by Mount Sinai's Institutional Animal Care and Use Committee.
Blood collection, blood counts, and bleeding time assay
Mice were anesthetized with methoxyflurane (Mallinckrodt Veterinary, Hazelwood, MO), and blood was collected into 0.1 volume of 3.8% sodium citrate by puncture of the retrobulbar venous plexus with a 12- to 15-mm-long glass capillary. Citrated whole blood was analyzed in either a Serono Diagnostic System 9018cp Analyzer (Serono Diagnostics, Allentown, PA) or a Mascot 800 (CDC Technologies, Oxford, CT) set to measure mouse blood cells. The bleeding time assay was performed as described8 13 by cutting the distal 2 mm from the tip of the tail; assays were terminated at 12 minutes if the tail was still bleeding.
Platelet preparation and aggregation studies
Citrated whole blood (350 μL) was centrifuged (800gfor 8 minutes) at 22°C to obtain platelet-rich plasma (PRP). After the PRP was removed, 200 μL modified Tyrode buffer (138 mM NaCl, 2.7 mM KCl, 0.4 mM NaH2PO4, 12 mM NaHCO3, 10 mM HEPES, 5 mM glucose, 0.35% bovine serum albumin, pH 7.35) was added to the red cell pellet, and the suspension was mixed. Platelet-rich buffer (PRB) was obtained by centrifuging (800g for 4 minutes) the suspension at 22°C, and PRB was then combined with the PRP in a mixture of PRP/PRB. Aggregation of platelets in PRP/PRB (1-3 × 108/mL) was performed as previously described.8 For flow cytometry, PRP/PRB was incubated with Alexa 488–labeled mAb 1B5 IgG (5 μg/mL) for 30 minutes at room temperature. Samples were analyzed on an Epics flow cytometer (Coulter, Hialeah, FL).
Thrombosis models
Large-vessel (carotid) artery thrombosis.
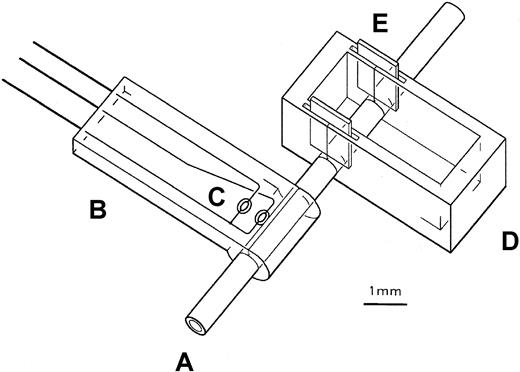
Male mice (10 to 12 weeks old) were anesthestized with methoxyflurane and maintained under anesthesia by inhaling through a nose cone a mixture of isoflurane-saturated air (3.2-5.2 mL/min) mixed with room air (100-130 mL/min). Body temperature was monitored with a rectal probe and maintained at 37°C ± 1°C by varying the output (from 0 to 12V AC) of an EPZ type halogen heat lamp (Sylvania, New York, NY) placed 16 cm from the mouse and aimed at the trunk. The left carotid artery was exposed, dissected free of surrounding tissue, and placed through slits into holes at the bottom of a rectangular “boat” cast from 0.55-mm-thick Sylgard 184 silicone elastomer (Dow Corning, Midland, MI) (2 mm × 2 mm × 4.5 mm) (Figure1). After insertion of the artery, the slits were sealed by inserting 2 mm × 1.5 mm gaskets of 0.13 mm polyester into slots in the walls at both ends of the boat (Figure 1). Changes in vessel temperature, an indicator of blood flow,14 were monitored with a temperature probe placed distal to the boat. The body of the probe was made of silicone elastomer reinforced with silk cloth and contained a transverse cylindrical groove (0.55-mm diameter) at its distal end to hold the artery. Temperature differences between 2 thermistors, one touching the groove and a second approximately 1 mm from the groove, were continuously measured and recorded with Lab View software (National Instruments, Austin, TX). Heat fluctuations were minimized by surrounding the probe with Surgilube and placing a 0.13-mm-thick metal heat conductor on top of the probe. Once a stable signal was obtained, the proximal portion of the artery was clamped for 30 seconds with a microvascular clamp to establish the extent of temperature change with occlusion. To initiate thrombosis, at least 2 minutes after releasing the clamp, 5 to 10 μL 20% (wt/wt) FeCl3 · 6 H2O in Surgilube was added to the boat. Time to maximal temperature reduction was defined as the time from the application of FeCl3 to the onset of the temperature deflection of a sustained reduction—that is, one in which there was a decrease in temperature equal to that observed after clamping the vessel and a duration longer than 30 seconds. Experiments were performed in a blinded manner.
Schematic diagram of instrumented mouse carotid artery.
The proximal portion of the exposed carotid artery (A) is placed in the boat (D) through slits in the side walls, and the distal portion of the artery is placed in the groove of the temperature probe (B). Once the artery is positioned, FeCl3 in Surgilube is placed in the boat to initiate thrombosis. A, artery; B, probe; C, thermistors; D, boat; E, gaskets.
Schematic diagram of instrumented mouse carotid artery.
The proximal portion of the exposed carotid artery (A) is placed in the boat (D) through slits in the side walls, and the distal portion of the artery is placed in the groove of the temperature probe (B). Once the artery is positioned, FeCl3 in Surgilube is placed in the boat to initiate thrombosis. A, artery; B, probe; C, thermistors; D, boat; E, gaskets.
Models of systemic intravascular thrombosis.
Wild-type (β3+/+) and β3-deficient mice matched for sex and age (10-16 weeks) were anesthetized with inhaled methoxyflurane, and 125 μL blood was collected into citrate for the determination of platelet count, hematocrit, and leukocyte count. The right jugular vein was exposed by a lateral neck incision, and a 27-gauge needle was used to inject over 10 seconds 0.1 mL normal saline, 5 μg ADP (Sigma Chemical) in saline, 25 μg collagen (equine tendon type I fibrillar collagen; Chronolog, Havertown, PA) plus 1 μg epinephrine (Sigma), or undiluted recombinant tissue factor reconstituted in sterile water according to the manufacturer's instructions (Innovin; Dade, Miami, FL). One minute after the injection was complete, a second sample of blood was collected. Three minutes after the injection, mice were euthanized by cervical dislocation.
Lung fibrin determination.
The sequential extraction procedure of Olman et al15 was used to quantify fibrin deposition in lung tissue. Lungs were harvested and then rinsed at 4°C in extraction buffer composed of 150 mM NaCl, 10 mM EDTA, 1 mM phenylmethylsulfonyl fluoride (Sigma), 10 U/mL aprotinin (Miles, West Haven, CT), 100 U/mL heparin (from porcine intestinal mucosa, grade I-A; Sigma), 0.1 M ε-aminocaproic acid (Fluka, Milwaukee, WI), and 10 mM Tris/HCl, pH 7.4. Samples were frozen in cryovials on the surface of liquid nitrogen and stored at −20°C until further use. Lung tissue was thawed (by immersing the cryovial in a 37°C waterbath for 15 seconds), minced, homogenized in extraction buffer (0.5 mL buffer/100 mg tissue) for 10 minutes, and incubated on ice for 4 hours. The pellet, obtained after centrifugation at 16 000g for 30 minutes at 4°C, was washed twice, resuspended in 1 mL 8 M urea–4% SDS–2% dithiothreitol, incubated for 18 hours at 37°C, and recentrifuged at 16 000g for 30 minutes at room temperature. The supernatant containing the extracted fibrin was separated by SDS–polyacrylamide gel electrophoresis on a 7.5% gel and transferred to polyvinylidene difluoride membrane (Millipore, Bedford, MA) for immunoblotting. Fibrin β chain was detected by reacting membranes first with a 1:200 dilution of mAb 350, followed by horseradish peroxidase–conjugated antimouse antibody. Bands were visualized with enhanced chemiluminescence (ECL; Amersham, Little Chalfont, Buckinghamshire, United Kingdom). To quantify fibrin, the optical density of bands derived from samples containing equal amounts of lung protein were compared to standard curves of uncross-linked mouse fibrin by scanning densitometry.
Histology, immunohistochemistry, and electron microscopy.
For light microscopy, specimens were fixed with 10% formalin in phosphate-buffered saline (PBS) overnight, embedded in paraffin, and stained with hematoxylin-eosin or modified elastic-tissue Masson trichome. For immunohistochemical analysis, 5-μm sections were deparaffinized, rinsed in xylene, rehydrated, and blocked first with 3% hydrogen peroxide and then with 2% ovalbumin in PBS. Sections were incubated at 37°C for 2 hours with either rabbit anti–mouse thrombocyte polyclonal antibody (1:20 000) or rabbit anti–human fibrinogen polyclonal antibody (1:2000). Sections were then incubated at room temperature for 30 minutes with biotin-conjugated antirabbit secondary antibody (Biogenics, Napa, CA). Bound antibody was detected by reaction with HRP-conjugated streptavidin and diaminobenzidine; specimens were counterstained with hematoxylin. To quantitate the accumulation of thrombi in the lung, the number of thrombi in half a dozen 40 × fields from 5 mice in each treatment group were averaged.
For electron microscopy of carotid arteries, mice were euthanized and then immediately perfused via a cannula in the left ventricle with 3 mL 4% paraformaldehyde in PBS. The injured area of the artery (or the corresponding area of the uninjured contralateral artery) was excised and fixed with 3% glutaraldehyde in 0.1 M cacodylate. Cross-sections of the artery were obtained and prepared for transmission electron microscopy. Blocks containing these sections were dehydrated in graded alcohol solutions and embedded in embed 812 (EMS; Fort Washington, PA.). Thin sections were stained with uranyl acetate and lead citrate and viewed with a JEM 100 CX microscope (JEOL, Tokyo, Japan). For scanning electron microscopy, the remaining artery was sectioned longitudinally, oriented with the lumen exposed, processed by critical point drying, mounted with silver paint, sputter coated with gold-palladium, and examined in a Hitachi S350 scanning electron microscope.
Statistical analysis
Results are expressed as the mean ± SD, unless otherwise indicated. In the thrombosis models, comparisons were made by Mann-Whitney U test. Other results were analyzed by Student t test. P < .05 was considered significant.
Results
Characterization of the in vivo effects of anti-αIIbβ3 mAb 1B5
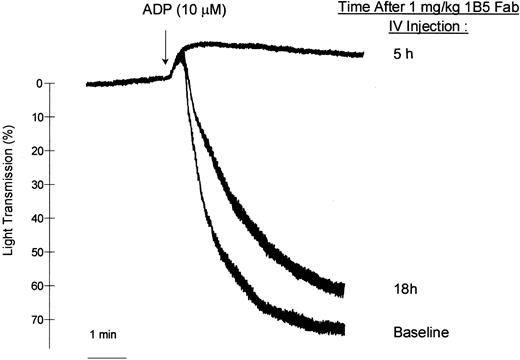
One hour after mice were injected with 1B5 Fab fragments (1 mg/kg, intraperitoneally [IP]) tail bleeding times were all greater than 12 minutes (n = 4), a result that was significantly different (P < .05) from the mean time of 1.5 minutes in untreated mice (n = 3). Neither 1B5 nor 1B5 F(ab′)2 had an effect on platelet count (data not shown). After a single intravenous bolus dose of 0.53 mg/kg 1B5 Fab, initial slopes of platelet aggregation induced by 10 μM ADP were inhibited 85% at 10 minutes, 80% at 1 hour, 50% at 24 hours, and 15% at 5 days. A higher intravenous dose of 1B5 Fab fragments (1 mg/kg) abolished ADP-induced aggregation at 1 hour and 5 hours after injection; 60% inhibition remained at 18 hours (Figure 2), as determined by measuring differences in the initial slope of aggregation. In vivo administration of 1B5 Fab blocked ex vivo binding of 1B5 IgG as measured by flow cytometry, with a time-course consistent with the inhibition of platelet aggregation (Table 1).
1B5 Fab fragments.
Administration of 1B5 Fab fragments (1 mg/kg, IV) inhibits ex vivo ADP (10 mM)–induced platelet aggregation.
1B5 Fab fragments.
Administration of 1B5 Fab fragments (1 mg/kg, IV) inhibits ex vivo ADP (10 mM)–induced platelet aggregation.
Mean fluorescence intensity of Alexa 488IB5 IgG binding to mouse platelets isolated before or at various times after in vivo administration of 1B5 Fab
| Time after injection . | Alexa 488IB5 IgG mean fluorescence intensity . | |
|---|---|---|
| 1B5 Fab 0.53 mg/kg IV . | 1B5 Fab 1 mg/kg IV . | |
| Baseline | 3.30 | 3.10 |
| 7 minutes | 0.68 | ND |
| 1 hour | 0.78 | 0.02 |
| 5 hours | ND | 0.04 |
| 18 hours | ND | 0.60 |
| 24 hours | 1.50 | ND |
| 5 days | 3.10 | ND |
| Time after injection . | Alexa 488IB5 IgG mean fluorescence intensity . | |
|---|---|---|
| 1B5 Fab 0.53 mg/kg IV . | 1B5 Fab 1 mg/kg IV . | |
| Baseline | 3.30 | 3.10 |
| 7 minutes | 0.68 | ND |
| 1 hour | 0.78 | 0.02 |
| 5 hours | ND | 0.04 |
| 18 hours | ND | 0.60 |
| 24 hours | 1.50 | ND |
| 5 days | 3.10 | ND |
Results are from 2 individual mice. Similar results were obtained through the first 6 hours after injection in 2 additional mice receiving 1B5 Fab (1 mg/kg, IP) and 4 mice receiving 1B5 F(ab′)2 (1-2 mg/kg, IP).
ND, not determined.
Carotid artery thrombosis
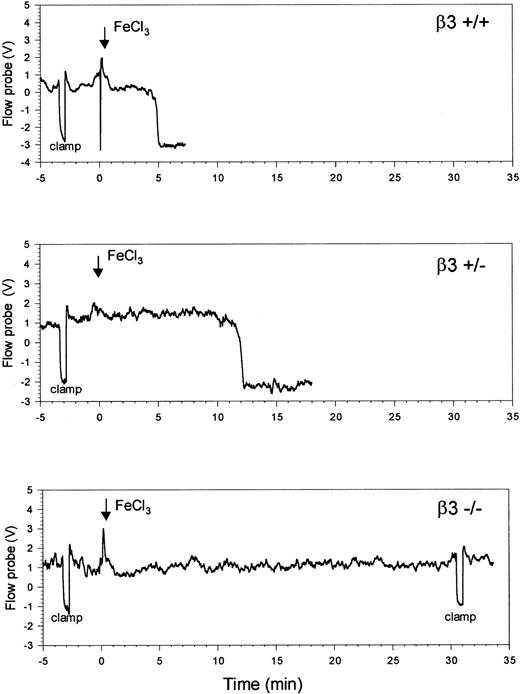
Figure 3 contains typical temperature probe readings from a carotid artery after applying FeCl3 to the mouse carotid artery. In β3+/+mice (n = 7), whose genetic background is a mixture of C57Bl/6 and 129Sv strains, the median time to maximal temperature reduction was 6.7 minutes (mean ± SD of 8.2 ± 3.3 minutes). Time to maximal temperature reduction in pure C57Bl/6 and 129Sv mice was similar to that in the mixed β3+/+ mice, suggesting that there was little variation between the strains (Table2). Median time to maximal temperature reduction in the heterozygous β3+/− mice (n = 8) was 9.8 minutes (mean ± SD of 9.8 ± 1.8 minutes), which was longer than the median time in the C57/129 β3+/+ mice, but the difference was not statistically significant (P = .4). In sharp contrast to the results in the β3+/+ and β3+/− mice, however, no change in temperature occurred for up to 30 minutes in any of carotid arteries of the 5 β3−/− mice tested (P < .001 vs C57/129 β3+/+ mice).
β3-null (β3−/−) mice are protected from FeCl3-induced thrombosis of the carotid artery.
To determine the extent of temperature change during the cessation of blood flow, the arteries were occluded for 30 seconds with a vascular clamp before the application of FeCl3 (arrow). Time to maximal temperature reduction occurred 5 minutes and 12 minutes after the initiation of injury in the β3+/+ mouse and the β3+/− mouse, respectively. Up to 30 minutes after the addition of FeCl3, no temperature reduction occurred in the β3−/− mouse, at which time the clamp was re-applied to document that the vessel was still patent.
β3-null (β3−/−) mice are protected from FeCl3-induced thrombosis of the carotid artery.
To determine the extent of temperature change during the cessation of blood flow, the arteries were occluded for 30 seconds with a vascular clamp before the application of FeCl3 (arrow). Time to maximal temperature reduction occurred 5 minutes and 12 minutes after the initiation of injury in the β3+/+ mouse and the β3+/− mouse, respectively. Up to 30 minutes after the addition of FeCl3, no temperature reduction occurred in the β3−/− mouse, at which time the clamp was re-applied to document that the vessel was still patent.
Time to maximal temperature reduction of carotid arteries after application of FeCl3
| . | Median time (min) . | Mean time ± SD (min) . | n . | P (vs β3+/+) . |
|---|---|---|---|---|
| C57B1/6 | 7.3 | 7.5 ± 0.9 | 4 | NS |
| 129Sv | 7.3 | 7.6 ± 2.4 | 4 | NS |
| β3+/+ C57/129 | 6.7 | 8.2 ± 3.3 | 7 | NS |
| β3+/− C57/129 | 9.8 | 9.8 ± 1.8 | 8 | NS |
| β3−/−C57/129 | > 30 | > 30 | 5 | < .001 |
| . | Median time (min) . | Mean time ± SD (min) . | n . | P (vs β3+/+) . |
|---|---|---|---|---|
| C57B1/6 | 7.3 | 7.5 ± 0.9 | 4 | NS |
| 129Sv | 7.3 | 7.6 ± 2.4 | 4 | NS |
| β3+/+ C57/129 | 6.7 | 8.2 ± 3.3 | 7 | NS |
| β3+/− C57/129 | 9.8 | 9.8 ± 1.8 | 8 | NS |
| β3−/−C57/129 | > 30 | > 30 | 5 | < .001 |
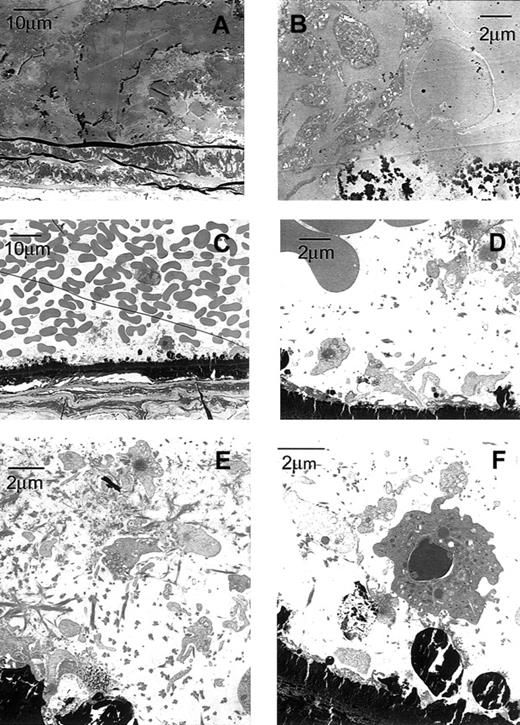
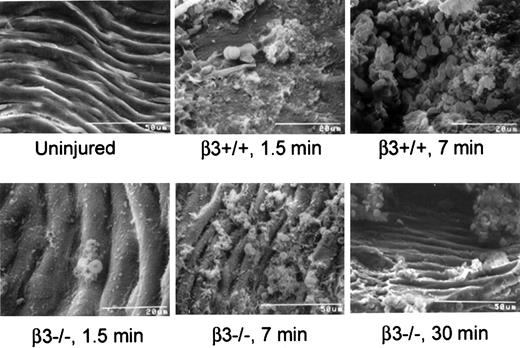
Microscopic evaluation of longitudinal sections of the arteries of wild-type mice (data not shown) revealed that the thrombus occluding the lumen was platelet- and fibrin-rich and, at the ends, contained trapped red cells. Electron microscopy (Figures4, 5) confirmed these findings. In contrast, the lumens of arteries from β3-null mice were lined by essentially a single layer of platelets with overlying red cells but no occlusive thrombus (Figures 4, 5).
Transmission electron micrographs of a carotid artery from a β3+/+ and a β3−/− mouse exposed to FeCl3 demonstrate platelet masses in the β3+/+ mouse and a platelet monolayer in the β3−/− mouse.
The carotid artery of the β3+/+ mouse (A-B) was fixed approximately 7 minutes after the application of FeCl3, whereas the artery in the β3−/− mouse (C-F) was fixed after 30 minutes. Dark-staining FeCl3 accumulated along the internal elastic lamina and, in the wild-type artery, within the thrombus. The vessel in the wild-type mouse was occluded by thrombus composed of platelets, fibrin, and erythrocytes (A-B). Primarily a single layer of platelets adhered to FeCl3-treated vessel wall of the in β3−/− mouse (C-D); fibrin and residual erythrocytes were also present. In addition to attaching directly to the damaged wall, platelets accumulated in areas rich in fibrin along the β3−/− artery (E). Along the damaged vessel in β3−/− mouse, adherent platelets recruited leukocytes, and additional platelets attached to the luminal surfaces of the leukocytes (F).
Transmission electron micrographs of a carotid artery from a β3+/+ and a β3−/− mouse exposed to FeCl3 demonstrate platelet masses in the β3+/+ mouse and a platelet monolayer in the β3−/− mouse.
The carotid artery of the β3+/+ mouse (A-B) was fixed approximately 7 minutes after the application of FeCl3, whereas the artery in the β3−/− mouse (C-F) was fixed after 30 minutes. Dark-staining FeCl3 accumulated along the internal elastic lamina and, in the wild-type artery, within the thrombus. The vessel in the wild-type mouse was occluded by thrombus composed of platelets, fibrin, and erythrocytes (A-B). Primarily a single layer of platelets adhered to FeCl3-treated vessel wall of the in β3−/− mouse (C-D); fibrin and residual erythrocytes were also present. In addition to attaching directly to the damaged wall, platelets accumulated in areas rich in fibrin along the β3−/− artery (E). Along the damaged vessel in β3−/− mouse, adherent platelets recruited leukocytes, and additional platelets attached to the luminal surfaces of the leukocytes (F).
Scanning electron micrographs of carotid arteries of β3+/+ and β3−/− mice exposed to FeCl3 for differing time intervals demonstrate an occlusive platelet- and erythrocyte-rich thrombus in the β3+/+ mice and a platelet monolayer with small erythrocyte accumulations in the β3−/− mice.
Scanning electron micrographs of carotid arteries of β3+/+ and β3−/− mice exposed to FeCl3 for differing time intervals demonstrate an occlusive platelet- and erythrocyte-rich thrombus in the β3+/+ mice and a platelet monolayer with small erythrocyte accumulations in the β3−/− mice.
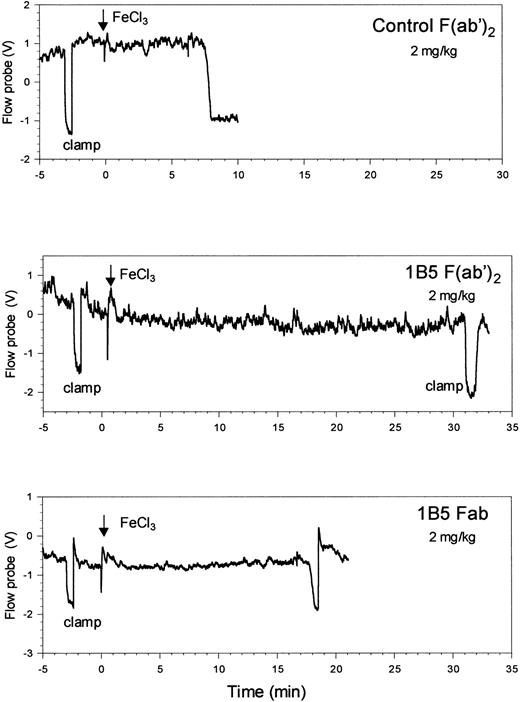
The effect of antibody 1B5 on thrombotic occlusion in this model was tested by injecting 1B5 or control antibodies into wild-type mice. Control hamster mAb Fab (9C2, an anti–mouse αIIbβ3 mAb that does not inhibit receptor function) did not prolong the time to maximal temperature reduction when compared to untreated wild-type animals (Tables 2, 3; mean ± SD time of 7.0 ± 1.5 vs 8.2 ± 3.3, respectively). At 1 mg/kg, 1B5 Fab increased the median time to maximal temperature reduction from the control value range of 7.8 to 8.7 minutes to 12 minutes (P = .2), and 1B5 Fab at 2 mg/kg increased the median time to 21.5 minutes (P = .006) (Table 3, Figure6). Control polyclonal hamster F(ab′)2 also had no effect on the time to maximal temperature reduction, whereas 1B5 F(ab′)2 at 2 mg/kg prolonged the median time to more than 30 minutes (P < .001). Microscopic evaluation demonstrated more thrombus formation than in the β3−/− mice but less than in the arteries of wild-type mice. Treatment of wild-type mice (n = 6) with aspirin at doses sufficient to inhibit ex vivo arachidonic acid-induced platelet aggregation (20 mg/kg, IP) had no effect on the time to maximal temperature reduction (data not shown). Moreover, aspirin did not prolong the time to maximal temperature reduction in mice receiving 2 mg/kg 1B5 Fab (data not shown).
Time to maximal temperature reduction of carotid arteries in wild-type mice pretreated with 1B5 Fab, 1B5 F(ab′)2, or control antibodies
| Treatment . | Median time (min) . | Mean time ± SD (min) . | n . | P . |
|---|---|---|---|---|
| Control 9C2 Fab (1 mg/kg) | 7.8 | 7.0 ± 1.5 | 3 | — |
| 1B5 Fab (1 mg/kg) | 12.0 | 12 ± 0.5 | 3 | .2 vs control Fab |
| 1B5 Fab (2 mg/kg) | 21.5 | 19.8 ± 5 | 3 | .006 vs control Fab |
| Control polyclonal F(ab′)2 (1 mg/kg) | 8.7 | 8.8 ± 1.1 | 3 | |
| 1B5 F(ab′)2 (1 mg/kg) | > 30 | > 30 | 3 | < .001 vs polyclonal F(ab′)2 |
| Treatment . | Median time (min) . | Mean time ± SD (min) . | n . | P . |
|---|---|---|---|---|
| Control 9C2 Fab (1 mg/kg) | 7.8 | 7.0 ± 1.5 | 3 | — |
| 1B5 Fab (1 mg/kg) | 12.0 | 12 ± 0.5 | 3 | .2 vs control Fab |
| 1B5 Fab (2 mg/kg) | 21.5 | 19.8 ± 5 | 3 | .006 vs control Fab |
| Control polyclonal F(ab′)2 (1 mg/kg) | 8.7 | 8.8 ± 1.1 | 3 | |
| 1B5 F(ab′)2 (1 mg/kg) | > 30 | > 30 | 3 | < .001 vs polyclonal F(ab′)2 |
Antibody 1B5 prolongs the time to occlusive thrombus formation in carotid arteries of wild-type mice.
Antibodies (2 mg/kg) were administered by intraperitoneal injection within 1 hour before arterial injury. Time to maximal temperature reduction occurred 8 minutes and 18.5 minutes after the initiation of injury in mice treated with control F (ab′)2 and 1B5 Fab, respectively. No temperature reduction occurred in the mouse treated with 1B5 F(ab′)2 for up to 30 minutes after the application of FeCl3.
Antibody 1B5 prolongs the time to occlusive thrombus formation in carotid arteries of wild-type mice.
Antibodies (2 mg/kg) were administered by intraperitoneal injection within 1 hour before arterial injury. Time to maximal temperature reduction occurred 8 minutes and 18.5 minutes after the initiation of injury in mice treated with control F (ab′)2 and 1B5 Fab, respectively. No temperature reduction occurred in the mouse treated with 1B5 F(ab′)2 for up to 30 minutes after the application of FeCl3.
Models of systemic intravascular thrombosis
To initiate systemic intravascular thrombosis, prothrombotic agents (collagen + epinephrine, tissue factor, or ADP) were administered intravenously, and the incorporation of platelets into thrombi was monitored by the decrease in circulating platelet count after 1 minute.
Baseline platelet counts were similar in β3−/− and β3+/+ mice (540 ± 150 and 530 ± 110 × 103/μL), as were basal white blood cell counts (4.5 ± 2.3 vs 5.9 ± 2.3 × 103/μL), but, as previously described,8 the baseline hematocrit levels were lower in the β3−/− mice (30% ± 8% vs 40% ± 7%).
Injecting saline produced median decreases of 62 × 103and 92 × 103 platelets per microliter in wild-type and β3-null mice. Three minutes after injection of ADP, collagen + epinephrine, or tissue factor, median platelet counts in the wild-type mice decreased by 289 × 103/μL, 424 × 103/μL, and 429 × 103/μL (Table4), indicating a gradation in prothrombotic effects. Essentially, β3−/− mice were completely protected from the effects of injecting ADP because the median platelet count decrease (90 × 103/μL) was no different from that produced by saline (92 × 103/μL). Wild-type mice pretreated with 1B5 Fab (2 mg/kg, IP) were also substantially protected from the effects of ADP, exhibiting minimal changes in median platelet count. In addition, β3−/−mice were partially, but significantly, protected from the effects of injecting collagen + epinephrine (Table 4; P < .01), whereas the β3−/− demonstrated minimal protection from the prothrombotic effects of tissue factor, sustaining a platelet count decrease almost as great as that of the control mice (Table 4).
Median decrease in platelet count (×10−3/μL) 3 minutes after indicated agents were injected
| . | Tissue factor . | Collagen + epinephrine . | ADP . | Saline . |
|---|---|---|---|---|
| WT (β3+/+) | 429 | 424 | 289 | 62 |
| n = 11 | n = 11 | n = 6 | n = 3 | |
| NULL (β3−/−) | 334 | 2684-150 | 904-150 | 92 |
| n = 8 | n = 11 | n = 5 | n = 5 | |
| WT + 1B5 Fab | ND | ND | 1144-150 | ND |
| n = 8 |
| . | Tissue factor . | Collagen + epinephrine . | ADP . | Saline . |
|---|---|---|---|---|
| WT (β3+/+) | 429 | 424 | 289 | 62 |
| n = 11 | n = 11 | n = 6 | n = 3 | |
| NULL (β3−/−) | 334 | 2684-150 | 904-150 | 92 |
| n = 8 | n = 11 | n = 5 | n = 5 | |
| WT + 1B5 Fab | ND | ND | 1144-150 | ND |
| n = 8 |
WT indicates wild type; ND, not determined.
P < .01 (vs WT β3+/+).
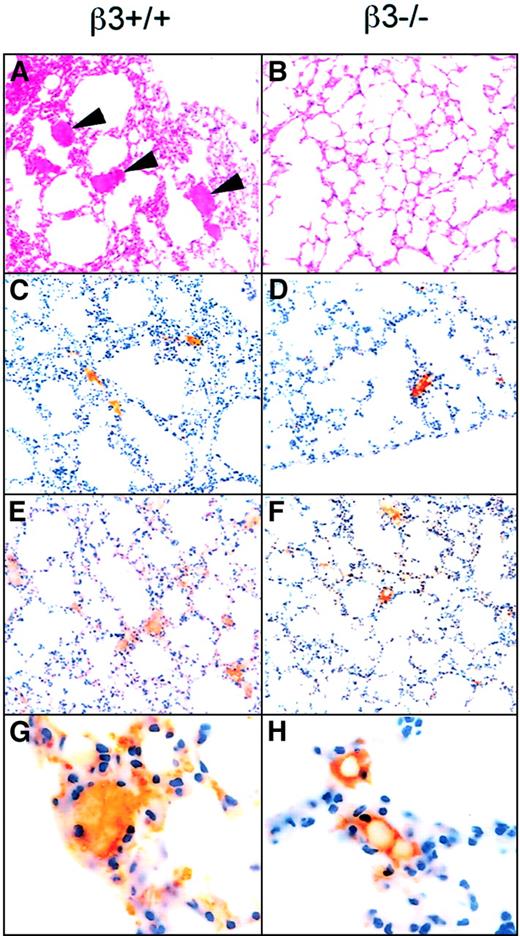
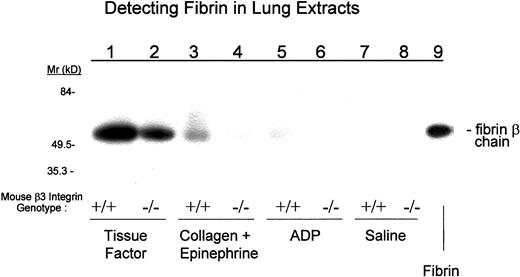
Because thrombi are composed of varying mixtures of platelets and fibrin, we analyzed the microvascular thrombi in the lung for both elements by immunohistochemistry (Figure7), and we analyzed the pulmonary vasculature for fibrin by immunoblotting (Figure8). In all cases, more thrombi were present in the lungs of the wild-type than the β3−/− mice. Tissue factor treatment resulted in a mean of 3 ± 0.4 and 1.7 ± 0.3 thrombi per high power field (hpf) in wild-type and β3−/− mice, respectively (P = .08); collagen + epinephrine resulted in 1.8 ± 0.5 and 0.5 ± 0.4 thrombi per hpf, respectively (P < .01); and ADP resulted in 0.5 ± 0.2 and 0.03 ± 0.2 thrombi per hpf, respectively (P < .01). The amount of fibrin extracted from the lungs of wild-type mice varied depending on the agent injected, with ADP resulting in the least and tissue factor resulting in the most. In all cases, less fibrin accumulated in the lungs of β3-null mice than of wild-type mice (Figure 9, Table5).
Histology and immunohistochemistry with antibodies to platelets and fibrin(ogen) reveal increased platelet- and fibrin(ogen)-rich thrombi in the lungs of β3+/+ mice injected with collagen + epinephrine as compared to β3−/− mice.
Mice were injected with collagen + epinephrine, and 3 minutes later sections of lung tissue were stained with hematoxylin and eosin (panels A-B, 40 ×). Arrowheads point to thrombi in the pulmonary vasculature of a β3+/+ mouse; thrombi were present in the central and peripheral fields of the lung. Fewer thrombi were present in β3−/− mice, and, in the β3−/− mice, the thrombi (more than 70%) tended to be confined to the periphery of the lung tissue. Immunohistochemistry demonstrated that the thrombi were composed of fibrin(ogen) (C-D) and platelets (brown staining; E-F, 40 ×; G-H, 100 ×).
Histology and immunohistochemistry with antibodies to platelets and fibrin(ogen) reveal increased platelet- and fibrin(ogen)-rich thrombi in the lungs of β3+/+ mice injected with collagen + epinephrine as compared to β3−/− mice.
Mice were injected with collagen + epinephrine, and 3 minutes later sections of lung tissue were stained with hematoxylin and eosin (panels A-B, 40 ×). Arrowheads point to thrombi in the pulmonary vasculature of a β3+/+ mouse; thrombi were present in the central and peripheral fields of the lung. Fewer thrombi were present in β3−/− mice, and, in the β3−/− mice, the thrombi (more than 70%) tended to be confined to the periphery of the lung tissue. Immunohistochemistry demonstrated that the thrombi were composed of fibrin(ogen) (C-D) and platelets (brown staining; E-F, 40 ×; G-H, 100 ×).
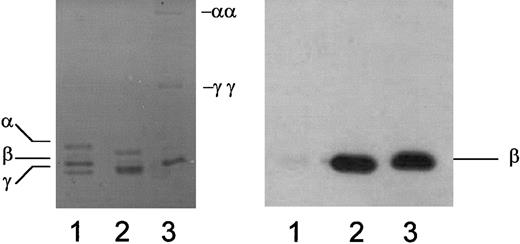
mAb 350 recognizes the β-chain of mouse fibrin but does not react with fibrinogen.
Mouse fibrinogen (lane 1), mouse fibrin prepared in the absence of calcium to limit endogenous factor XIII–mediated cross-linking (lane 2), or mouse fibrin prepared in the presence of calcium to promote endogenous factor XIII–mediated cross-linking (lane 3) was separated by polyacrylamide gel electrophoresis and visualized with Coomassie staining (left) or subjected to immunoblot analysis with mAb 350 (right). γγ, γ-dimer; αα, α polymer.
mAb 350 recognizes the β-chain of mouse fibrin but does not react with fibrinogen.
Mouse fibrinogen (lane 1), mouse fibrin prepared in the absence of calcium to limit endogenous factor XIII–mediated cross-linking (lane 2), or mouse fibrin prepared in the presence of calcium to promote endogenous factor XIII–mediated cross-linking (lane 3) was separated by polyacrylamide gel electrophoresis and visualized with Coomassie staining (left) or subjected to immunoblot analysis with mAb 350 (right). γγ, γ-dimer; αα, α polymer.
After the injection of prothrombotic agents, more fibrin deposits in the lungs of β3+/+ mice than in those of β3−/− mice.
Lung extracts from mice injected with the indicated agents were separated by polyacrylamide gel electrophoresis and subjected to immunoblot analysis with mAb 350 to detect fibrin.
After the injection of prothrombotic agents, more fibrin deposits in the lungs of β3+/+ mice than in those of β3−/− mice.
Lung extracts from mice injected with the indicated agents were separated by polyacrylamide gel electrophoresis and subjected to immunoblot analysis with mAb 350 to detect fibrin.
Lung fibrin deposition 3 minutes after injection of prothrombic agents in wild-type (β3+/+) or β3-null (β3−/−) mice
| . | Fibrin (mg/mL) . | |
|---|---|---|
| WT (β3+/+) . | Null (β3−/−) . | |
| Saline | 2 | 2 |
| ADP | 3 | 2 |
| Collagen + epinephrine | 10 | 3 |
| Tissue factor | 150 | 100 |
| . | Fibrin (mg/mL) . | |
|---|---|---|
| WT (β3+/+) . | Null (β3−/−) . | |
| Saline | 2 | 2 |
| ADP | 3 | 2 |
| Collagen + epinephrine | 10 | 3 |
| Tissue factor | 150 | 100 |
WT indicates wild type.
Discussion
Mice lacking or overexpressing specific genes are uniquely powerful and attractive animals for developing models of human thrombotic disease. However, mouse platelets differ from human platelets in a number of fundamental ways—including smaller size, greater number, alternative thrombin receptors, differences in signal transduction (reviewed in Tsakiris et al16)—and mouse vascular rheology is likely to differ from human as a result of the much higher pulse rate (approximately 600 beats per minute). Therefore, it is unclear whether there are fundamental differences in the thrombotic processes in mice and humans that would invalidate the use of mouse models. Moreover, it is important to recognize the major biologic differences between large-vessel thrombosis and systemic intravascular thrombosis. The current study was designed to address these issues by analysis of the role of the αIIbβ3 platelet receptor in a number of different mouse models of thrombosis. We found that β3-null mice are variably protected from thrombosis based on the method used to initiate the thrombosis.
In the model of carotid artery thrombosis, FeCl3penetrates the blood vessel wall and initiates the production of oxygen radicals, resulting in damage to the endothelium.17 This led to occlusive thrombosis in the carotid arteries of wild-type but not β3-null mice. To improve reproducibility of exposing the carotid artery to FeCl3, we constructed a small boatlike structure to house the FeCl3 and localize its effects. Because the exposed carotid artery was too short to accommodate both the boat and a flow probe, we used a temperature probe to monitor blood flow in the artery. Previous studies by Kurz et al14 demonstrated that reductions in blood flow as measured by a flow probe were highly correlated with changes in temperature. They also demonstrated that reductions in flow velocity did not occur until there was nearly an 80% reduction in the outer diameter of the carotid artery. Our unpublished observations correlating data from a flow probe and our temperature probe indicate that complete or near-complete cessation of blood flow is required to elicit a maximum temperature reduction as measured by our temperature probe. Thus, we conclude that FeCl3 induces occlusive or near-occlusive thrombus formation in the carotid arteries of wild-type but not β3-null mice. Microscopic examination of the arteries supported our conclusions and revealed extensive platelet thrombi, fibrin, and red cells that obstructed the lumen of the carotid arteries in wild-type mice, whereas arteries from β3-null mice, which lack both αIIbβ3 and αVβ3, were patent and lined primarily with only a single layer of platelets. Studies using our mAb 1B5, which blocks the αIIbβ3 receptor but not the αVβ3 receptor, support the interpretation that the findings in β3−/− mice reflect the loss of the αIIbβ3 receptor. They also make it unlikely that the results in the β3−/− mice were due to anemia rather than to the αIIbβ3 defect. Thus, 1B5 prolonged the bleeding time, inhibited ex vivo platelet aggregation, and protected mice from the effects of thrombosis induced by FeCl3. Because the results of these experiments are similar to those obtained with other models of arterial injury5,6 and with percutaneous coronary interventions in large arteries in humans,7 and because there was concordance in the antithrombotic effects using β3-null mice and the mAbs to αIIbβ3, we conclude that insights obtained on large-artery thrombosis with β3-null mice are likely to be relevant to the inhibition of human αIIbβ3 with mAbs or other antagonists.
Many important clinical syndromes involve the entire systemic vasculature rather than just large arteries, among them diffuse intravascular coagulation, thrombotic thrombocytopenic purpura, and sepsis-related microvascular thrombosis. The relative contribution of αIIbβ3 receptors to these phenomena has not been systematically described, making it difficult to assess whether blockade of αIIbβ3 receptors is likely to be of therapeutic value. As a first step in addressing this question, we tested wild-type and β3−/−mice in 3 different models of systemic intravascular thrombosis. These models lack the complexity of the human syndromes, which likely involve several agonists acting in concert. However, by choosing agonists that span the spectrum—from an effect primarily on platelet aggregation (ADP) to an effect primarily on thrombin generation (tissue factor)—they provide insight into the role of αIIbβ3-mediated platelet function in response to these prototypic agonists. Essentially, β3−/− mice were completely protected from the effect of ADP injected intravenously. In contrast, they were only partially protected from the effects of collagen + epinephrine and only minimally protected from the effects of tissue factor. These differences probably reflect differences in the nature of the challenging agent. ADP probably exerts most of its effects by activating platelet αIIbβ3, thereby causing intravascular platelet aggregation. In contrast, tissue factor injection most likely results more directly in fibrin formation, which may then trap platelets in an αIIbβ3-independent manner, perhaps in part through an interaction of platelet GPIb with von Willebrand factor adherent to fibrin.18-20 The decrease in platelet count in wild-type mice after injecting collagen + epinephrine likely reflects 2 separate processes, platelet adhesion to collagen fibrils (through α2β1, GPVI, and perhaps other receptors21 22) and αIIbβ3-mediated platelet–platelet interactions resulting from the release of ADP, thromboxane A2, and perhaps other agents from the adherent platelets. The partial protection against thrombocytopenia in the β3-null mice is consistent with this interpretation given that their platelets are not deficient in collagen receptors, but they are unable to support αIIbβ3-mediated platelet–platelet interactions.
Data from the systemic intravascular thrombosis model initiated by injecting tissue factor are consistent with previous studies demonstrating that Glanzmann thrombasthenia platelets can interact with fibrin but not with fibrinogen.23 They are also consistent with our previous studies in which administering antibody 7E3 F(ab′)2, which inhibits αIIbβ3, to baboons had little impact on the thrombocytopenia and defibrination caused by injecting a sublethal dose of Escherichia coli along with C4b-binding protein (which lowers free protein S).3 The 7E3 F(ab′)2-treated animals did, however, show protection from developing microangiopathic hemolysis and renal insufficiency, suggesting that 7E3 F(ab′)2 prevented intravascular platelet aggregate deposition and resultant ischemic organ damage and the damage to erythrocytes traversing the partially obstructed blood vessels.3 Our data are also in accord with the clinical observation that patients with Glanzmann thrombasthenia are not protected from developing disseminated intravascular coagulation. Seligsohn observed this syndrome in one patient after major blood loss after cesarean section and in another patient after a hemolytic transfusion reaction (U. Seligsohn, personal communication, 2000).
Our observations about the ability of tissue factor to overcome the deficiency of αIIbβ3 in initiating thrombus formation also provide a potential explanation for the reported success in using recombinant activated factor VII, which presumably augments the effects of tissue factor, to treat patients with Glanzmann thrombasthenia who have uncontrolled hemorrhage.24-27 The reverse implication is that optimal therapy of systemic intravascular thrombosis may require inhibition of both tissue factor–mediated fibrin deposition and platelet αIIbβ3. This last consideration takes on particular importance in light of the recent announcement of the premature termination of a clinical trial evaluating the effect of activated protein C on sepsis-related mortality as a result of a beneficial effect of the treatment.28 These findings suggest that systemic intravascular thrombosis may be a major contributor to sepsis-related mortality, which raises the possibility that combined anticoagulant and anti-αIIbβ3 therapy may be more efficacious than treatment with either alone.
B.S.C. has declared financial interest in a drug whose sales may be affected by the results of this present work.
Supported in part by National Heart, Lung and Blood Institute grants 19278 and 54469 (B.S.C.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Barry S. Coller, Laboratory of Blood and Vascular Biology, The Rockefeller University, 1230 York Ave, New York, NY 10021; e-mail: collerb@mail.rockefeller.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal