Human T-cell leukemia virus type I is the etiologic agent of adult T-cell leukemia/lymphoma. The Tax protein of this virus is thought to contribute to cellular transformation and tumor development. In this report, we have used a Tax transgenic mouse model of tumorigenesis to study the contribution of nuclear factor (NF)-κB activity to spontaneous tumor cell proliferation and resistance to apoptosis. We have demonstrated elevated expression levels of NF-κB–inducible cytokines, including interleukin (IL)-6, IL-10, IL-15, and interferon (IFN)-γ, in freshly isolated primary tumors from Tax transgenic mice. Inhibitors of NF-κB activity, sodium salicylate and cyclopentenone prostaglandins (prostaglandin A1 and 15-deoxy-Δ(12,14)-prostaglandin J2), blocked spontaneous proliferation of Tax transgenic mouse spleen cells. In addition, Tax-induced tumor cells, which are resistant to irradiation-induced apoptosis, became sensitive to apoptosis in the presence of sodium salicylate and prostaglandins. These results strongly suggest that Tax-mediated induction of NF-κB activity contributes to tumorigenesis in vivo.
Introduction
Human T-cell leukemia virus type I (HTLV-I) is the causative agent of adult T-cell leukemia/lymphoma (ATLL) and is associated with a variety of inflammatory diseases, including HTLV-I–associated myelopathy/tropical spastic paraparesis.1-4 HTLV-I Tax is a nuclear phosphoprotein that transactivates viral gene expression by activating cellular transcription factors which, in turn, bind Tax-responsive elements located in the viral long terminal repeat.5-7 Tax has been shown to transactivate cellular gene transcription by acting on proteins such as cAMP response element/activating transcription factor family members,6,8 serum response factors,9and Rel/nuclear factor (NF)-κB proteins.10-12 This is thought to contribute to deregulated T-cell proliferation and transformation.
The Rel/NF-κB proteins in mammalian cells consist of 5 members: p50, p52, p65, c-Rel, and RelB.13,14 These family members contain a Rel homology domain, a conserved region of approximately 300 amino acids, which is involved in dimerization, DNA binding, and nuclear localization. These proteins form homodimers or heterodimers with other family members and positively regulate a number of cellular genes whose products mediate immune and inflammatory responses as well as lymphoproliferation. In particular, NF-κB stimulates production of cytokines, including interleukin (IL)-1, IL-2, IL-6, IL-8, IL-10, IL-15, tumor necrosis factor (TNF), and interferon (IFN).13-16 In resting lymphocytes, NF-κB dimers are sequestered in the cytoplasm in an inactive form by association with an inhibitory IκB subunit. Following cellular activation, multiple kinase cascades lead to serine phosphorylation of IκB by IκB kinases (IKKs) and proteosome-mediated degradation, resulting in the release of an active NF-κB complex that translocates to the nucleus. In the nucleus, NF-κB transactivates genes containing specific consensus sequences in their 5′ transcriptional regulatory regions.13-15
There is mounting evidence that NF-κB dysregulation is important for tumorigenesis. For instance, NF-κB activity was shown to protect Epstein-Barr virus (EBV)-infected cells from apoptosis induced by TNF-α, contributing to increased proliferation of B lymphoblastoid cells.17 TNF-α coactivates the NF-κB pathway, which is then able to limit apoptosis, presumably through up-regulation of anti–apoptotic gene transcription.18 However, in the absence of new protein synthesis, TNF-α–induced apoptosis proceeds through caspase activation. Indeed, activation of NF-κB has been reported to block caspase-8 activity.18,19 TNF-α can also induce apoptosis in EBV-transformed lymphocytes in which NF-κB activity is inhibited, and this is associated with decreased expression of the anti–apoptotic bcl-2 and bcl-xlgenes.20 Research indicates the latent membrane protein (LMP)-1 of EBV activates NF-κB by associating with TNF receptor (TNFR)-associated factors (TRAFs 1 and 2) and TNFR-associated death domain protein (TRADD).21,22 NF-κB has also been shown in transfection experiments to suppress p53-mediated apoptosis by competition for limiting quantities of transcription factors, specifically p300 and CREB binding protein.23
Several studies have indicated that NF-κB is constitutively active in Tax-expressing and HTLV-I–infected T-cell lines as well as primary ATLL cells.24,25 Furthermore, HTLV-I–transformed cells and tumor cells from ATLL patients have been reported to express elevated levels of cytokines containing NF-κB enhancer elements. These include IL-1, IL-2, IL-6, IL-8, IL-10, IL-15, TNF-α, and IFN-γ.4,16,24-28 There is evidence that Tax induces the degradation of IκBα, which may allow for constitutive activation of NF-κB in HTLV-I–infected T-cell lines.29-31 An additional step in this process is thought to involve formation of nuclear structures that contain Tax colocalized with NF-κB subunits p50 and RelA along with components of the cellular transcription and splicing machinery.32 Studies utilizing an infectious HTLV-I clone have demonstrated that mutations in Tax that disrupt NF-κB activation prevent cellular immortalization, while mutations that disrupt CREB/ATF and SRF activation have no effect on immortalization.60,61 Tax-induced activation of NF-κB has also recently been shown to correlate with inactivation of p53 function in T cells.33 Importantly, p53 inactivation could be overcome by addition of an IκB mutant that prevents NF-κB activation or by expressing Tax in p65-deficient cells.33Furthermore, Tax-mediated induction of Bcl-xl expression through NF-κB was reported to be associated with development of IL-2 independence and resistance to apoptosis in mouse T cells.34 35
We have previously reported that transgenic mice expressing HTLV-I Tax from the human granzyme B promoter develop primary, peripheral lymphomas at 6 to 9 months of age, and these lymphomas spread to the mesenteric lymph nodes, bone marrow, spleen, liver, and lungs.36 These tumors consist primarily of CD8+ T cells and natural killer (NK) cells. Tumor cells exhibit elevated production of IL-1α and IL-1β, IFN-γ, granulocyte-macrophage colony-stimulating factor, and constitutive cell surface expression of intercellular adhesion molecule-1, lymphocyte function–associated antigen-1, and very late antigen-4.26We have since reported that fresh tumors and tumor-derived cell lines from Tax transgenic mice are resistant to irradiation-induced apoptosis and that tumor and spleen cells from Tax transgenic mice proliferate spontaneously when placed in culture.62 In this study, we have more closely analyzed cytokine expression in tumor cells from Tax transgenic mice and have used anti-inflammatory inhibitors of NF-κB, sodium salicylate and cyclopentenone prostaglandins, to determine the contribution of this pathway to spontaneous proliferation of tumor cells and resistance to apoptosis.
Materials and methods
Reagents
Sodium salicylate was obtained from Sigma (St Louis, MO), and 1 M stock solutions were prepared. Prostaglandin A1 (PGA1) and 15-deoxy-Δ(12,14)-prostaglandin J2 (15dPGJ2) were purchased from Cayman Chemical (Ann Arbor, MI). Neutralizing rat antimouse monoclonal antibodies to IL-6 and IL-10 were purchased from PharMingen (San Diego, CA). Rabbit antimouse polyclonal antibodies to IFN-γ and GST were a generous gift from Dr Bob Schreiber (Washington University, St. Louis, MO). Monoclonal antibodies to the murine IL-2 receptor β (IL-2Rβ) chain were produced from a hybridoma (TM-β1)37 and purified on immobilized protein G columns (Pierce, Rockford, IL).
Mouse tissues
Granzyme B–Tax transgenic mice were generated as previously described.36 All mice were bred and maintained under pathogen-free conditions in accordance with Washington University animal care guidelines. Fresh tumors and tissues removed from mice were released into RPMI (Life Technologies) supplemented with 10% fetal bovine serum, 1% L-glutamine, 1% sodium pyruvate, and 1% penicillin/streptomycin. Erythrocytes in splenocyte preparations were lysed with 155 mM ammonium chloride, and cells were washed prior to culture at 37°C. The tumor-derived F8 and SC large granular lymphocytic (LGL) cell lines have been described elsewhere and were maintained in RPMI.26 36 When indicated, splenocytes from nontransgenic (NT) control mice were treated with 500 U/mL IL-2 and 10 μg/mL phytohemagglutinin (PHA).
Nuclear extracts
Tissues from transgenic mice were dispersed in 1 mL of cell lysis buffer (40 mM KCl, 10 mM Hepes, pH 7.0, 3 mM MgCl2, 1 mM DTT, 5% glycerol, 7 μg/mL aprotinin, 2 μg/mL leupeptin, 0.5 mM PMSF, and 0.2% Nonidet P40 (NP-40) (vol/vol), incubated for 10 minutes at 4°C, and nuclei pelleted at 14 000g for 10 minutes. Nuclei were resuspended in nuclear extract solution (20 mM Hepes, pH 7.9, 0.42 M KCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, 0.5 mM PMSF, 25% glycerol (vol/vol), incubated for 30 minutes at 4°C, dialyzed for 24 hours at 4°C against 20 mM Hepes, pH 7.9, 0.1 M KCl, 0.2 mM EDTA, 0.5 mM DTT, 0.5 mM PMSF, 20% glycerol (vol/vol), quantitated by the Bradford assay, and stored at −80°C.
NF-κB DNA binding activity
DNA binding activity was measured by 2 techniques. Electrophoretic mobility gel shift assays were performed with [32P]end-labeled and -annealed oligonucleotides corresponding to the NK-κB binding site of the murine Ig kappa gene with the following sequence: CCGGTTAACAGAGGGGGCTTTCCGAG. Binding reactions were performed for 15 minutes at room temperature with or without 100-fold excess of cold oligonucleotide with 5 μg of nuclear extract, 1 μg of poly (dI-dC) (Amersham) and 1 × Superdex buffer (25 mM Hepes, pH 7.9, 12.5 mM MgCl2, 10 uM ZnSO4, 150 mM KCl, 4 mM 2-mercaptoethanol, 20% [vol/vol] glycerol, 0.1% NP-40) and 50 000 cpm of labeled oligonucleotide. The DNA-protein complexes were resolved by electrophoresis on a 5% polyacrylamide gel for 4 hours at 165 V at 4°C in 1 × TGE buffer (25 mM Tris-Cl, pH 8.5, 190 mM glycine, 1 mM EDTA).
Alternatively, using an ELISA assay, p65-p50 DNA binding activity was measured with 10 μg of nuclear extract with the Trans-AMTM kit according to the manufacturer's recommendations (Active Motif, Rixensart, Belgium). Background levels obtained using 100-fold levels of cold competitor were subtracted from each experimental determination.
RNase protection assays
Total RNA was extracted either directly from mouse tissues or from cultured cells using TRI reagent (Sigma). Cultured mouse spleen and tumor cells were treated with sodium salicylate or blocking antibodies for 16 hours at 37°C prior to RNA extraction. RNA (10 μg) was used in ribonuclease (RNase) protection assays using the Riboquant multiprobe cytokine system (PharMingen).
ELISAs
Cells from mouse spleen or primary tumor tissues (10 × 106 or 5 × 106 cells per well, respectively) were added to 6-well plates in 3 mL RPMI, cultured for 16 hours at 37°C, and supernatants were collected. Standard enzyme-linked immunosorbent assays (ELISAs) using the OptE1A mouse IL-6 and IL-10 systems were carried out according to the manufacturer's instructions (PharMingen). The absorbance was read at 450 nm using an ELISA reader.
Thymidine incorporation assays
Mouse spleen cells (10 × 106 cells per well) were added to 6-well plates in 3 mL RPMI and cultured for 4 hours following sodium salicylate treatment or 2 hours following prostaglandin treatment. Then, 1.1 MBq per well of [3H]thymidine was added to cultures and incubated for 14 hours at 37°C. Cells were harvested onto glass filters, and thymidine incorporation was quantitated by liquid scintillation counting. Cell viability was also determined by trypan blue exclusion prior to cell harvest.
Apoptosis assays
Fresh tumor and spleen cell suspensions were incubated in the presence or absence of 10 mM sodium salicylate for 20 hours prior to treatment with 20 Gy (2000 rad) γ-irradiation. Five hours postirradiation, 1 × 105 cells were dual-stained with fluorescein isothiocyanate–conjugated antibody against annexin V and propidium iodide as described by the manufacturer (PharMingen). Apoptotic cells were measured by FACS analysis on a FACScan flow cytometer (Becton Dickinson). Fresh tumor and spleen cells treated with PGA1and 15dPGJ2 were incubated 16 hours prior to annexin V staining and FACS analysis.
Results
NF-κB activity in Tax-induced tumors
We have previously shown that tumors from Tax transgenic mice express elevated levels of IFN-γ and GM-CSF, cytokines known to be activated by Tax through the NF-κB pathway.26 Therefore, we sought to determine whether NF-κB activity is elevated in Tax transgenic tumors compared to uninvolved tissues. For this purpose, electrophoretic mobility shift analyses were performed with a double-stranded radiolabeled oligonucleotide corresponding to the NF-κB binding site of the murine Ig kappa gene, in the presence or absence of 100-fold excess of unlabeled competitor oligonucleotide. Figure 1 shows retarded NF-κB-DNA complexes derived from nuclear extracts from peripheral tumors arising on the nose, leg, or foot, as well as extracts from fresh splenocytes. Control tissues without tumor infiltration, eg heart, showed no NF-κB activity (Figure 1).
NF-κB activity in Tax transgenic mouse tumors.
Nuclear extracts were prepared from Tax transgenic tissues, and representative results from 3 animals (#524, #542, and #579) are shown. Tissues used included tumors arising on the nose, leg, or foot, as well as spleens with tumor involvement or uninvolved heart tissue. Electrophoretic mobility gel-shift assays were performed in the absence or presence of 100-fold excess of unlabeled oligonucleotide (indicated as “+ Cold Oligo”). The positions of the retarded NF-κB-DNA bands are indicated by the arrows on the left, indicating the positions of DNA bound to the p65-p50 heterodimer and p50-p50 homodimer of NF-κB.
NF-κB activity in Tax transgenic mouse tumors.
Nuclear extracts were prepared from Tax transgenic tissues, and representative results from 3 animals (#524, #542, and #579) are shown. Tissues used included tumors arising on the nose, leg, or foot, as well as spleens with tumor involvement or uninvolved heart tissue. Electrophoretic mobility gel-shift assays were performed in the absence or presence of 100-fold excess of unlabeled oligonucleotide (indicated as “+ Cold Oligo”). The positions of the retarded NF-κB-DNA bands are indicated by the arrows on the left, indicating the positions of DNA bound to the p65-p50 heterodimer and p50-p50 homodimer of NF-κB.
An ELISA format was also used to examine DNA binding activity of p65-p50 NF-κB heterodimers in tissues from Tax transgenic mice (Table1). Significantly higher levels of NF-κB activity were seen in peripheral tumors than splenocytes from Tax transgenic mice, and both were higher than uninvolved tissues, including heart, kidney, and brain. The effects of several inhibitors of NF-κB induction were also examined using this assay (Table2). Concentrations of 1 mM or 10 mM sodium salicylate were tested and resulted in 19% and 32% inhibition of NF-κB activity, respectively. Prostaglandin A1(PGA1) and 15-deoxy-Δ12,14-prostaglandin J2 (15dPGJ2), both tested at 20 μM, resulted in inhibition of NF κB activity of 46% and 32%, respectively.
NF-κB activity in Tax transgenic mouse tissues
| Tissue . | Optical density1-153 . |
|---|---|
| Peripheral tumors* | 0.493 ± 0.186 |
| Splenocytes† | 0.140 ± 0.012 |
| Control tissues‡ | 0.015 ± 0.010 |
| Tissue . | Optical density1-153 . |
|---|---|
| Peripheral tumors* | 0.493 ± 0.186 |
| Splenocytes† | 0.140 ± 0.012 |
| Control tissues‡ | 0.015 ± 0.010 |
Nuclear extracts were prepared from 6 peripheral tumors from 6 different animals, of which 5 tumors arose on an extremity and 1 tumor arose on the nose.
Nuclear extracts were prepared from uncultured splenocytes of 3 different animals.
Nuclear extracts were prepared from 4 tissues from 2 animals (from heart, 2 kidneys, and brain).
p50-p65 NF-κB DNA binding activity was measured in an ELISA format. Means and standard deviations are listed.
NF-κB activity in cultured splenocytes from Tax transgenic mice
| Culture . | Inhibition . |
|---|---|
| 1 mM sodium salicylate | 19% |
| 10 mM sodium salicylate | 32% |
| 20 μM PGA1 | 46% |
| 20 μM 15 dPGJ2 | 31% |
| Culture . | Inhibition . |
|---|---|
| 1 mM sodium salicylate | 19% |
| 10 mM sodium salicylate | 32% |
| 20 μM PGA1 | 46% |
| 20 μM 15 dPGJ2 | 31% |
Splenocytes were cultured from 2 different mice for 18 hours in the indicated concentrations of each inhibitor, and the mean level of inhibition is listed.
Cytokine expression in Tax-induced tumors
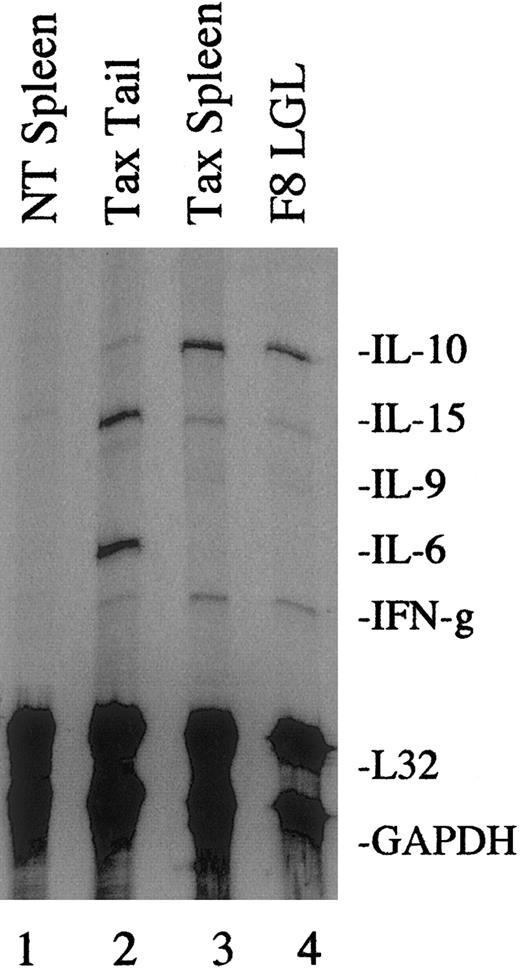
We have previously observed that tumor cells from Tax transgenic mice undergo spontaneous proliferation when placed in culture in the absence of growth factors;62 therefore, we utilized RNAse protection assays with multiple cytokine probes to perform a more extensive analysis of cytokine mRNA expression in freshly isolated tumors. As shown in a representative gel in Figure2, primary tail tumors (lane 2), spleen tumors (lane 3), and the F8 tumor–derived cell line (lane 4) each expressed elevated levels of IL-10, IL-15, and IFN-γ compared with spleens from NT control littermates (lane 1). Primary, peripheral tumors also expressed elevated levels of IL-6 (lane 2).
Cytokine mRNA expression in Tax transgenic mouse tumors.
Total RNA (10 μg) was extracted from fresh NT (lane 1) and Tax transgenic mouse tissues (lanes 2 and 3) or from the tumor-derived F8 LGL cell line (lane 4) and used in RNase protection assays using a multiprobe cytokine system. The L32 and GAPDH probes were used to demonstrate equivalent RNA levels in each sample. A total of 4 primary Tax-induced tumors and 5 Tax spleens were analyzed, and a representative gel is shown.
Cytokine mRNA expression in Tax transgenic mouse tumors.
Total RNA (10 μg) was extracted from fresh NT (lane 1) and Tax transgenic mouse tissues (lanes 2 and 3) or from the tumor-derived F8 LGL cell line (lane 4) and used in RNase protection assays using a multiprobe cytokine system. The L32 and GAPDH probes were used to demonstrate equivalent RNA levels in each sample. A total of 4 primary Tax-induced tumors and 5 Tax spleens were analyzed, and a representative gel is shown.
Levels of IL-6 and IL-10 secreted from tumor cells were verified by ELISA following overnight growth in culture. Unfortunately, we were unable to test for production of IL-15 by this method because of a lack of reliable antibodies to mouse IL-15. As shown in Figure3A, IL-6 secretion was elevated in primary Tax tail and foot tumors and spleen cells compared with spleen cells from NT control mice. As shown in Figure 3B, IL-10 secretion was abundant in the F8 and SC tumor–derived cell lines grown in culture. However, IL-10 levels in Tax tumors and spleen cells were similar to levels in NT control spleens following overnight growth in culture. The discrepancy between IL-10 mRNA and protein expression is likely due to changes in cytokine expression that occur when cells are grown in vitro, which may not reflect the levels expressed in tumors in vivo.
IL-6 and IL-10 production in Tax transgenic mouse tumors.
A total of 107 cells from NT and Tax transgenic mouse spleen or primary tumor tissues and tumor-derived F8 and SC LGL cell lines were cultured for 16 hours. Supernatants were used in ELISAs to measure (A) IL-6 and (B) IL-10 production. Error bars represent the percent SE of 3 separate experiments using tissues from 3 NT and 3 Tax transgenic mice.
IL-6 and IL-10 production in Tax transgenic mouse tumors.
A total of 107 cells from NT and Tax transgenic mouse spleen or primary tumor tissues and tumor-derived F8 and SC LGL cell lines were cultured for 16 hours. Supernatants were used in ELISAs to measure (A) IL-6 and (B) IL-10 production. Error bars represent the percent SE of 3 separate experiments using tissues from 3 NT and 3 Tax transgenic mice.
To determine whether the cytokines produced by Tax-induced tumors contribute to autocrine cell growth as demonstrated in other systems,38-41 we treated cells with neutralizing antibodies to IL-6, IL-10, and IFN-γ, either alone or in combination. Additionally, we used an antibody generated against the IL-2Rβ chain, which has been shown to block IL-15 activity.37 Following a 16-hour treatment with antibodies, the levels of [3H]thymidine incorporation were measured. Addition of blocking antibodies, either alone or in combination, did not affect proliferation of Tax tail tumor or spleen cells (data not shown). These experiments were repeated for incubation periods ranging from 16 to 48 hours and with doses of neutralizing antibodies ranging from 0.5 to 20 μg/mL, and no significant difference was observed compared with untreated cultures (data not shown).
Sodium salicylate abrogates proliferation and cytokine expression in Tax-induced tumor cells
Because IL-6, IL-10, IL-15, and IFN-γ have each been described as NF-κB–responsive genes,13,14,16 we set out to determine whether NF-κB activity specifically contributes to spontaneous proliferation of Tax-induced tumor cells. To do this, we treated tumor cells with sodium salicylate, an anti-inflammatory inhibitor of IKKβ activity.42 Following a 16-hour treatment with sodium salicylate, the levels of [3H] incorporation were measured. For these experiments, we measured proliferation of spleen cells only, because their proliferation levels were consistently 3- to 4-fold higher than those of primary tumor cells (Figure 4). Spleen cells from NT control mice were also stimulated with IL-2 and PHA for 16 hours while Tax spleen cells were left untreated. As shown in Figure 4A, proliferation of Tax spleen cells was significantly inhibited by treatment with 10 mM sodium salicylate but not with 1 mM sodium salicylate. The percentage of viable cells was determined by trypan blue exclusion after treatment with 10 mM sodium salicylate, and no effect on viability was seen after treatment for 18 hours (Figure 4B).
Sodium salicylate and cyclopentenone prostaglandins inhibit spontaneous proliferation of Tax transgenic mouse splenocytes.
(A) A total of 107 spleen cells from NT and Tax transgenic mice were cultured for 4 hours following sodium salicylate or mock treatment. NT splenocytes were also stimulated with 500 U/mL IL-2 plus 10 μg/mL PHA. Then, 1.1 MBq per well of [3H]thymidine was added to cultures, and thymidine incorporation was measured after a 16-hour incubation. (B) The percent cell viability was determined by trypan blue exclusion prior to cell harvest. (C) A total of 107 spleen cells from NT and Tax transgenic mice were cultured for 2 hours following PGA1, 15dPGJ2, or mock treatment. NT splenocytes were also stimulated with 500 U/mL IL-2 plus 10 μg/mL PHA. [3H]thymidine was added to cultures, and thymidine incorporation was measured 14 hours later. All experiments were performed in triplicate, and error bars represent the percent SE.
Sodium salicylate and cyclopentenone prostaglandins inhibit spontaneous proliferation of Tax transgenic mouse splenocytes.
(A) A total of 107 spleen cells from NT and Tax transgenic mice were cultured for 4 hours following sodium salicylate or mock treatment. NT splenocytes were also stimulated with 500 U/mL IL-2 plus 10 μg/mL PHA. Then, 1.1 MBq per well of [3H]thymidine was added to cultures, and thymidine incorporation was measured after a 16-hour incubation. (B) The percent cell viability was determined by trypan blue exclusion prior to cell harvest. (C) A total of 107 spleen cells from NT and Tax transgenic mice were cultured for 2 hours following PGA1, 15dPGJ2, or mock treatment. NT splenocytes were also stimulated with 500 U/mL IL-2 plus 10 μg/mL PHA. [3H]thymidine was added to cultures, and thymidine incorporation was measured 14 hours later. All experiments were performed in triplicate, and error bars represent the percent SE.
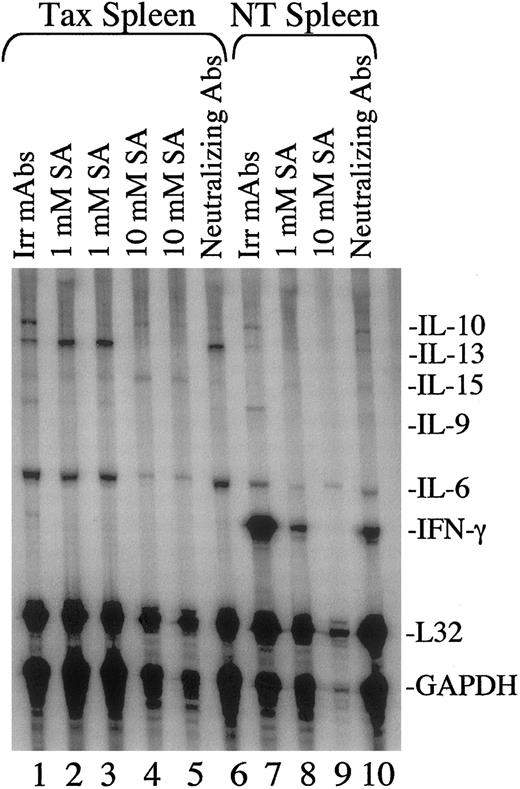
The ability of sodium salicylate to inhibit cytokine expression in tumors was then measured by RNase protection assays. As shown in Figure5, the patterns of cytokine expression in Tax spleen cells was altered when cells were placed in culture overnight, which correlates with results shown in Figure 3. This includes lower levels of IFN-γ mRNA and higher levels of IL-6 and IL-10 mRNA and protein levels in cultured versus uncultured Tax splenocytes (compare Figures 2, 3, and 5). Cytokine expression in cultured Tax spleen cells resembled that of IL-2/PHA–stimulated NT spleen cells, which demonstrated strong expression of IL-6, IL-9, IL-10, IL-13, IL-15, and IFN-γ (Figure 5, compare lanes 1 and 7). IFN-γ expression was significantly enhanced in stimulated control spleen cells when compared with Tax spleen cells, while IL-6, IL-10, and IL-13 were expressed at higher levels in Tax spleen cells (Figure5, compare lanes 1 and 7). Upon addition of 1 mM sodium salicylate, the levels of IL-10 mRNA were noticeably reduced in Tax spleen cells (Figure 5B, lanes 1-3) and the levels of IL-10, IL-6, and IFN-γ were reduced in stimulated NT spleen cells (Figure 5, lanes 7 and 8). Following addition of 10 mM sodium salicylate, levels of IL-6, IL-10, IL-13, and IFN-γ in Tax spleen cells were reduced (Figure 5, lanes 4-5). But levels of IL-15 mRNA were not significantly altered by sodium salicylate treatment. Treatment with blocking antibodies to IL-6, IL-10, IL-15, and IFN-γ did not appear to have a significant effect on cytokine mRNA expression (Figure 5B, lanes 6 and 10).
Sodium salicylate inhibits cytokine mRNA expression in mouse splenocytes.
Total RNA (10 μg) was extracted from NT (lanes 7-10) and Tax transgenic (lanes 1-6) mouse spleens following treatment with sodium salicylate (SA, lanes 2-5 and 8-9) or neutralizing cytokine antibodies (lanes 6 and 10) for 16 hours. NT splenocytes were also stimulated with 500 U/mL IL-2 plus 10 μg/mL PHA. Neutralizing antibodies included monoclonal antibodies to IL-6 and IL-10 (1 μg/mL), polyclonal antibodies to IFN-γ (10 μg/mL), and monoclonal antibodies to the IL-2Rβ chain (10 μg/mL). Irrelevant GST monoclonal antibodies (10 μg/mL) were used as negative controls (lanes 1 and 7). RNase protection assays were performed as described in Figure 1. A total of 3 spleens from NT mice and 3 spleens from Tax transgenic mice were tested, and a representative gel is shown. Lanes 2 and 3 and lanes 4 and 5 are each duplicate samples of the same cultured splenocytes.
Sodium salicylate inhibits cytokine mRNA expression in mouse splenocytes.
Total RNA (10 μg) was extracted from NT (lanes 7-10) and Tax transgenic (lanes 1-6) mouse spleens following treatment with sodium salicylate (SA, lanes 2-5 and 8-9) or neutralizing cytokine antibodies (lanes 6 and 10) for 16 hours. NT splenocytes were also stimulated with 500 U/mL IL-2 plus 10 μg/mL PHA. Neutralizing antibodies included monoclonal antibodies to IL-6 and IL-10 (1 μg/mL), polyclonal antibodies to IFN-γ (10 μg/mL), and monoclonal antibodies to the IL-2Rβ chain (10 μg/mL). Irrelevant GST monoclonal antibodies (10 μg/mL) were used as negative controls (lanes 1 and 7). RNase protection assays were performed as described in Figure 1. A total of 3 spleens from NT mice and 3 spleens from Tax transgenic mice were tested, and a representative gel is shown. Lanes 2 and 3 and lanes 4 and 5 are each duplicate samples of the same cultured splenocytes.
The effects of sodium salicylate on cytokine secretion in Tax spleen cells were also measured by collecting cell supernatants at the time of RNA isolation. As shown in Figure 6A and in agreement with Figure 5, IL-6 production was elevated in Tax spleen cells (2000 pg/mL) compared with IL-2/PHA–stimulated NT control spleen cells (900 pg/mL). IL-6 production was inhibited approximately 2.5-fold in Tax spleen cells and stimulated NT spleen cells following treatment with 10 mM sodium salicylate. In agreement with results shown in Figure5, IL-10 production was much lower than IL-6 secretion from both Tax spleen (190 pg/mL) and stimulated control spleen cells (100 pg/mL). A similar inhibition in IL-10 secretion was observed following treatment of Tax spleen cells with 10 mM as well as 1 mM sodium salicylate (Figure 6B). This effect was not as dramatic in stimulated NT control spleen cells.
Sodium salicylate inhibits IL-6 and IL-10 production in mouse splenocytes.
A total of 107 cells from NT and Tax transgenic mouse spleens were treated with sodium salicylate (1 mM or 10 mM) for 16 hours. NT splenocytes were also stimulated with 500 U/mL IL-2 plus 10 μg/mL PHA. Cell supernatants were then used in ELISAs to measure (A) IL-6 and (B) IL-10 production. Error bars represent the percent SE of 3 separate experiments using spleens from 3 NT and 3 Tax transgenic mice.
Sodium salicylate inhibits IL-6 and IL-10 production in mouse splenocytes.
A total of 107 cells from NT and Tax transgenic mouse spleens were treated with sodium salicylate (1 mM or 10 mM) for 16 hours. NT splenocytes were also stimulated with 500 U/mL IL-2 plus 10 μg/mL PHA. Cell supernatants were then used in ELISAs to measure (A) IL-6 and (B) IL-10 production. Error bars represent the percent SE of 3 separate experiments using spleens from 3 NT and 3 Tax transgenic mice.
Cyclopentenone prostaglandins inhibit proliferation of Tax-induced tumor cells
We used additional inhibitors of IKKβ activity to demonstrate the specificity of the antiproliferative effects we observed using sodium salicylate. Cyclopentenone prostaglandins, including PGA1 and 15dPGJ2, have recently been demonstrated to directly inhibit IKKβ in human cells.43As shown in Figure 4C, PGA1 and 15dPGJ2significantly inhibited proliferation of splenocytes from Tax transgenic mice at concentrations of 10 μM and 20 μM but demonstrated no effect on proliferation of IL-2– and PHA-stimulated splenocytes from NT control mice.
NF-κB inhibitors render tumor cells sensitive to apoptosis
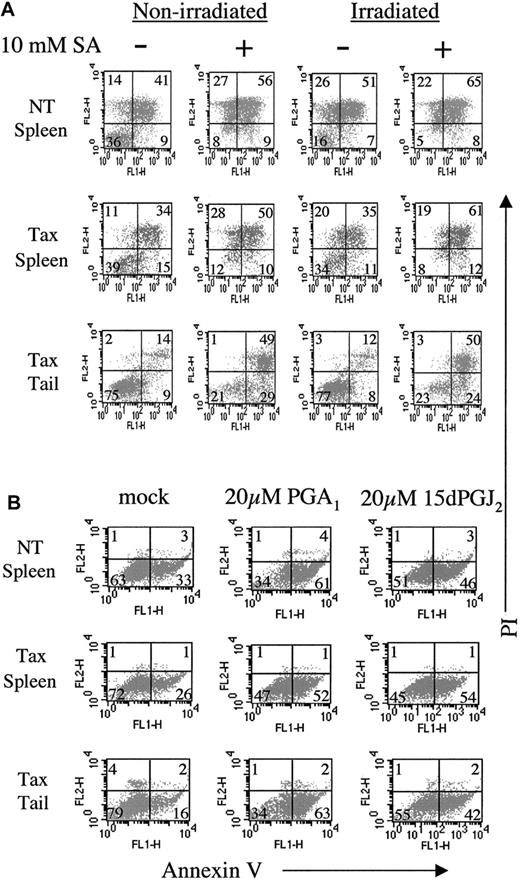
We have previously demonstrated that tumor cells from Tax transgenic mice are resistant to γ-irradiation–induced apoptosis.62 To determine whether NF-κB activation contributes to apoptosis resistance, we used the anti-inflammatory inhibitors sodium salicylate, PGA1, and 15dPGJ2to inactivate this pathway. Others have shown that sodium salicylate inhibits NF-κB activation and induces apoptosis in human cells.44 45 Spleen cells from NT mice were sensitive to apoptosis induced by sodium salicylate or irradiation, as demonstrated by annexin V and propidium iodide dual positivity (Figure7A, top row, 56% and 51%, respectively). NT control spleen cells also exhibited apoptosis following manipulation and growth in vitro with no additional treatment (41%). In contrast, spleen cells from Tax transgenic mice were relatively resistant to apoptosis induced by irradiation (Figure 7A, middle row, 35%) but more sensitive to sodium salicylate treatment (50%). The resistance to irradiation-induced apoptosis was less pronounced in Tax spleen cells compared with primary Tax tail tumor cells (Figure 7A, bottom row) because of the presence of normal nonmalignant cells in the spleen. Tax tail tumor cells were completely resistant to apoptosis induced by irradiation (12%) but sensitive to sodium salicylate treatment (49%), suggesting that inhibiting NF-κB activity renders Tax-induced tumor cells sensitive to apoptosis.
NF-κB inhibitors induce apoptosis in Tax transgenic tumor cells.
(A) Fresh tumor and spleen cell suspensions from nontransgenic (NT) and Tax transgenic mice were incubated in the presence (+) or absence (−) of 10 mM sodium salicylate (SA) for 20 hours prior to treatment with 20 Gy (2000 rad) irradiation. Five hours post-irradiation, 105cells were stained with fluorescein isothiocyanate-conjugated antibodies against annexin V (FL1-H) and propidium iodide (PI, FL2-H). Late-stage apoptotic cells are indicated by dual positivity with annexin V and PI, and the percentage of positive cells in each quadrant is shown. (B) Fresh tumor and spleen cell suspensions from nontransgenic (NT) and Tax transgenic mice were incubated in the presence or absence of 10 μM or 20 μM prostaglandin A1 (PGA1) or 15-deoxy-Δ12,14-prostaglandin J2 (15dPGJ2) for 16 hours prior to annexin V and propidium iodide staining. Early-stage apoptotic cells are indicated by annexin V single positive staining, and the percentage of positive cells in each quadrant is shown.
NF-κB inhibitors induce apoptosis in Tax transgenic tumor cells.
(A) Fresh tumor and spleen cell suspensions from nontransgenic (NT) and Tax transgenic mice were incubated in the presence (+) or absence (−) of 10 mM sodium salicylate (SA) for 20 hours prior to treatment with 20 Gy (2000 rad) irradiation. Five hours post-irradiation, 105cells were stained with fluorescein isothiocyanate-conjugated antibodies against annexin V (FL1-H) and propidium iodide (PI, FL2-H). Late-stage apoptotic cells are indicated by dual positivity with annexin V and PI, and the percentage of positive cells in each quadrant is shown. (B) Fresh tumor and spleen cell suspensions from nontransgenic (NT) and Tax transgenic mice were incubated in the presence or absence of 10 μM or 20 μM prostaglandin A1 (PGA1) or 15-deoxy-Δ12,14-prostaglandin J2 (15dPGJ2) for 16 hours prior to annexin V and propidium iodide staining. Early-stage apoptotic cells are indicated by annexin V single positive staining, and the percentage of positive cells in each quadrant is shown.
Similarly, treatment of spleen and tumor cells for a shorter time (16 hours vs 25 hours) with PGA1 or 15dPGJ2 induces apoptosis, as indicated by annexin V single-positive staining (Figure7B). Spleen cells from NT mice were sensitive to PGA1 and 15dPGJ2 treatment (33% to 61% and 33% to 46%, respectively). Comparable sensitivity to prostaglandin treatment was observed in splenocytes (26% to 52% and 26% to 54%) and tumor cells (16% to 63% and 16% to 42%) from Tax transgenic mice, again suggesting that NF-κB activity contributes to apoptosis resistance in these tumor cells.
Discussion
In this report, we have analyzed cytokine expression and the contribution of NF-κB activity to spontaneous proliferation of tumor cells from Tax transgenic mice. More importantly, we show that anti-inflammatory inhibitors of the NF-κB pathway, sodium salicylate and cyclopentenone prostaglandins, render previously resistant tumor cells sensitive to apoptosis. These results strongly suggest that Tax-mediated induction of NF-κB activity contributes to tumorigenesis in vivo.
We have demonstrated elevated expression levels of NF-κB–inducible cytokines, including IL-6, IL-10, IL-15, and IFN-γ,26 in freshly isolated primary tumors from Tax transgenic mice. Freshly isolated spleen cells from Tax transgenic mice demonstrated expression of IL-10, IL-15, and IFN-γ but typically lacked expression of IL-6. We have also shown that the cytokine profiles of tumor cells changed when cells were placed in culture. Thus, the RNase protection assays, performed on freshly isolated tumors, provide a more accurate assessment of gene expression in vivo. Interestingly, we were unable to block cellular proliferation of Tax tumor cells by adding increasing doses of neutralizing antibodies specific for IL-6, IL-10, IL-15, and IFN-γ, either alone or in combination. This is in contrast to numerous reports that suggest that these cytokines, particularly IL-6 and IL-10, contribute to autonomous growth of tumor cells.38-41 However, these tumors were of B-cell origin, and IL-6 and IL-10 are generally considered to be B-cell growth factors and immunosuppressive for cell-mediated immunity.46,47 In contrast, tumors in our model system are of CD8+ T-cell and NK-cell lineage. It is possible that production of these cytokines influences antitumor immunity and contributes to end-stage disease, which has been described in other in vivo tumor models.48-50
IL-15 is a proinflammatory cytokine that promotes B- and T-cell proliferation, lymphokine-activated killer cell induction, and B-cell differentiation.51 Overexpression of IL-15 mRNA as a result of NF-κB activation has been described in HTLV-1–infected T cells; however, the effects of IL-15 production on HTLV-I–mediated transformation were not previously assessed.16 Our results suggest that IL-15 production is not required for spontaneous proliferation of tumor cells, because treatment with neutralizing antibodies against the IL-2Rβ chain was unable to inhibit cell proliferation. These antibodies did, however, inhibit IL-15–induced proliferation of IL-2–dependent CTLL-2 cells by 66% after 16 hours of treatment (data not shown). Similarly, IFN-γ, another proinflammatory cytokine produced mainly by NK cells, did not appear to contribute to spontaneous proliferation of Tax tumor cells. However, a recent study has demonstrated that IFN-γ contributes to progression of EBV-infected NK leukemia cells by preventing apoptosis without inducing cell proliferation and independent of bcl-2 or bcl-xl.52Therefore, we have not ruled out the possibility that IFN-γ is involved in resistance to apoptosis, and this could be overcome by inhibiting NF-κB activity.
To determine the contribution of NF-κB activation to spontaneous tumor cell proliferation and resistance to apoptosis, we treated tumor cells with known inhibitors of NF-κB activity, sodium salicylate and cyclopentenone prostaglandins. Sodium salicylate has been shown to prevent degradation of the NF-κB repressor, IκB-α, in a variety of cell types, including lymphoid cells, and enhances TNF-α–mediated apoptosis.44,53,54 It functions by directly inhibiting the activity of IKK-β, the kinase that phosphorylates IκB.42 In CD28-costimulated primary human CD4+ T cells, sodium salicylate also induces apoptosis and blocks NF-κB nuclear localization and bcl-xl expression.55 In this study, we were able to block spontaneous cellular proliferation of Tax transgenic spleen cells by treatment with 10 mM sodium salicylate for 18 hours without affecting cellular viability. A dose of 1 mM appeared to inhibit IL-10 production but not cellular proliferation. At 10 mM, mRNA expression of all cytokines tested was inhibited, indicating that these cytokines were induced by NF-κB. After 25 hours of sodium salicylate treatment, tumors cells previously resistant to irradiation-induced apoptosis became sensitive to apoptosis in the presence of sodium salicylate, even in the absence of irradiation. Our results suggest that sodium salicylate triggers a slow process of apoptotic death because tumor cells were viable by trypan blue exclusion at 18 hours but positive for annexin V staining 25 hours after treatment.
Cyclopentenone prostaglandins PGA1 and 15dPGJ2have also been shown to inhibit NF-κB activation in TNF-α–stimulated human cells by directly inhibiting IKKβ.43 Treatment of splenocytes from Tax transgenic mice with either of these agents for 16 hours was shown to inhibit spontaneous proliferation and induce apoptosis. It is recognized that the effects of sodium salicylate and cyclopentenone prostaglandins may not be specific for the NF-κB pathway. Although we have not excluded effects on the CREB or SRF pathways, the finding that both inhibitors blocked cell proliferation and induced apoptosis at doses that inhibited NF-κB responsive genes strongly suggest that NF-κB activity contributes to spontaneous proliferation and apoptosis resistance in Tax-induced tumors.
Activation of the NF-κB pathway by viral proteins has been well established, particularly in studies involving EBV LMP-1 and HTLV-I Tax. Interestingly, both of these proteins appear to stimulate the activity of upstream kinases in the NF-κB pathway. EBV LMP-1 associates with the TNF-associated TRAFs and TRADD, activating a pathway whose members include NF-κB–inducing kinase (NIK), IKK-α, and IKK-β.56 Similarly, HTLV-I Tax expression has been shown to promote phosphorylation and activation of IKK-α and IKK-β by increasing the activity of the upstream kinases mitogen-activated protein/extracellular signal–related kinase kinase-1 and NIK.57,58 In our in vivo model of tumorigenesis, Tax expression may be inducing NF-κB activity in a similar manner, which may explain why treatment with sodium salicylate or cyclopentenone prostaglandins, direct inhibitors of IKK-β, appears to reverse spontaneous proliferation and resistance to apoptosis in Tax-induced tumors. In support of our findings, a study by Kitajima et al demonstrates that antisense oligonucleotides to NF-κB inhibit proliferation of Tax-transformed tumor cells transplanted into mice.59
Taken together, our findings have important implications regarding prevention and/or treatment of tumors induced by HTLV-I Tax. It will be interesting to see whether specific blockade of NF-κB activation delays or inhibits growth of tumors in vivo in Tax transgenic mice.
We thank Dr Bob Schreiber for reagents.
Supported by National Institutes of Health grants CA-63417 and RR-14324, a National Heart, Lung, and Blood Institute Training Grant Award, and a Leukemia and Lymphoma Society fellowship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Lee Ratner, Box 8069, Washington University, 660 S Euclid Ave, St Louis, MO 63110; e-mail: lratner@imgate.wustl.edu.



![Fig. 4. Sodium salicylate and cyclopentenone prostaglandins inhibit spontaneous proliferation of Tax transgenic mouse splenocytes. / (A) A total of 107 spleen cells from NT and Tax transgenic mice were cultured for 4 hours following sodium salicylate or mock treatment. NT splenocytes were also stimulated with 500 U/mL IL-2 plus 10 μg/mL PHA. Then, 1.1 MBq per well of [3H]thymidine was added to cultures, and thymidine incorporation was measured after a 16-hour incubation. (B) The percent cell viability was determined by trypan blue exclusion prior to cell harvest. (C) A total of 107 spleen cells from NT and Tax transgenic mice were cultured for 2 hours following PGA1, 15dPGJ2, or mock treatment. NT splenocytes were also stimulated with 500 U/mL IL-2 plus 10 μg/mL PHA. [3H]thymidine was added to cultures, and thymidine incorporation was measured 14 hours later. All experiments were performed in triplicate, and error bars represent the percent SE.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/4/10.1182_blood.v98.4.1200/6/m_h81611431004.jpeg?Expires=1765891102&Signature=JSCv7kxT5TvGaxHv5j0a8m88BnRNjsidivfww8ofOasyPMNccSHiERir1tNW9RYcdwy1tliv8tr96y8WsGR9iq2Ka9HhsewyrDI9p~P0Cv7qjft5mdL2Cf-L7haViEcVxwOHywqcuOiBQTDsZ0yG20mka2Pd7TCIU0z7wzQOcMdCmP6DjsNrq6l51qx4zli~4YT~YnL6MVVUuClR-94Wrd30iAwPynxiYbUgCEoB1BXomQTDsEwi2VGEvs9WeE0dJVdxRgwrYOOKXARLIF1f6mMlZP5xZjKnxiDX8VM7XxF5xrLUVkHRVW49QK5AVTHoFfTmfc9cBzeCpLbdAJmDpw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal