Treatment of cells with the HIV drugs ritonavir, saquinavir, or nelfinavir (Nfv) inhibits apoptosis induced by a variety of stimuli. Because these drugs are protease inhibitors, they have been postulated to inhibit apoptosis by blocking caspase activity. This study shows that Nfv has no effect on caspase activity or on the transcription or synthesis of a variety of apoptosis regulatory molecules. Instead, Nfv inhibits mitochondrial transmembrane potential loss (Δψm) and the subsequent release of apoptotic mediators. Consequently, the antiapoptotic effects of Nfv are restricted to apoptotic pathways that involve Δψm.
Introduction
Current therapies used in the treatment of HIV-infected patients include combinations of inhibitors of reverse transcriptase and HIV protease. Multidrug therapy has reduced morbidity and mortality among patients infected with HIV1-3 and has led to quantitative and qualitative improvements in host immune function.4-8 Although increases in CD4 T-cell counts typically occur in parallel with a reduction in viral replication, these effects may be seen before significant changes in plasma RNA levels9 or in the absence of antiviral effect.10-13 These and other findings suggest that the protease inhibitor (PI) class of drugs may influence T-cell turnover in a manner that is independent of their role in viral suppression.
Nonviral effects of HIV PIs have been evaluated recently. PIs influence proteasome activity, antigen presentation, cytotoxic T-lymphocyte activity,14 lipoprotein metabolism,15adipocyte differentiation,16,17 cytochrome P450 activity,18 and adenosine triphosphate (ATP)–dependent drug export mechanisms.19 Furthermore, PIs may affect the ability of cells to undergo both spontaneous apoptosis and apoptosis induced by a variety of stimuli.9,20-23 These data are consistent with the in vivo observation that apoptosis in both peripheral blood24 and in lymphatic tissues25is rapidly decreased following initiation of PI-based therapy. In this study, we have evaluated the mechanism by which PIs inhibit apoptosis.
Materials and methods
Cells
Jurkat T cells and H9 cells (American Type Culture Collection, Rockville, MD) were cultured in RPMI 1640 medium (Gibco, Burlington, ON, Canada) supplemented with 10% or 20% fetal calf serum (Gibco), respectively, together with penicillin, streptomycin, and glutamine (Sigma-Aldrich, Oakville, ON, Canada), and were incubated at 37°C in a 5% CO2 humidified environment. Cells were seeded in media supplemented with drugs as indicated at 0.3 × 106/mL and passaged every 2 days.
Drugs
Azidothymidine (AZT) was purchased from Sigma (Oakville, ON, Canada) and resuspended in water. Nelfinavir (Nfv), ritonavir, and saquinavir were generous gifts from Agouron Pharmaceuticals (La Jolla, CA), Abbott Laboratories (Abbott Park, IL), and Roche Laboratories (Nutley, NJ), respectively, and were resuspended in methanol.
Induction and measurement of apoptosis and mitochondrial membrane permeability changes
Jurkat or H9 cells were cultured in the presence of AZT, Nfv, or diluent at indicated concentrations for periods of time ranging from 1 to 5 days before induction of apoptosis. Apoptosis was induced with actinomycin D (ActD; 10 μg/mL; Sigma, Oakville, ON, Canada), camptothecin (CPT; 10 μM; Sigma), an agonistic anti-Fas antibody (CH11; 0.5 μg/mL; Beckman Coulter, Mississauga, ON, Canada), or recombinant synthetic Vpr peptide. For Vpr (Genemed Systems, San Francisco, CA) stimulation, cells were incubated in isotonic buffer with 2.5 μM synthetic Vpr peptide (residues 52-96) for 30 minutes, followed by culture in complete media. After treatment, apoptosis was measured by annexin-V and propidium iodide staining26; mitochondrial membrane permeability changes (Δψm) were determined with the use of 3,3′-dihexyloxacarbocyanine iodide (DiOC6; 40 mM; Molecular Probes, Eugene, OR).27 Samples were analyzed using an Epics ELITE flow cytometer (Beckman Coulter, Missisauga, ON, Canada), and 10 000 events were acquired for each sample. Where indicated, protein synthesis was inhibited by a 1-hour pretreatment with cycloheximide (CHX; 10 μg/mL; Sigma). Protein synthesis was evaluated by biosynthetic labeling with35S-labeled methionine and cysteine. 35S incorporation was measured with the 1450 microbeta liquid scintillation counter (Wallac, Turku, Finland).
Ribonuclease protection assays
Total RNA was isolated with an RNeasy mini kit (Qiagen, Mississauga, ON, Canada) from cells treated with methanol, AZT, or Nfv for 3 days and evaluated by ribonucleotide protection assay (RPA; Pharmingen, Mississauga, ON, Canada). We probed 40 μg RNA with the use of 2 custom templates containing probes for caspase-3, -8, and -9; DCR-1; death receptor 4 (DR-4) and -5; BID; Bfl-1; tumor necrosis factor (TNF)–related apoptosis-inducing factor (TRAIL); TNF receptor (TNFRp55); X-linked inhibitor of apoptosis protein (XIAP); neuronal apoptosis inhibitory protein (NAIP); FAS-associated phosphatase (FAP); cellular inhibitor of apoptosis protein 1 (cIAP-1) and -2; Bcl-2; BclXL; and BclXS. Following gel resolution, band intensities were quantified using the ImageQuant software and normalized to the band intensity of glyceraldehyde-3-phosphate dehydrogenase.
Immunoblotting
Following indicated treatments, protein expression of pro–caspases-3 and -8 was assessed by washing cells in cold phosphate-buffered saline (PBS) and lysing them in buffer (50 mM Tris-HCl, pH 7.4, 1% NP-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 μg/mL aprotinin, leupeptin, and pepstatin, 1 mM Na3VO4, 1 mM NaF) before determining the protein concentration (Pierce, Rockford, IL). Subsequently, 40 μg of protein was subjected to 4% to 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membrane (Millipore, Mississauga, ON, Canada). Membranes were blocked overnight with 5% nonfat dry milk powder in TBST (50 mM Tris-HCl, pH 7.4,150 mM NaCl, 0.1% Tween 20) and immunoblotted for 1 hour at room temperature using antibodies specific for caspase-3 (1:1000; Transduction Laboratories, Lexington, KY) or caspase-8 (1:15; gift from Dr P. Krammer).28 Membranes were washed 5 times with TBST for 5 minutes and exposed to various dilutions of anti-IgG–horseradish peroxidase conjugate (Amersham, Pharmacia Biotech, Oakville, ON, Canada) for 1 hour at room temperature. Proteins were visualized by enhanced chemiluminescence (Amersham, Pharmacia Biotech). The loading and transfer of equal amounts of protein were confirmed by blotting with an anti–proliferating cell nuclear antigen (PCNA) antibody (PC-10, 1:1000; Santa-Cruz Biotechnologies, Santa Cruz, CA).
Cytochrome-c release assay
Jurkat cells were treated overnight with methanol, AZT, or Nfv before being stimulated to undergo apoptosis with CPT. After induction of apoptosis, cells were washed twice with ice-cold PBS, pH 7.4, and pelleted by centrifugation at 200g for 5 minutes. The pellet was resuspended in caspase buffer (100 mM HEPES, pH 7.5, 250 mM sucrose [wt/vol], 0.5 mM EDTA, and 10 mM dithiothreitol [DTT] and PIs) before being lysed in a dounce homogenizer (Kontes Glass, Vineland, NJ). Cytosolic and mitochondrial fractions were separated by centrifugation at 14 000g for 15 minutes29 and blotted for cytochrome c (1:500; Pharmingen). To ensure that the cytosolic fraction contained only cytochrome c that was released from mitochondria, we blotted both cytosolic and mitochondrial fractions for the mitochondrial protein Hsp 70.30
Determination of caspase-1, -3, -6, -7, and -8 activity
The effect of Nfv on the activity of recombinant active caspase-1, -3, -6, and -8 (Biomol, Plymouth Meeting, PA) was assayed with the use of 7-amino-4-trifluoromethyl coumarin (AFC)–conjugated substrates, and the activity of recombinant active caspase-7 was assessed using MCA (7-methoxy coumarin-4 acetyl)–conjugated substrate. Nfv, methanol alone, or caspase inhibitor (100 μm zVAD-FMK for caspase-1, -6, and -7; 100 μm DEVD-FMK for caspase-3; and 100 μm IETD-FMK for caspase-8; Enzyme System Products, Livermore, CA) was added to caspase buffer containing 50 U recombinant enzyme and fluorogenic caspase-specific substrate (WEHD-AFC for caspase-1, DEVD-AFC for caspase-3, VEID-AFC for caspase-6, IETD-AFC for caspase-8, Enzyme System Products; and MCA-VDQVDGW[K-DNP] for caspase-7, Calbiochem, San Diego, CA). Caspase activity was determined after excitation at 400 nm or 325 nm by measuring the fluorescence emission at 505 nm or 392 nm for AFC or DNP conjugates, respectively, using a Cytofluor 2300 Series (Millipore). Results are shown as relative fluorescence relative to controls. As a control for Nfv activity, the ability of Nfv to inhibit cleavage of a fluorogenicgag-pol substrate by HIV protease was also assayed. Recombinant HIV-1 protease (50 U; Bachem, King of Prussia, PA) was added to protease buffer (50 mM NaOAc, pH 4.9, 200 mM NaCl, 5 mM DTT, and 10% glycerol) containing the gag-pol substrate and either Nfv or methanol.
Isolation of Jurkat T-cell mitochondria
Mitochondria from Jurkat T cells were isolated as described previously.31 32 Briefly, Jurkat T cells were resuspended in isolation buffer (0.32 M sucrose, 1.0 mM EDTA, 10 mM Tris-HCl, pH 7.4) and homogenized by hand in a glass dounce homogenizer using 4 up-and-down strokes with the A pestle (0.12 mm clearance) and 8 strokes with the B pestle (0.05 mm clearance). Homogenates were centrifuged at 4000g for 3 minutes, and supernatants were poured into a clean centrifuge tube. The pellet was resuspended in isolation buffer and spun at 4000g for 3 minutes. Supernatants were combined and spun at 16 000g for 10 minutes. The pellet was resuspended in 15% Percoll and layered on a discontinuous density gradient (bottom 40%, middle 23%). Tubes were centrifuged at 19 000g for 5 minutes, and the material at the interface of the lower 2 Percoll layers was diluted 1:4 with isolation buffer and centrifuged at 14 000g for 10 minutes. The pellet was then resuspended in incubation buffer (100 mM KCl, 75 mM mannitol, 25 mM sucrose, 5 mM phosphate [adjusted to pH 7.4 with Tris], 0.05 mM EDTA, and 10 mM Tris-HCl, pH 7.4). All steps were performed at 4°C or on ice.
Statistics
For comparisons of apoptosis between treatment groups, Student t tests were performed. Data presented represent means of experiments with SDs.
Results
Nfv inhibits T-cell apoptosis in a time- and dose-dependent manner
We and others have previously determined that both ritonavir9,20-23 and saquinavir9 have potent antiapoptotic effects in different cellular systems. To test whether these are a generalized effect of this class of drugs, we assessed the antiapoptotic effects of Nfv, an HIV PI whose chemical structure differs from those of ritonavir and saquinavir.33-35Jurkat T cells were incubated with methanol (vehicle control), AZT (non-PI antiviral drug control), ritonavir, saquinavir, or Nfv for 3 days at doses up to 10 μM, and cells were stimulated to undergo apoptosis through Fas receptor ligation. All 3 PIs tested demonstrated a dose-dependent inhibition of apoptosis (data not shown), indicating that the antiapoptotic effects of PIs are common to these members of the PI class.
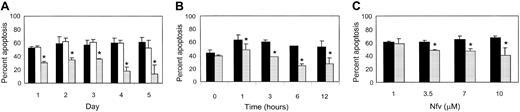
Next we analyzed the effect of Nfv34,35 on apoptosis induced by a variety of other stimuli. Jurkat T cells were pretreated with methanol, AZT, or Nfv, and the percentage of annexin-V–positive, propidium iodide–negative cells was determined daily for 5 days in aliquots of treated cells that were stimulated to undergo apoptosis with ActD, CPT, or an agonistic anti-Fas antibody (clone CH11). Inhibition of CPT-induced apoptosis by Nfv was observed as early as day 1, whereas methanol or AZT had no effect (Figure1A). Similar results were seen with ActD and CH11 (data not shown). Strikingly, resistance to apoptosis was seen within 1 hour of treatment with Nfv (Figure 1B) at a minimal dose of 3.5 μM, and concentrations of 7 μM and 10 μM caused significant inhibition of apoptosis (Figure 1C). Nfv concentrations of 7 μM are similar to trough levels of Nfv achieved in patients receiving treatment36 37; consequently, we chose to use this dose for the remainder of our experiments.
Kinetics of inhibition of apoptosis by Nfv in Jurkat cells.
(A) Jurkat cells were treated with MeOH (diluent control; ▪), 10 μM AZT (non-PI antiviral control; ■), or 7 μM Nfv (░). Apoptosis was induced with 10 μM CPT every day for 5 days and assessed after annexin-V and propidium iodide staining by flow cytometry. Apoptosis was significantly inhibited by Nfv as early as day 1 (P = .01). (Day 2, P = .04; day 3,P = .005; day 4, P = .001; day 5,P = .03; n = 3.) Similar results were also obtained after stimulation with ActD (10 μg/mL) and CH11 (0.5 μg/mL) (data not shown). (B) Jurkat cells were treated with MeOH (▪) or 7 μM Nfv (░) for 0, 1, 3, 6, or 12 hours and stimulated with 10 μM CPT. Apoptosis was assessed as in (A). Nfv inhibited apoptosis after 1 hour of treatment (P = .01), 3 hours (P = .03), 6 hours (P = .02), and 12 hours (P = .004; n = 3). (C) Dose-response curves. Jurkat cells were treated with increasing doses of MeOH (▪) or Nfv (░) overnight and then stimulated with 10 μM CPT. Apoptosis was assessed as in (A). Whereas 1 μM Nfv did not inhibit apoptosis, 3.5 μM (P = .006; n = 3), 7 μM (P = .02; n = 3), and 10 μM (P = .03; n = 3) all inhibited apoptosis.
Kinetics of inhibition of apoptosis by Nfv in Jurkat cells.
(A) Jurkat cells were treated with MeOH (diluent control; ▪), 10 μM AZT (non-PI antiviral control; ■), or 7 μM Nfv (░). Apoptosis was induced with 10 μM CPT every day for 5 days and assessed after annexin-V and propidium iodide staining by flow cytometry. Apoptosis was significantly inhibited by Nfv as early as day 1 (P = .01). (Day 2, P = .04; day 3,P = .005; day 4, P = .001; day 5,P = .03; n = 3.) Similar results were also obtained after stimulation with ActD (10 μg/mL) and CH11 (0.5 μg/mL) (data not shown). (B) Jurkat cells were treated with MeOH (▪) or 7 μM Nfv (░) for 0, 1, 3, 6, or 12 hours and stimulated with 10 μM CPT. Apoptosis was assessed as in (A). Nfv inhibited apoptosis after 1 hour of treatment (P = .01), 3 hours (P = .03), 6 hours (P = .02), and 12 hours (P = .004; n = 3). (C) Dose-response curves. Jurkat cells were treated with increasing doses of MeOH (▪) or Nfv (░) overnight and then stimulated with 10 μM CPT. Apoptosis was assessed as in (A). Whereas 1 μM Nfv did not inhibit apoptosis, 3.5 μM (P = .006; n = 3), 7 μM (P = .02; n = 3), and 10 μM (P = .03; n = 3) all inhibited apoptosis.
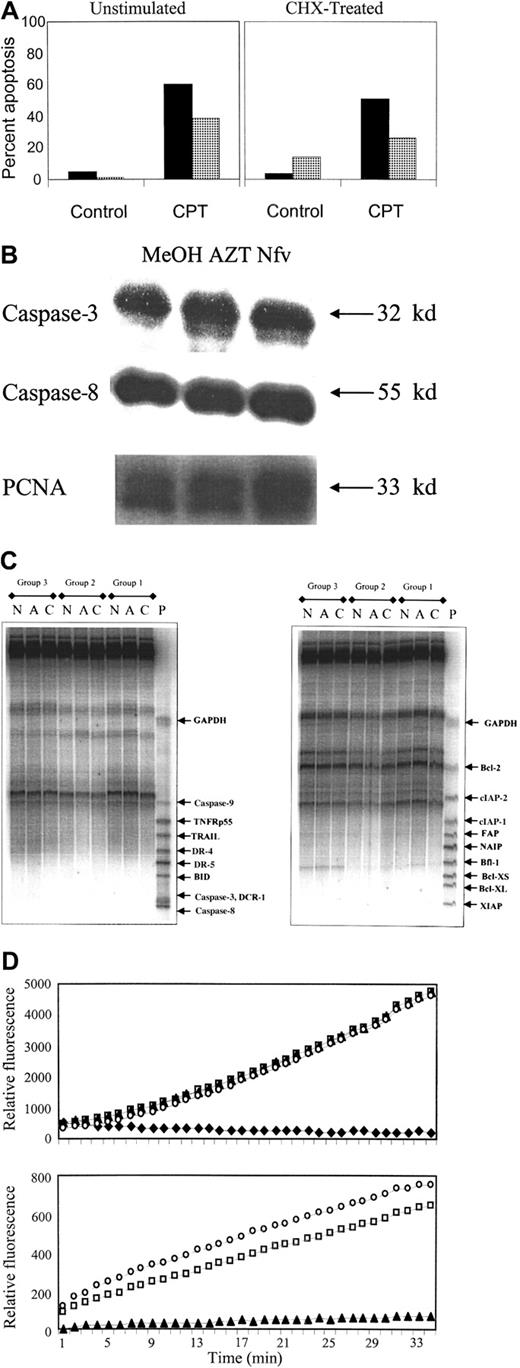
Nfv inhibition of apoptosis is independent of protein and RNA synthesis
Inhibition of apoptosis within 1 hour of Nfv treatment suggests that it acts through a mechanism that is independent of transcription or protein synthesis. To confirm this, we performed 3 different sets of experiments. First, we determined a dose of CHX (10 μg/mL) that inhibits protein synthesis in Jurkat T cells (35S incorporation in Nfv-treated cells without CHX 125 554 CPM,35S incorporation in Nfv-treated cells with 10 fg/mL CHX 64 777 CPM; 51% of control). Cells coincubated with CHX and Nfv showed the same degree of apoptosis inhibition as cells incubated with Nfv alone (Figure 2A), demonstrating that Nfv inhibition of apoptosis is independent of protein synthesis. Next, we assessed 2 caspase family members, pro–caspase-8 (full length 55 kd) and pro–caspase-3 (full length 32 kd), which are necessary for apoptosis after Fas ligation. As shown in Figure 2B, pro–caspase-8 and pro–caspase-3 levels were not altered after incubation with Nfv compared with control cells. Finally, RPAs failed to show differences at the mRNA level for a cross-section of antiapoptotic proteins and proapoptotic proteins in cells pretreated with methanol, AZT, or Nfv for 3 days (Figure 2C). Data from 3 independent experiments (groups 1, 2, 3) failed to show differences between treatments in the amount of mRNA present for caspase-3, -8, or -9; DCR-1; DR-4 or -5; BID; Bfl-1; TRAIL; TNFRp55; XIAP; NAIP; FAP; cIAP-1 or -2; Bcl-2; BclXL; or BclXS.
Nfv does not alter protein or mRNA expression or caspase activity.
(A) Apoptosis was induced by stimulation with 10 μM CPT and assessed after annexin-V and propidium iodide staining by flow cytometry (data representative of 3 independent experiments). (B) Immunoblots of pro–caspase-3 and -8 from whole-cell lysates (40 μg of protein per lane) of Jurkat cells treated overnight with MeOH, 10 μM AZT, or 7 μM Nfv. The PCNA immunoblot confirms equal loading. Jurkat control cells (left panel) or cells pretreated for 1 hour with 10 μg/mL CHX (right panel) before overnight culture with MeOH (▪) or 7 μM Nfv (░). (C) RPA of mRNA (40 μg per lane) from Jurkat cells treated for 3 days with MeOH, 10 μM AZT, or 7 μM Nfv. P indicates probe; C, MeOH; A, AZT; N, Nfv; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (D) Active human recombinant caspase-3 was incubated with its fluorogenic substrate (DEVD-AFC) alone (■) or in the presence of 100 μM DEVD-FMK (♦), 7 μM Nfv (▴), or MeOH (○), and the kinetics were assessed over 30 minutes (top panel). Similar results were also obtained with active human recombinant caspase-1, -6, -7, and -8 (data not shown). Data are representative of at least 3 independent experiments for each caspase tested. HIV-1 protease cleavage of gag/pol fluorogenic substrate was assessed alone (■), with 7 μM Nfv (▴), or with methanol (○) (bottom panel).
Nfv does not alter protein or mRNA expression or caspase activity.
(A) Apoptosis was induced by stimulation with 10 μM CPT and assessed after annexin-V and propidium iodide staining by flow cytometry (data representative of 3 independent experiments). (B) Immunoblots of pro–caspase-3 and -8 from whole-cell lysates (40 μg of protein per lane) of Jurkat cells treated overnight with MeOH, 10 μM AZT, or 7 μM Nfv. The PCNA immunoblot confirms equal loading. Jurkat control cells (left panel) or cells pretreated for 1 hour with 10 μg/mL CHX (right panel) before overnight culture with MeOH (▪) or 7 μM Nfv (░). (C) RPA of mRNA (40 μg per lane) from Jurkat cells treated for 3 days with MeOH, 10 μM AZT, or 7 μM Nfv. P indicates probe; C, MeOH; A, AZT; N, Nfv; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (D) Active human recombinant caspase-3 was incubated with its fluorogenic substrate (DEVD-AFC) alone (■) or in the presence of 100 μM DEVD-FMK (♦), 7 μM Nfv (▴), or MeOH (○), and the kinetics were assessed over 30 minutes (top panel). Similar results were also obtained with active human recombinant caspase-1, -6, -7, and -8 (data not shown). Data are representative of at least 3 independent experiments for each caspase tested. HIV-1 protease cleavage of gag/pol fluorogenic substrate was assessed alone (■), with 7 μM Nfv (▴), or with methanol (○) (bottom panel).
Nfv does not inhibit caspase activity
Because PIs inhibit the proteolytic activity of the viral protease, it has been postulated that they might have a similar effect on cellular proteases.20 21 Because caspases (cysteine-specific aspartyl proteases) are key regulators of the apoptotic cascade, we chose to assess whether Nfv could inhibit caspase activity. Recombinant active caspase-1, -3, -6, -7, or -8 was incubated in the presence or absence of Nfv and fluorogenic tetrapeptide substrates specific for each respective caspase. Following cleavage of the fluorogenic substrate by active caspases, fluorescence is released, allowing assessment of caspase activity as a function of time. As shown in Figure 2D, Nfv did not inhibit caspase-3 activity (top panel); similarly, Nfv had no effect on the activity of caspase-1, -6, -7, or -8 (data not shown). To confirm the activity of Nfv, we demonstrated that Nfv inhibited HIV protease cleavage of a fluorogenicgag/pol consensus site (bottom panel).
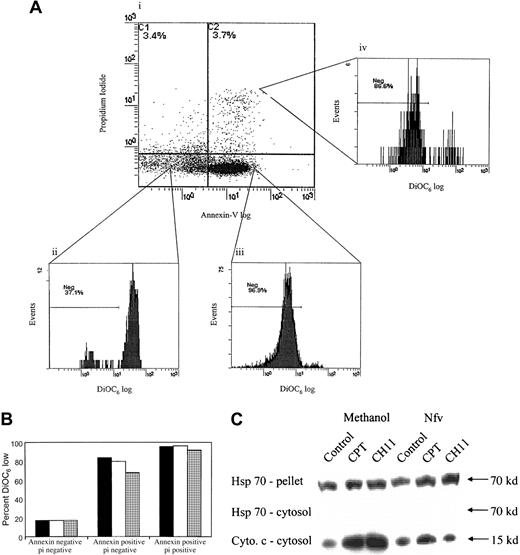
Nfv blocks loss of mitochondrial transmembrane potential
Mitochondrial transmembrane potential loss (Δψm) is seen in many forms of apoptosis and serves to link activation of early initiator caspases with activation of downstream effector proteases and nucleases. We therefore analyzed whether Nfv-induced inhibition of apoptosis occurs above or below the level of mitochondrial permeability changes. Jurkat T cells were incubated in the presence of methanol, AZT, or Nfv and stimulated to undergo apoptosis with ActD, CPT, or CH11. Apoptosis was determined using annexin-V and propidium iodide, and Δψm was assessed using the cationic lipophilic dye DiOC6.27 38DiOC6 stains mitochondria with intact mitochondrial transmembrane potential, whereas mitochondria that have lost transmembrane potential lose their ability to retain the dye (DiOC6 low). In Nfv-treated samples, the apoptotic fraction (annexin-V positive, propidium iodide negative) had a decreased proportion of DiOC6-low cells (Figure3A,B), indicating that Nfv-treated cells that became apoptotic had less loss of transmembrane potential than untreated cells. Together our data suggest that Nfv may inhibit apoptosis by virtue of inhibiting mitochondrial Δψm.
Nfv inhibits loss of mitochondrial membrane potential (Δψm) as well as cytochrome-c release and caspase-8 cleavage.
(A) Jurkat cells were treated overnight with 10 μM CPT. Δψm of cells in the (i) dead (annexin negative propidium iodide positive), (ii) healthy (annexin negative propidium iodide negative), (iii) apoptotic (annexin positive propidium iodide negative), and (iv) early dead (annexin positive propidium iodide positive) cell populations were simultaneously assessed using DiOC6. (B) Cumulative data from (A) refer to the percentage of cells exhibiting a DiOC6-low phenotype. Data are representative of 3 independent experiments. pi indicates propidium iodide. (C) Nfv treatment inhibits cytochrome-c release. Jurkat cells were treated overnight with MeOH or 7 μM Nfv, and apoptosis was induced by stimulation with 10 μM CPT or 0.5 μg/mL CH11 for 8 hours, as indicated. Immunoblots were then performed of Hsp 70 in the mitochondrial pellet (upper) and cytosol (middle), and of cytochrome-c release in cytosol (lower; 40μg of protein per lane). Blots are representative of 2 independent experiments.
Nfv inhibits loss of mitochondrial membrane potential (Δψm) as well as cytochrome-c release and caspase-8 cleavage.
(A) Jurkat cells were treated overnight with 10 μM CPT. Δψm of cells in the (i) dead (annexin negative propidium iodide positive), (ii) healthy (annexin negative propidium iodide negative), (iii) apoptotic (annexin positive propidium iodide negative), and (iv) early dead (annexin positive propidium iodide positive) cell populations were simultaneously assessed using DiOC6. (B) Cumulative data from (A) refer to the percentage of cells exhibiting a DiOC6-low phenotype. Data are representative of 3 independent experiments. pi indicates propidium iodide. (C) Nfv treatment inhibits cytochrome-c release. Jurkat cells were treated overnight with MeOH or 7 μM Nfv, and apoptosis was induced by stimulation with 10 μM CPT or 0.5 μg/mL CH11 for 8 hours, as indicated. Immunoblots were then performed of Hsp 70 in the mitochondrial pellet (upper) and cytosol (middle), and of cytochrome-c release in cytosol (lower; 40μg of protein per lane). Blots are representative of 2 independent experiments.
Nfv inhibits the release of apoptotic mediators from mitochondria
To determine whether Nfv affects the mitochondrial events of apoptosis, we examined cytochrome-c release in cells induced to undergo apoptosis, with or without prior Nfv treatment. CPT induces apoptosis via a direct effect on mitochondria, resulting in permeability transition pore complex (PTPC) opening, loss of Δψm, and subsequent release of cytochrome c and apoptosis-inducing factor (AIF), resulting in postmitochondrial activation of caspase-9, -3, and -7.39-41 Cytosolic and mitochondrial fractions of Jurkat T cells stimulated with CPT were blotted for cytochrome c. Nfv-pretreated cells demonstrated a decrease in cytochrome-c release from the mitochondria as compared with control cells (Figure 3C, bottom). This suggests that Nfv inhibits apoptosis at or above the level of loss of mitochondrial Δψm.
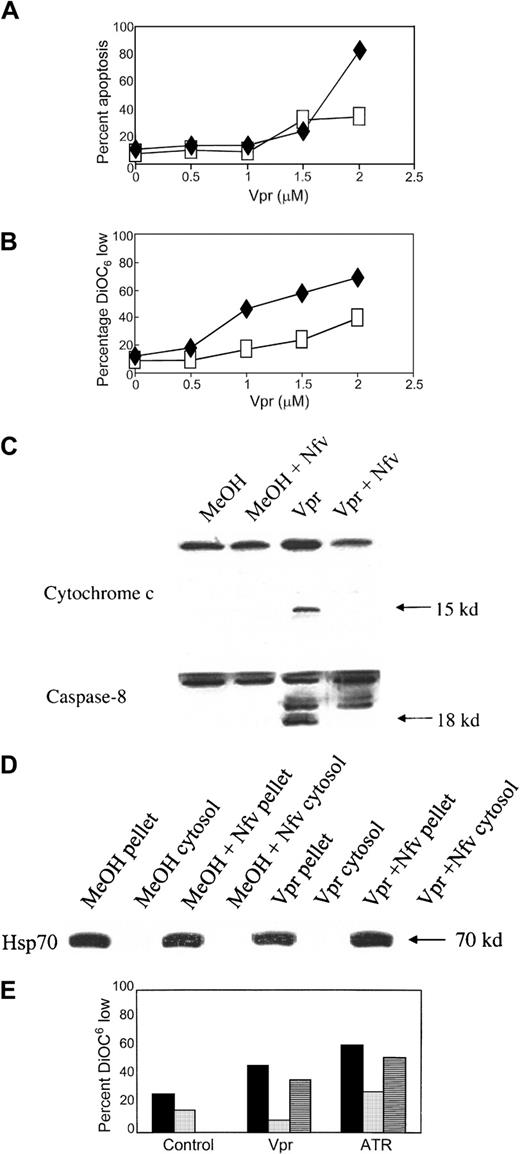
Nfv inhibits mitochondrial PTPC opening
To confirm the postulate that Nfv inhibits apoptosis at the mitochondrial level, we analyzed the effects of Nfv on apoptosis induced by factors that directly open the mitochondrial PTPC. The PTPC is a multiprotein channel that is believed to consist of a voltage-dependent anion channel (VDAC), adenine nucleotide translocator (ANT), cyclophilin D, and peripheral benzodiazepine receptor.38,42 PTPC opening results in loss of mitochondrial Δψm and the release of cytochrome c into the cytosol.38 Cytochrome c, together with Apaf-1 and in the presence of dATP, forms the apoptosome,43 which initiates apoptotic signaling downstream of the mitochondria by mediating autocatalysis of pro–caspase-9.44 The HIV accessory molecule Vpr, when added to intact cells or isolated mitochondria, causes Δψm and consequent release of cytochrome c and AIF,45 possibly mediated by binding of Vpr to VDAC or ANT. These effects of Vpr are inhibited by both Bcl and bongkrekic acid (BA), which is a ligand for ANT and inhibits PTPC opening.46
To determine whether Nfv inhibits PTPC opening, we incubated intact Jurkat cells with recombinant Vpr (peptides 52-96) in the presence or absence of Nfv. If Nfv functions to prevent PTPC opening, it should block Vpr-induced cell death. Wild-type Vpr at a concentration of 2 μM induced significant apoptosis that was inhibited by Nfv (Figure4A). Furthermore, Nfv treatment resulted in a decrease in the percentage of cells that exhibited a DiOC6-low phenotype (Figure 4B), indicating that Nfv inhibited loss of mitochondrial transmembrane potential in these cells. Western blot analyses confirmed that cells coincubated with Vpr and Nfv had less release of cytochrome c and less pro–caspase-8 cleavage than cells treated with Vpr alone (Figure 4C).
Nfv inhibits apoptosis induced by PTPC opening.
(A) Jurkat T cells were pretreated with MeOH (♦) or 7 μM Nfv (■) for 1 hour before overnight stimulation with the indicated doses of Vpr. Apoptosis was assessed by annexin-V and propidium iodide. Data are representative of 3 independent experiments. (B) Mitochondrial Δψm was assessed using DiOC6. Data are representative of 3 independent experiments. ♦, Vpr; ■, Vpr + Nfv. (C) Jurkat cells were treated with MeOH or 7 μM Nfv for 1 hour before overnight stimulation with 2.5 μM bovine serum albumin (BSA) or 2.5 μM Vpr and immunoblotted for cytochrome-c release (upper) and pro–caspase-8 cleavage (lower) in the cytosolic fraction. (D) Immunoblot of Hsp 70 in pellet and cytosolic fractions. (E) Isolated mitochondria were treated overnight with methanol (▪), 7 μM Nfv (░), or 50 μM BA (▤) before being stimulated with 2.5 μM Vpr or 5 mM ATR. Loss of Δψm was assessed using DiOC6.
Nfv inhibits apoptosis induced by PTPC opening.
(A) Jurkat T cells were pretreated with MeOH (♦) or 7 μM Nfv (■) for 1 hour before overnight stimulation with the indicated doses of Vpr. Apoptosis was assessed by annexin-V and propidium iodide. Data are representative of 3 independent experiments. (B) Mitochondrial Δψm was assessed using DiOC6. Data are representative of 3 independent experiments. ♦, Vpr; ■, Vpr + Nfv. (C) Jurkat cells were treated with MeOH or 7 μM Nfv for 1 hour before overnight stimulation with 2.5 μM bovine serum albumin (BSA) or 2.5 μM Vpr and immunoblotted for cytochrome-c release (upper) and pro–caspase-8 cleavage (lower) in the cytosolic fraction. (D) Immunoblot of Hsp 70 in pellet and cytosolic fractions. (E) Isolated mitochondria were treated overnight with methanol (▪), 7 μM Nfv (░), or 50 μM BA (▤) before being stimulated with 2.5 μM Vpr or 5 mM ATR. Loss of Δψm was assessed using DiOC6.
To characterize further the effect of Nfv on mitochondrial PTPC opening, we induced PTPC opening in isolated mitochondria. Atractyloside (ATR) interacts with ANT and directly causes loss of Δψm with the release of AIF and cytochrome c,46 and these effects of ATR are directly inhibited by BA. Mitochondria were isolated from Jurkat T cells and incubated overnight with BA, Nfv, or methanol (as vehicle control). Following this incubation, the mitochondria were treated with ATR (5 mM) or Vpr (2.5 μM), and PTPC opening was assessed by DiOC6staining. Although BA partially inhibited PTPC opening in response to both Vpr and ATR (Figure 4E), Nfv also blocked PTPC opening in response to both Vpr and ATR, to even greater degrees than BA alone (Figure 4E). Our cumulative data therefore indicate that Nfv inhibits apoptosis via a direct effect on PTPC opening.
Effect of Nfv on type-I Fas signaling
If PIs block apoptosis through inhibition of PTPC opening, then apoptosis that does not depend on mitochondrial activation should be unaffected by PI pretreatment. Fas receptor ligation may activate one of 2 different pathways that lead to apoptosis.47-49 Cells with a type-II Fas signaling pathway recruit Fas-associated death domain (FADD) to the trimerized Fas receptor, causing caspase-8 activation and cleavage of BID, which in turn leads to PTPC opening and loss of mitochondrial Δψm. This results in release of cytochrome c; activation of caspase-9, -7, and -3; and finally DNA fragmentation and cell death.39-41 By contrast, in cells with a type-I Fas signaling pathway, caspase-8 activation immediately causes caspase-3 activation, thereby bypassing the requirement for loss of mitochondrial Δψm. Jurkat T cells use a type-II pathway in response to Fas receptor ligation, whereas H9 cells use a type-I signal transduction pathway.47-49 We therefore evaluated the effect of Nfv on H9 cells treated with either Fas, which induces apoptosis independent of changes in mitochondrial Δψm, or with CPT, which induces apoptosis via loss of mitochondrial Δψmχιτ50 (Figure5). Whereas Nfv-pretreated H9 cells underwent the same amount of apoptosis as control cells in response to Fas ligation, Nfv inhibited CPT-induced apoptosis in H9 cells. Thus, Nfv inhibits apoptotic pathways that require mitochondrial PTPC opening, but does not inhibit pathways that are independent of mitochondria.
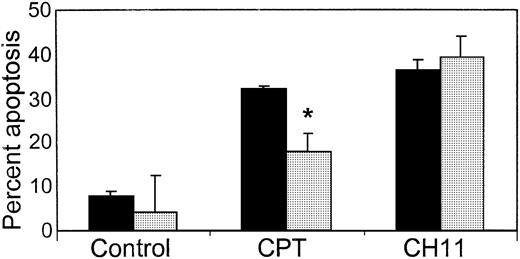
Nfv inhibits CPT- but not CH11-mediated apoptosis in type-I cells.
H9 cells were treated overnight with MeOH (▪) or 7 μM Nfv (░) and stimulated with 10 μM CPT or 0.5 μg/mL CH11, and apoptosis was assessed using annexin-V and propidium iodide. Nfv treatment significantly inhibited CPT-induced (P = .03) but not CH11-induced apoptosis (n = 3).
Nfv inhibits CPT- but not CH11-mediated apoptosis in type-I cells.
H9 cells were treated overnight with MeOH (▪) or 7 μM Nfv (░) and stimulated with 10 μM CPT or 0.5 μg/mL CH11, and apoptosis was assessed using annexin-V and propidium iodide. Nfv treatment significantly inhibited CPT-induced (P = .03) but not CH11-induced apoptosis (n = 3).
Discussion
HIV principally infects CD4+ lymphocytes and cells of the monocyte/macrophage lineage.51,52 The genome of HIV codes for several propeptides that must undergo posttranslational proteolytic cleavage to yield functional proteins. This is accomplished by a virally encoded aspartyl protease. Inhibition of HIV protease blocks this process and, consequently, has become a major therapeutic strategy. Several factors make HIV protease an ideal therapeutic target, including the observation that structural and enzymatic proteins generated after proteolytic cleavage of the propeptides are necessary for viral maturation and infectivity53 and that HIV protease is not present in uninfected cells.54 Also, current HIV PIs demonstrate specificity and do not significantly inhibit the human aspartyl proteases pepsin, renin, gastricin, and cathepsins E and D.55
This study demonstrates that the HIV PI Nfv inhibits Jurkat T-cell apoptosis induced by a variety of different stimuli, including ActD (a chemotherapeutic agent),56 CPT (a topoisomerase I inhibitor),50 and an agonistic anti-Fas antibody (clone CH11).57 We further show that inhibition of apoptotic death is not related to alterations at the mRNA expression level of a variety of pro- and antiapoptotic factors, as determined by RPA, and does not depend on protein synthesis. Whereas others have reported that PIs may affect the generation of active caspase-1 (ICE) or caspase-3 (CPP32),20 21 our experiments show that Nfv has no effect on the ability of recombinant caspase-1, -3, -6, -7, and -8 to cleave their tetrapeptide consensus cleavage sequences.
The mitochondrial transmembrane potential that results from the asymmetric distribution of ions on both sides of the inner mitochondrial membrane is normally maintained by the mitochondrial membrane PTPC.58,59 Soon after receiving an apoptotic signal, the mitochondrial PTPC opens, disrupting transmembrane potential46,60-62 and releasing apoptogenic factors including cytochrome c,63 pro–caspase-9,64and AIF.46 65 We analyzed Nfv-treated Jurkat cells following CPT induction of apoptosis and monitored Δψmwith DiOC6. Our results demonstrate maintenance of mitochondrial transmembrane potential levels in the annexin-V–positive, propidium iodide–negative (therefore apoptotic) Nfv-treated cell population, suggesting that Nfv inhibits loss of Δψm, a concept that is supported by the finding that Nfv pretreatment also inhibits cytochrome-c release and caspase-8 activation in CPT-treated Jurkat cells.
To confirm that Nfv inhibits apoptosis by influencing mitochondria, we next assessed the effects of Nfv on Vpr-induced apoptosis. Vpr is cell permeable and directly interacts with the PTPC, resulting in its opening and the subsequent release of apoptogenic factors45 from mitochondria. Vpr-induced apoptosis was inhibited by Nfv, therefore supporting the hypothesis that Nfv inhibits apoptosis by preventing PTPC opening. Indeed, these data are further confirmed by experiments using isolated mitochondria, in which Nfv inhibited PTPC opening induced by both ATR and Vpr, to degrees greater than that seen by BA, a known inhibitor of PTPC opening. Finally, we treated H9 cells with apoptotic stimuli that either require (CPT) or bypass (Fas ligation) mitochondrial PTPC opening. Whereas Nfv inhibited CPT-induced apoptosis in H9 cells, it did not inhibit apoptosis induced by Fas ligation, therefore confirming that Nfv inhibits apoptosis via an effect on mitochondrial PTPC opening.
The clinical significance of antiapoptotic effects of PIs remains to be fully defined; however, both the antiapoptotic effect on CD4+ T cells9,20-23 and putative antiapoptotic effects on CD34+ progenitor cells22 may serve to increase CD4+ cell number in HIV-infected patients independent of the drug's effects on viral replication. Whether this effect is significant in vivo has been disputed,66 yet it is supported by several distinct lines of evidence: (1) enhanced CD4+ T-cell levels with PI-containing versus -sparing regimens that achieve equivalent suppression of viral replication,10,67 (2) increases in CD4+ T-cell number despite no observable antiviral effect,11-13 (3) greater increases in CD8+ T-cell number after initiation of PI therapy than non-PI therapy,68 and (4) dramatic decreases in CD4+ T-cell number after discontinuation of PI-containing therapy, even when PI therapies were unsuccessful in achieving viral suppression.11 69 Together with in vitro observations of antiapoptotic effects, these observations argue in favor of a clinically significant effect of PI-containing therapy on CD4+ T-cell number as compared with PI-sparing therapies. However, until results from ongoing prospective trials of PI-containing versus -sparing therapies of equally high potency are available, these effects remain speculative.
Our cumulative data are consistent with previous reports9,20-23 in which Nfv as well as ritonavir and saquinavir have been shown to inhibit apoptosis; however, in contrast to previous reports suggesting that HIV PIs inhibit the enzymatic activity of caspases,20 21 we demonstrate that Nfv inhibits apoptosis via an effect on mitochondrial PTPC opening. These results have implications not only for the care of patients infected with HIV, but potentially for the therapy of other diseases associated with enhanced levels of apoptosis.
We gratefully acknowledge the expert administrative assistance of Ms A. Carisse, as well as helpful discussions and careful manuscript review by Dr D. Lynch, Dr R. Ogden, Dr B. Badley, and the entire Badley lab. B.N.P. and J.J.L. have received Studentship Awards from the OHTN.
Supported by the Medical Research Council of Canada, the Canadian Foundation for AIDS Research (CANFAR), the AIDS Program Committee of Ontario, and the Doris Duke Foundation (A.D.B.). A.D.B. has received a Career Scientist Award from the Ontario HIV Treatment Network (OHTN).
A.D.B. has received consultant fees from Agouron, Roche, and Abbott and unrestricted educational grants from Abbott and Agouron for studies unrelated to this paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Andrew D. Badley, Division of Infectious Diseases, Ottawa Hospital Research Institute, 501 Smyth Road, Ottawa, ON K1H 8L6, Canada; e-mail: abadley@ottawahospital.on.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal