The role of rituximab, a chimeric monoclonal antibody directed against the CD20 antigen, in the treatment of patients with chronic idiopathic thrombocytopenic purpura (ITP) has not been determined. The effectiveness and side effects of this therapeutic modality were investigated in a cohort of 25 individuals with chronic ITP. All patients had ITP that had been resistant to between 2 and 5 different therapeutic regimens, including 8 patients who had already failed splenectomy. Patients were scheduled to receive intravenous rituximab at the dose of 375 mg/m2 once weekly for 4 weeks. Rituximab infusion-related side effects were observed in 18 patients, but were of modest intensity and did not require discontinuation of treatment. A complete response (platelet count greater than 100 × 109/L) was observed in 5 cases, a partial response (platelet count between 50 and 100 × 109/L) in 5 cases, and a minor response (platelet count below 50 × 109/L, with no need for continued treatment) in 3 cases, with an overall response rate of 52%. In 7 cases, responses were sustained (6 months or longer). In 2 patients with relapsed disease, repeat challenge with rituximab induced a new response. In patients with a complete or partial response, a significant rise in platelet concentrations was observed early during the course of treatment, usually 1 week after the first rituximab infusion. No clinical or laboratory parameter was found to predict treatment outcome, although there was a suggestion that women and younger patients have a better chance of response. In conclusion, rituximab therapy has a limited but valuable effect in patients with chronic ITP. In view of its mild toxicity and the lack of effective alternative treatments, its use in the setting of chronic refractory ITP is warranted.
Introduction
Idiopathic thrombocytopenic purpura (ITP) is an immune-mediated disorder in which platelets are opsonized by autoantibodies and prematurely destroyed by the reticuloendothelial system.1 Approximately 25% to 30% of adult patients develop a chronic disease that will become refractory to corticosteroids and splenectomy, as well as other available agents. Because the 10-year mortality rate for this group of refractory patients ranges from 10% to 20%,2-5 there is a need for more effective therapeutic interventions.
Novel approaches to treatment of chronic refractory ITP are based on a better understanding of the immunologic abnormalities associated with this disorder,6,7 as well as the availability of sophisticated biotechnology products.8 One such approach involves the use of monoclonal antibodies directed against the CD20 antigen, a transmembrane protein found on the surface of normal and malignant B cells, but not on hematopoietic stem cells, pro-B cells, normal plasma cells, or other normal tissues. The CD20 molecule appears particularly suitable for targeted therapy because it does not shed from the cell surface and does not internalize upon antibody binding.9
Rituximab is a genetically engineered chimeric murine/human anti-CD20 monoclonal antibody currently used for the treatment of non-Hodgkin lymphoma. The antibody is an IgG1 kappa immunoglobulin containing murine light- and heavy-chain variable region sequences and human constant region sequences.10 The Fab domain of rituximab binds to the CD20 antigen on B lymphocytes, and the Fc domain recruits immune effector functions to mediate B-cell lysis in vitro. Possible mechanisms of cell lysis include complement-dependent cytotoxicity, antibody-dependent cellular cytotoxicity, and induction of apoptosis. Rituximab is highly effective for in vivo depletion of B cells,10 and the rationale for its use in chronic ITP rests on the attempt to eliminate autoreactive B-cell clones.
In the current report, we detail the effectiveness and side effects of rituximab treatment in a cohort of 25 individuals with chronic ITP.
Patients and methods
Patients
The clinical and laboratory characteristics of patients with chronic ITP enrolled in this study are summarized in Table1. The diagnosis of ITP was one of exclusion. Disorders known to cause shortened survival or decreased production of platelets were ruled out, including multisystem autoimmune diseases, lymphoproliferative disorders, drug-induced thrombocytopenia, myelodysplastic syndromes, chronic liver diseases, human immunodeficiency virus (HIV) infection, and other viral as well as bacterial infections. A diagnostic bone marrow aspirate had been performed in all patients at presentation.
Summary of patients' pretreatment characteristics
| No. of patients | 25 |
| Men | 9 |
| Women | 16 |
| Age, y | |
| Median | 46 |
| Range | 22-74 |
| Baseline platelet count, × 109/L | |
| Median | 13 |
| Range | 3-25 |
| Previous therapies | |
| Prednisone* | 25 |
| Intravenous immunoglobulin | 25 |
| High-dose dexamethasone | 11 |
| Splenectomy | 8 |
| Vincristine† | 5 |
| Pulse cyclophosphamide | 4 |
| Vitamin C | 2 |
| Anti-Rh globulin | 1 |
| ITP duration before rituximab treatment, mo | |
| Median | 22 |
| Range | 9-56 |
| Elevated PAIgG | 19/19 |
| Elevated ACA/LLA | 11 |
| ANA positive | 0 |
| Coombs tests positive | 0 |
| No. of patients | 25 |
| Men | 9 |
| Women | 16 |
| Age, y | |
| Median | 46 |
| Range | 22-74 |
| Baseline platelet count, × 109/L | |
| Median | 13 |
| Range | 3-25 |
| Previous therapies | |
| Prednisone* | 25 |
| Intravenous immunoglobulin | 25 |
| High-dose dexamethasone | 11 |
| Splenectomy | 8 |
| Vincristine† | 5 |
| Pulse cyclophosphamide | 4 |
| Vitamin C | 2 |
| Anti-Rh globulin | 1 |
| ITP duration before rituximab treatment, mo | |
| Median | 22 |
| Range | 9-56 |
| Elevated PAIgG | 19/19 |
| Elevated ACA/LLA | 11 |
| ANA positive | 0 |
| Coombs tests positive | 0 |
ITP indicates idiopathic thrombocytopenic purpura; PAIgG, platelet-associated IgG; ACA/LLA, anticardiolipin antibodies/lupuslike anticoagulant; ANA, antinuclear antibodies.
1 to 2 mg · kg−1 · d−1.
2 mg/wk × 4.
In accordance with the guidelines published by the American Society of Hematology, treatment was considered appropriate for these patients either because of a platelet count less than 20 × 109/L, regardless of their symptoms, or because of the presence of purpura or significant mucous membrane bleeding, regardless of platelet count.2 3 All patients had already received at least 2 lines of treatment, with a median of 3. Previous treatments included: standard dose (1 to 2 mg · kg−1 · d−1) oral prednisone and intravenous immunoglobulins (0.5 g · kg−1 · d−1 on 2 consecutive days for at least 4 cycles) for all patients, high-dose dexamethasone (40 mg/d for 4 days monthly for up to 6 cycles) in 11 patients, vincristine (2 mg weekly for 4 cycles) in 5 cases, pulse cyclophosphamide (1 g/m2 monthly for up to 3 cycles) in 4 cases, oral vitamin C (2 g/d) in 2 cases, and anti-D(Rh) immunoglobulin in 1 case. Eight patients had previously undergone splenectomy. It is noteworthy that all patients in this series had achieved at least a partial (transient) response to intravenous immunoglobulins (Table2), a finding that further supports the autoimmune nature of their disease.
Response to intravenous immunoglobulin in patients with chronic idiopathic thrombocytopenic purpura
| Case no. . | Platelet count before IVIg, × 109/L . | Peak platelet count after IVIg, × 109/L . |
|---|---|---|
| 1 | 22 | 62 |
| 2 | 13 | 218 |
| 3 | 8 | 325 |
| 4 | 18 | 57 |
| 5 | 7 | 129 |
| 6 | 16 | 93 |
| 7 | 10 | 75 |
| 8 | 17 | 106 |
| 9 | 5 | 82 |
| 10 | 12 | 59 |
| 11 | 6 | 83 |
| 12 | 17 | 66 |
| 13 | 11 | 251 |
| 14 | 15 | 406 |
| 15 | 14 | 48 |
| 16 | 24 | 77 |
| 17 | 13 | 105 |
| 18 | 9 | 45 |
| 19 | 21 | 189 |
| 20 | 13 | 134 |
| 21 | 15 | 61 |
| 22 | 8 | 196 |
| 23 | 6 | 52 |
| 24 | 27 | 94 |
| 25 | 10 | 113 |
| Case no. . | Platelet count before IVIg, × 109/L . | Peak platelet count after IVIg, × 109/L . |
|---|---|---|
| 1 | 22 | 62 |
| 2 | 13 | 218 |
| 3 | 8 | 325 |
| 4 | 18 | 57 |
| 5 | 7 | 129 |
| 6 | 16 | 93 |
| 7 | 10 | 75 |
| 8 | 17 | 106 |
| 9 | 5 | 82 |
| 10 | 12 | 59 |
| 11 | 6 | 83 |
| 12 | 17 | 66 |
| 13 | 11 | 251 |
| 14 | 15 | 406 |
| 15 | 14 | 48 |
| 16 | 24 | 77 |
| 17 | 13 | 105 |
| 18 | 9 | 45 |
| 19 | 21 | 189 |
| 20 | 13 | 134 |
| 21 | 15 | 61 |
| 22 | 8 | 196 |
| 23 | 6 | 52 |
| 24 | 27 | 94 |
| 25 | 10 | 113 |
On study entry, patients had signed an institutional review board–approved informed consent.
Treatment regimen
Rituximab (Mabthera; Roche, Milan, Italy) at a dose of 375 mg/m2 was administered intravenously once weekly for a total of 4 infusions (days 1, 8, 15, and 22) on an outpatient basis. The drug was reconstituted in normal saline to a concentration of no more than 1 to 4 mg/mL. The initial infusion rate was 50 mg/h, with subsequent infusion-rate increase if no toxicity was seen. Premedication with oral acetaminophen 500 mg and diphenhydramine 50 mg was given to all patients. Those who experienced any treatment-related nausea or vomiting with the first treatment received subsequent premedication with a serotonin receptor antagonist. Administration of corticosteroids was avoided.
Laboratory studies
Laboratory evaluation before enrollment included: complete hemogram; serum chemistry profiles; direct and indirect Coombs test; prothrombin time; partial thromboplastin time; fibrinogen levels; and serologic tests for hepatitis B and C, HIV, cytomegalovirus, and toxoplasmosis. Immunology studies included tests for C3 and C4 fractions of complement, antinuclear antibodies, anti–double-stranded DNA antibodies and anticardiolipin antibodies (ACA), lymphocyte subsets, and serum immunoglobulin (IgG, IgA, and IgM) concentrations. ACA and the lupuslike anticoagulant were determined as described previously.11 Platelet-associated IgG (PAIgG) was quantified by an enzyme-linked immunosorbent assay. Briefly, blood was collected into EDTA and platelet concentrates were obtained by differential centrifugation. Afterward, platelets were incubated for 5 minutes at room temperature (RT) with ammonium oxalate to remove residual erythrocytes, washed twice, and resuspended in a Ringer's/2-mM EDTA solution. Platelet suspensions were then disrupted by 3 cycles of freeze-thawing, sonicated, and centrifuged, and the supernatant was employed for IgG detection. Fifty-microliter aliquots of the platelet extracts at appropriate dilutions were pipetted into each well of a microtiter plate and incubated at RT for 3 hours. The plate had been coated with anti–human IgG (Dakopatts A/S, Glostrup, Denmark) and treated overnight with 1% bovine serum albumin–phosphate-buffered saline (PBS) (Sigma Chemical, St Louis, MO) to block residual binding sites. After triple washing with PBS-1% Tween (Sigma), 50 μL horseradish peroxidase–conjugated rabbit anti–human IgG (Dakopatts A/S) was added to each well and further incubated at RT for 90 minutes. A substrate solution of O-phenylenediamine dihydrochloride (Sigma) was added, the reaction was stopped after 30 minutes with 1 N H2SO4, and the color reaction was read at 490 nm. A standard curve for IgG was made in each plate by using reference standard sera (Dakopatts A/S). The intra-assay and interassay variabilities were 8.3% and 10.4%, respectively. The upper limit of normal was 10 ng/106 platelets.
Monitoring of patients included a weekly hemogram and determination of lymphocyte subsets, and monthly determinations of serum immunoglobulin and PAIgG concentrations.
Response criteria
Response was evaluated after the 4 weeks of treatment. Criteria for response were defined as follows: (1) complete response (CR), a rise of platelets to normal counts (ie, more than 100 × 109/L); (2) partial response (PR), a rise of platelets to counts between 50 and 100 × 109/L; (3) minor response (MR), a rise of platelets to counts below 50 × 109/L with no need for continued treatment; and (4) no response or refractory disease, when there was no rise in platelet count or a rise that did not exceed 50 × 109/L, with a need for continued treatment. Responses were considered sustained when they lasted at least 6 months.
Statistical analysis
Statistical analysis was carried out with the STATISTICA for Windows (Kalmia, Cambridge, MA) software package on an IBM-compatible computer. Comparisons between the groups were performed with Mann-Whitney U test or the Kruskal-Wallis test, and correlation analysis with the Spearman rank correlation test. AP value of .05 or less was designated as statistically significant.
Results
Response
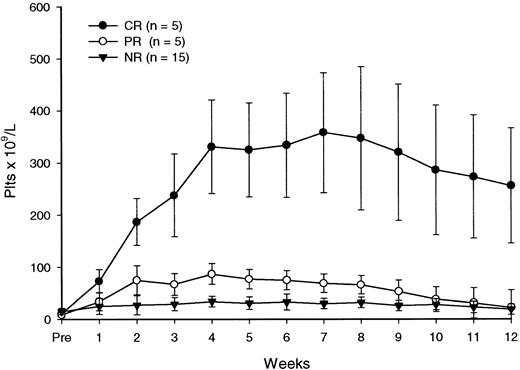
All patients completed the scheduled treatment. As illustrated in Table 3, after the 4 courses of rituximab, the platelet count was at least 50 × 109/L in 10 patients (40%), with 5 achieving a CR and 5 a PR. Three additional patients (nos. 4, 24, 25) had a clinical improvement classified as MR because their platelet counts did not rise above 50 × 109/L but they had no need for continued treatment. Six of the patients with a major (CR+PR) response (nos. 2, 5, 11, 14, 19, 22) and one with a minor response (no. 4) have remained in remission with stable platelet counts during follow-up intervals of 6 to more than 27 months after the end of treatment. In 4 patients who achieved either a CR (no. 3) or a PR (nos. 6, 13, 20), response was not sustained. In 2 other patients (nos. 24 and 25) with a minor response, the follow-up was less than 6 months. There were no late responses (ie, responses observed after the end of therapy). In patients with a major response, an increase in platelet counts occurred during rituximab therapy, with peak responses observed up to 4 weeks following the end of treatment (Table 3, Figure 1). The median peak platelet count in these individuals was 117 × 109/L (range, 62-395 × 109/L) 1 week after the last infusion of rituximab.
Clinical and hematologic characteristics of patients with chronic idiopathic thrombocytopenic purpura
| Case no. . | Age/sex . | Prior therapy . | Duration of disease, mo . | Platelet count before therapy, × 109/L3-150 . | Platelet counts after therapy, × 109/L . | Response duration, wk . | Subsequent therapy . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wk 1 . | Wk 2 . | Wk 3 . | Wk 4 . | Wk 8 . | Wk 12 . | |||||||
| 1 | 51/M | P, Ig, DXM | 15 | 12 | 25 | 28 | 27 | 35 | 32 | 13 | — | P, VC |
| 2 | 44/F | P, Ig | 22 | 6 | 65 | 113 | 238 | 301 | 347 | 256 | 108+ | — |
| 3 | 27/F | P, Ig | 17 | 19 | 107 | 145 | 127 | 139 | 72 | 22 | 6 | Rtx, P, VC |
| 4 | 52/M | P, Ig | 12 | 16 | 35 | 27 | 29 | 25 | 31 | 24 | 94+ | — |
| 5 | 74/F | P, Ig, V, Cy | 28 | 4 | 73 | 187 | 290 | 331 | 268 | 193 | 49 | Rtx |
| 6 | 45/M | P, Ig, S | 35 | 11 | 33 | 51 | 64 | 90 | 69 | 22 | 5 | Rtx, P |
| 7 | 32/F | P, Ig, V, DXM | 37 | 8 | 15 | 18 | 17 | 21 | 19 | 13 | — | S |
| 8 | 55/M | P, Ig, S, Cy | 41 | 25 | 31 | 58 | 44 | 40 | 39 | 21 | — | VC |
| 9 | 48/F | P, Ig, V, S | 33 | 7 | 19 | 20 | 18 | 27 | 30 | 17 | — | P |
| 10 | 36/F | P, Ig, S, DXM | 39 | 14 | 17 | 5 | 14 | 12 | 17 | 8 | — | P |
| 11 | 57/F | P, Ig, VC | 10 | 9 | 34 | 75 | 67 | 81 | 66 | 73 | 29 | Rtx, V, P |
| 12 | 24/F | P, Ig | 13 | 24 | 22 | 34 | 38 | 27 | 15 | 21 | — | S |
| 13 | 22/M | P, Ig | 17 | 9 | 25 | 40 | 48 | 62 | 44 | 19 | 3 | VC |
| 14 | 25/F | P, Ig, V, DXM | 25 | 13 | 105 | 218 | 339 | 356 | 423 | 282 | 83+ | — |
| 15 | 46/F | P, Ig, S | 34 | 21 | 25 | 18 | 39 | 46 | 33 | 10 | — | V, VC |
| 16 | 53/M | P, Ig, DXM | 13 | 15 | 56 | 43 | 31 | 37 | 35 | 19 | — | S |
| 17 | 64/F | P, Ig, DXM | 11 | 19 | 35 | 27 | 43 | 40 | 32 | 12 | — | Ig, S |
| 18 | 69/M | P, Ig, DXM | 17 | 3 | 27 | 13 | 16 | 23 | 18 | 9 | — | S |
| 19 | 29/F | P, Ig, V, S, Cy | 39 | 11 | 59 | 212 | 234 | 395 | 372 | 285 | 45+ | — |
| 20 | 57/F | P, Ig, VC, S | 44 | 7 | 49 | 81 | 103 | 87 | 31 | 13 | 2 | P, Cy |
| 21 | 35/M | P, Ig, DXM, Cy | 51 | 10 | 25 | 32 | 28 | 34 | 41 | 19 | — | P |
| 22 | 28/F | P, Ig, DXM | 19 | 21 | 69 | 112 | 84 | 95 | 72 | 85 | 30 | P |
| 23 | 59/F | P, Ig, S, aRh | 56 | 13 | 18 | 13 | 16 | 20 | 19 | 24 | — | P |
| 24 | 44/F | P, Ig | 9 | 18 | 25 | 33 | 29 | 41 | 37 | 33 | 19+ | — |
| 25 | 61/M | P, Ig, DXM | 12 | 17 | 72 | 76 | 58 | 45 | 47 | 31 | 14+ | — |
| Case no. . | Age/sex . | Prior therapy . | Duration of disease, mo . | Platelet count before therapy, × 109/L3-150 . | Platelet counts after therapy, × 109/L . | Response duration, wk . | Subsequent therapy . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wk 1 . | Wk 2 . | Wk 3 . | Wk 4 . | Wk 8 . | Wk 12 . | |||||||
| 1 | 51/M | P, Ig, DXM | 15 | 12 | 25 | 28 | 27 | 35 | 32 | 13 | — | P, VC |
| 2 | 44/F | P, Ig | 22 | 6 | 65 | 113 | 238 | 301 | 347 | 256 | 108+ | — |
| 3 | 27/F | P, Ig | 17 | 19 | 107 | 145 | 127 | 139 | 72 | 22 | 6 | Rtx, P, VC |
| 4 | 52/M | P, Ig | 12 | 16 | 35 | 27 | 29 | 25 | 31 | 24 | 94+ | — |
| 5 | 74/F | P, Ig, V, Cy | 28 | 4 | 73 | 187 | 290 | 331 | 268 | 193 | 49 | Rtx |
| 6 | 45/M | P, Ig, S | 35 | 11 | 33 | 51 | 64 | 90 | 69 | 22 | 5 | Rtx, P |
| 7 | 32/F | P, Ig, V, DXM | 37 | 8 | 15 | 18 | 17 | 21 | 19 | 13 | — | S |
| 8 | 55/M | P, Ig, S, Cy | 41 | 25 | 31 | 58 | 44 | 40 | 39 | 21 | — | VC |
| 9 | 48/F | P, Ig, V, S | 33 | 7 | 19 | 20 | 18 | 27 | 30 | 17 | — | P |
| 10 | 36/F | P, Ig, S, DXM | 39 | 14 | 17 | 5 | 14 | 12 | 17 | 8 | — | P |
| 11 | 57/F | P, Ig, VC | 10 | 9 | 34 | 75 | 67 | 81 | 66 | 73 | 29 | Rtx, V, P |
| 12 | 24/F | P, Ig | 13 | 24 | 22 | 34 | 38 | 27 | 15 | 21 | — | S |
| 13 | 22/M | P, Ig | 17 | 9 | 25 | 40 | 48 | 62 | 44 | 19 | 3 | VC |
| 14 | 25/F | P, Ig, V, DXM | 25 | 13 | 105 | 218 | 339 | 356 | 423 | 282 | 83+ | — |
| 15 | 46/F | P, Ig, S | 34 | 21 | 25 | 18 | 39 | 46 | 33 | 10 | — | V, VC |
| 16 | 53/M | P, Ig, DXM | 13 | 15 | 56 | 43 | 31 | 37 | 35 | 19 | — | S |
| 17 | 64/F | P, Ig, DXM | 11 | 19 | 35 | 27 | 43 | 40 | 32 | 12 | — | Ig, S |
| 18 | 69/M | P, Ig, DXM | 17 | 3 | 27 | 13 | 16 | 23 | 18 | 9 | — | S |
| 19 | 29/F | P, Ig, V, S, Cy | 39 | 11 | 59 | 212 | 234 | 395 | 372 | 285 | 45+ | — |
| 20 | 57/F | P, Ig, VC, S | 44 | 7 | 49 | 81 | 103 | 87 | 31 | 13 | 2 | P, Cy |
| 21 | 35/M | P, Ig, DXM, Cy | 51 | 10 | 25 | 32 | 28 | 34 | 41 | 19 | — | P |
| 22 | 28/F | P, Ig, DXM | 19 | 21 | 69 | 112 | 84 | 95 | 72 | 85 | 30 | P |
| 23 | 59/F | P, Ig, S, aRh | 56 | 13 | 18 | 13 | 16 | 20 | 19 | 24 | — | P |
| 24 | 44/F | P, Ig | 9 | 18 | 25 | 33 | 29 | 41 | 37 | 33 | 19+ | — |
| 25 | 61/M | P, Ig, DXM | 12 | 17 | 72 | 76 | 58 | 45 | 47 | 31 | 14+ | — |
M indicates male; P, prednisone; Ig, intravenous immunoglobulins; VC, vitamin C; DXM, high-dose dexamethasone; F, female; Rtx, rituximab; V, vincristine; Cy, pulse cyclophosphamide; S, splenectomy; aRh, anti-Rh immunoglobulin.
Nadir platelet count before rituximab.
Platelet response to rituximab.
Each data point represents the mean (± SD) platelet count at a particular time.
Platelet response to rituximab.
Each data point represents the mean (± SD) platelet count at a particular time.
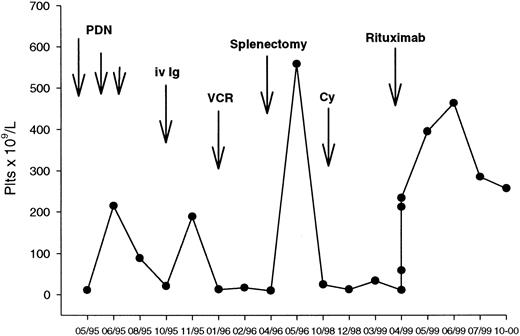
A representative course of a responding patient (no. 19) is shown in Figure 2. This patient had a 3-year history of ITP that had relapsed after conventional-dose steroids, intravenous immunoglobulins, and splenectomy, and had not responded to vincristine and pulse cyclophosphamide. She was dependent on 50 mg/d of oral prednisone to keep a safe platelet count. A significant rise in the platelet count was observed after the first antibody infusion, with a progressive increase until a platelet peak of 463 × 109/L recorded in week 7. She is still in remission with a stable platelet count at 9 months after the end of treatment.
Clinical course of a patient with chronic ITP responding to rituximab.
(See text for details.)
Clinical course of a patient with chronic ITP responding to rituximab.
(See text for details.)
Four relapsed patients (nos. 3, 5, 6, 11) were rechallenged with rituximab at the same dose and schedule. Repeat treatment resulted in a new response in 2 patients (nos. 6, 11), with one patient (no. 6) having maintained counts in excess of 100 × 109/L for 8 months.
The relation between patient variables and their response to rituximab is reported in Table 4. None of the parameters analyzed (age, sex, pretreatment platelet values, duration of disease, number and type of previous treatments, and PAIgG levels) were related to treatment outcome (P value not significant for all parameters). From our data, however, there is at least a suggestion that women and younger patients respond better to rituximab.
Patients' characteristics based on response to treatment
| . | CR (n = 5) . | PR (n = 5) . | MR (n = 3) . | NR (n = 12) . | P . |
|---|---|---|---|---|---|
| Sex (F/M) | 5/0 | 3/2 | 1/2 | 7/5 | .2368 |
| Median age, y | 29 | 45 | 52 | 49.5 | .3185 |
| Median baseline platelet count, × 109/L | 11 | 9 | 17 | 13.5 | .4370 |
| Median ITP duration before rituximab treatment, mo | 25 | 19 | 12 | 33.5 | .5167 |
| Prior splenectomy (n = 8) | 1 | 2 | 0 | 5 | .9342 |
| . | CR (n = 5) . | PR (n = 5) . | MR (n = 3) . | NR (n = 12) . | P . |
|---|---|---|---|---|---|
| Sex (F/M) | 5/0 | 3/2 | 1/2 | 7/5 | .2368 |
| Median age, y | 29 | 45 | 52 | 49.5 | .3185 |
| Median baseline platelet count, × 109/L | 11 | 9 | 17 | 13.5 | .4370 |
| Median ITP duration before rituximab treatment, mo | 25 | 19 | 12 | 33.5 | .5167 |
| Prior splenectomy (n = 8) | 1 | 2 | 0 | 5 | .9342 |
CR indicates complete response; PR, partial response; MR, minor response; NR, no response.
Adverse events
Eighteen patients experienced a total of 27 adverse events during therapy (Table 5). Most of these side effects were recorded during the first infusion and were typically brief and of mild intensity (grades 1 and 2 according to the National Cancer Institute criteria). The antibody infusion was temporarily stopped only once in the single patient who had grade 2 chills (no. 18). In this patient, adjustment of subsequent infusion rates avoided recurrence of symptoms.
Adverse events during therapy
| Event . | NCI grade . | |
|---|---|---|
| 1 . | 2 . | |
| Any | 26 | 1 |
| General | ||
| Fever | 6 | |
| Chills | 8 | 1 |
| Headache | 1 | |
| Dizziness | 1 | |
| Asthenia | 1 | |
| Digestive | ||
| Nausea | 5 | |
| Vomiting | 1 | |
| Cardiovascular | ||
| Hypotension | 2 | |
| Sinus tachycardia | 1 | |
| Event . | NCI grade . | |
|---|---|---|
| 1 . | 2 . | |
| Any | 26 | 1 |
| General | ||
| Fever | 6 | |
| Chills | 8 | 1 |
| Headache | 1 | |
| Dizziness | 1 | |
| Asthenia | 1 | |
| Digestive | ||
| Nausea | 5 | |
| Vomiting | 1 | |
| Cardiovascular | ||
| Hypotension | 2 | |
| Sinus tachycardia | 1 | |
NCI indicates National Cancer Institute.
Three patients had hemoglobin levels decrease to as low as 10 to 11 g/dL at 6 to 8 weeks upon conclusion of rituximab infusions; recovery occurred in a median of 7 days. One patient (no. 15) had granulocytes decrease to a level of 1 to 1.5 × 109/L 4 weeks after therapy, with recovery by 10 days.
No infections occurred during therapy. Those that occurred for up to a year thereafter were predominantly bacterial, and the majority were of minor clinical relevance (16 of 19 grade 1; none grade 3 or 4). The respiratory tract was the source in 16 and the urinary tract in 2; gastroenteritis occurred in 1. Viral infections included herpes zoster in one patient.
Monitoring of immunologic parameters
Increased PAIgG levels before rituximab treatment were detected in all 19 patients in whom this analysis could be performed. No relation was observed between PAIgG concentrations and clinical parameters. Serial determinations for up to 6 months after treatment indicated that the clinical response was not associated with normalization or significant reduction of PAIgG. In particular, PAIgG declined to normal levels in 3 of the responders and in 5 of the nonresponders (Table6).
Monitoring of immunologic parameters
| Case no. . | C3, mg/dL . | Serum IgG, mg/dL . | Serum IgM, mg/dL . | PAIgG, ng/106 platelets . | (CD19+) B cells, × 106/L . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline . | Nadir . | At 48 wk . | Baseline . | Nadir . | At 48 wk . | Baseline . | Nadir . | At 48 wk . | Baseline . | Nadir . | At 48 wk . | Baseline . | Nadir . | At 48 wk . | |
| 1 | 93 | 88 | 97 | 1235 | 1037 | 1120 | 189 | 156 | 174 | 141 | 102 | 158 | 265 | 10 | 210 |
| 2 | 115 | 102 | 138 | 964 | 885 | 1078 | 157 | 91 | 126 | ND | ND | ND | 310 | 5 | 376 |
| 3 | 101 | 96 | 99 | 1641 | 1290 | 1476 | 220 | 138 | 242 | 298 | 276 | 342 | 62 | 3 | 96 |
| 4 | 76 | 38 | 92 | 727 | 575 | 842 | 86 | 52 | 71 | 459 | 315 | 387 | 123 | 11 | 179 |
| 5 | 132 | 125 | 147 | 1190 | 1165 | 1327 | 147 | 128 | 193 | 167 | 3 | 5 | 104 | 6 | 158 |
| 6 | ND | ND | ND | 1320 | 981 | 1252 | 243 | 105 | 1272 | ND | ND | ND | ND | ND | ND |
| 7 | 94 | 75 | 81 | 1050 | 733 | 977 | 175 | 74 | 110 | 382 | 364 | 335 | 418 | 26 | 295 |
| 8 | 158 | 79 | 79 | 863 | 605 | 912 | 204 | 98 | 183 | 85 | 10 | 14 | 82 | 4 | 106 |
| 9 | 131 | 122 | 168 | 1267 | 880 | 1125 | 113 | 104 | 238 | ND | ND | ND | 259 | 6 | 185 |
| 10 | 62 | 40 | 122 | 1315 | 1171 | 1263 | 197 | 154 | 186 | 649 | 8 | 21 | 324 | 7 | 252 |
| 11 | 80 | 82 | 135 | 984 | 848 | 920 | 236 | 129 | 164 | 215 | 4 | 106 | 128 | 9 | 105 |
| 12 | 119 | 87 | 110 | 1100 | 836 | 1057 | 258 | 87 | 134 | ND | ND | ND | 189 | 8 | 176 |
| 13 | 133 | 101 | 128 | 1548 | 1316 | 1391 | 186 | 159 | 171 | 294 | 231 | 270 | 213 | 18 | 134 |
| 14 | 157 | 56 | 129 | 1491 | 1284 | 1342 | 264 | 235 | 270 | 452 | 2 | 6 | 238 | 7 | 296 |
| 15 | ND | ND | ND | 1050 | 825 | 936 | 129 | 104 | 163 | 298 | 263 | 312 | 56 | 5 | 72 |
| 16 | 125 | 96 | 109 | 1732 | 1362 | 1459 | 184 | 166 | 219 | 190 | 157 | 276 | 202 | 12 | 166 |
| 17 | 142 | 120 | 131 | 1256 | 954 | 1173 | 328 | 112 | 178 | 337 | 8 | 174 | 146 | 9 | 121 |
| 18 | 138 | 113 | 154 | 895 | 840 | 1066 | 182 | 176 | 244 | 848 | 536 | 901 | 78 | 6 | 105 |
| 19 | 110 | 91 | 127 | 1071 | 483 | 738 | 145 | 65 | 106 | 249 | 7 | 10 | 304 | 10 | 260 |
| 20 | ND | ND | ND | ND | 945 | 1053 | ND | 156 | 217 | 515 | 342 | 437 | 221 | 15 | 143 |
| 21 | 86 | 54 | 93 | 1420 | 1308 | 1395 | 233 | 186 | 225 | ND | ND | ND | ND | ND | ND |
| 22 | 145 | 130 | 163 | 820 | 673 | 744 | 171 | 128 | 206 | 271 | 180 | 239 | 95 | 8 | 67 |
| 23 | 169 | 120 | 151 | 1346 | 1158 | 1274 | 202 | 194 | 246 | 134 | 117 | 126 | 164 | 4 | 112 |
| 24 | 107 | 90 | 96 | 1189 | 976 | 1060 | 95 | 74 | 114 | 75 | 5 | 27 | 242 | 9 | 281 |
| 25 | ND | ND | ND | ND | 1264 | 1381 | ND | 146 | 177 | ND | ND | ND | 137 | 3 | 174 |
| Case no. . | C3, mg/dL . | Serum IgG, mg/dL . | Serum IgM, mg/dL . | PAIgG, ng/106 platelets . | (CD19+) B cells, × 106/L . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline . | Nadir . | At 48 wk . | Baseline . | Nadir . | At 48 wk . | Baseline . | Nadir . | At 48 wk . | Baseline . | Nadir . | At 48 wk . | Baseline . | Nadir . | At 48 wk . | |
| 1 | 93 | 88 | 97 | 1235 | 1037 | 1120 | 189 | 156 | 174 | 141 | 102 | 158 | 265 | 10 | 210 |
| 2 | 115 | 102 | 138 | 964 | 885 | 1078 | 157 | 91 | 126 | ND | ND | ND | 310 | 5 | 376 |
| 3 | 101 | 96 | 99 | 1641 | 1290 | 1476 | 220 | 138 | 242 | 298 | 276 | 342 | 62 | 3 | 96 |
| 4 | 76 | 38 | 92 | 727 | 575 | 842 | 86 | 52 | 71 | 459 | 315 | 387 | 123 | 11 | 179 |
| 5 | 132 | 125 | 147 | 1190 | 1165 | 1327 | 147 | 128 | 193 | 167 | 3 | 5 | 104 | 6 | 158 |
| 6 | ND | ND | ND | 1320 | 981 | 1252 | 243 | 105 | 1272 | ND | ND | ND | ND | ND | ND |
| 7 | 94 | 75 | 81 | 1050 | 733 | 977 | 175 | 74 | 110 | 382 | 364 | 335 | 418 | 26 | 295 |
| 8 | 158 | 79 | 79 | 863 | 605 | 912 | 204 | 98 | 183 | 85 | 10 | 14 | 82 | 4 | 106 |
| 9 | 131 | 122 | 168 | 1267 | 880 | 1125 | 113 | 104 | 238 | ND | ND | ND | 259 | 6 | 185 |
| 10 | 62 | 40 | 122 | 1315 | 1171 | 1263 | 197 | 154 | 186 | 649 | 8 | 21 | 324 | 7 | 252 |
| 11 | 80 | 82 | 135 | 984 | 848 | 920 | 236 | 129 | 164 | 215 | 4 | 106 | 128 | 9 | 105 |
| 12 | 119 | 87 | 110 | 1100 | 836 | 1057 | 258 | 87 | 134 | ND | ND | ND | 189 | 8 | 176 |
| 13 | 133 | 101 | 128 | 1548 | 1316 | 1391 | 186 | 159 | 171 | 294 | 231 | 270 | 213 | 18 | 134 |
| 14 | 157 | 56 | 129 | 1491 | 1284 | 1342 | 264 | 235 | 270 | 452 | 2 | 6 | 238 | 7 | 296 |
| 15 | ND | ND | ND | 1050 | 825 | 936 | 129 | 104 | 163 | 298 | 263 | 312 | 56 | 5 | 72 |
| 16 | 125 | 96 | 109 | 1732 | 1362 | 1459 | 184 | 166 | 219 | 190 | 157 | 276 | 202 | 12 | 166 |
| 17 | 142 | 120 | 131 | 1256 | 954 | 1173 | 328 | 112 | 178 | 337 | 8 | 174 | 146 | 9 | 121 |
| 18 | 138 | 113 | 154 | 895 | 840 | 1066 | 182 | 176 | 244 | 848 | 536 | 901 | 78 | 6 | 105 |
| 19 | 110 | 91 | 127 | 1071 | 483 | 738 | 145 | 65 | 106 | 249 | 7 | 10 | 304 | 10 | 260 |
| 20 | ND | ND | ND | ND | 945 | 1053 | ND | 156 | 217 | 515 | 342 | 437 | 221 | 15 | 143 |
| 21 | 86 | 54 | 93 | 1420 | 1308 | 1395 | 233 | 186 | 225 | ND | ND | ND | ND | ND | ND |
| 22 | 145 | 130 | 163 | 820 | 673 | 744 | 171 | 128 | 206 | 271 | 180 | 239 | 95 | 8 | 67 |
| 23 | 169 | 120 | 151 | 1346 | 1158 | 1274 | 202 | 194 | 246 | 134 | 117 | 126 | 164 | 4 | 112 |
| 24 | 107 | 90 | 96 | 1189 | 976 | 1060 | 95 | 74 | 114 | 75 | 5 | 27 | 242 | 9 | 281 |
| 25 | ND | ND | ND | ND | 1264 | 1381 | ND | 146 | 177 | ND | ND | ND | 137 | 3 | 174 |
Normal values are: C3, 60-180 mg/dL; serum IgG, 800-1800 mg/dL; serum IgM, 60-280 mg/dL; PAIgG, 0-10 ng/106 platelets; (CD19+) B cells, 35-350 × 106/L.
ND indicates not done.
Median serum IgG, IgA, and IgM levels remained within normal limits throughout the study, although 6 patients had reductions of IgM, IgG, or both to subnormal levels. A nonsignificant decrease from baseline in the C3 component of serum complement was noted in 8 patients.
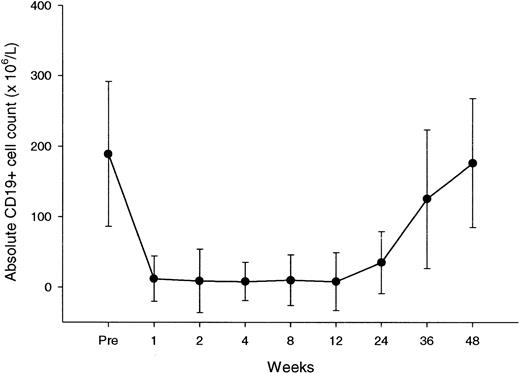
Peripheral blood B cells, evaluated by flow cytometry as CD19+ cells, had a median baseline count of 189 × 106/L (range, 56-418 × 106/L). This count declined with treatment, to subnormal levels after the first dose for the majority of patients (Figure 3). In all patients, we recorded nadir values of B cells below the normal range (Table 6). Recovery of B cells started between 6 and 9 months, reaching normal values between 9 and 12 months.
CD19+ lymphocyte counts over the course of rituximab treatment and during follow-up.
Each data point represents the mean (± SD) CD19+ cell count at a particular time.
CD19+ lymphocyte counts over the course of rituximab treatment and during follow-up.
Each data point represents the mean (± SD) CD19+ cell count at a particular time.
Median absolute T-cell counts in peripheral blood, using CD3, CD4, and CD8 as well as natural-killer-cell counts, remained stable during the study period.
Discussion
Rituximab was administered to 25 patients with chronic ITP over a 4-week period. All patients had ITP that had been resistant to between 2 and 5 different therapeutic regimens, including 8 patients who had already failed splenectomy. Ten patients (40%) had a PR or CR to treatment, and 3 (12%) had a clinical improvement classified as MR. In 7 patients (28%), responses had a duration of 6 months or longer. No clinical or laboratory parameter was found to predict treatment outcome.
The rituximab schedule employed in the current study has been borrowed from earlier trials in which this antibody was used to treat relapsed or refractory low-grade or follicular B-cell non-Hodgkin lymphomas.12-14 With this schedule, adverse events were mild and did not require discontinuation of treatment. Side effects occurred mainly during the first infusion, and typically included moderate and brief fever and chills. By the second and subsequent infusions, the majority of patients experienced no further infusion-related toxicities. Because this agent depletes normal B cells, it is also notable that the infectious episodes observed during the follow-up period had modest clinical relevance.
Apart from anecdotal reports, the only 2 other studies of rituximab therapy in chronic ITP with a significant number of patients have been published in abstract form. Perotta et al15 described 10 patients, of whom 6 had already failed splenectomy. Using the antibody with the same schedule as in the present study, they attained 1 PR and 5 CRs. Response duration ranged from 1 to more than 14 months. Saleh et al16 reported the preliminary results of a phase I/II dose-escalation trial. Objective responses were observed only in 3 of the 13 patients (23%) who received the highest dose level of 375 mg · m−2 · wk−1 for 4 weeks. In both reports, no severe adverse events were recorded.
The modality of response raises some issues about the mechanism of action of rituximab in chronic ITP. In responders, the rise in platelet counts is observed rapidly, usually 1 week after the first rituximab dose. This is unlikely to be due to a fall in circulating antiplatelet antibodies. In fact, only a minority of patients develop reductions of IgM and IgG below the normal range, seen 5 to 11 months after administration. On the other hand, our flow cytometry studies have confirmed previous data that a depletion of peripheral blood B cells occurs after just one antibody infusion.12-14 Thus, it is tempting to speculate that opsonized B cells can inhibit macrophage Fc-receptor function and clearance of IgG-coated platelets, a mechanism that has analogy with the proposed effect of reticuloendothelial blockade by anti-D immuglobulin.17 This effect eventually adds to the suppression of autoreactive B-cell clones that can account for the sustained remission observed in some patients. Unfortunately, we were not able to monitor patients for the development of human anti–chimeric antibodies (HACA) because this assay has not yet been made commercially available. However, from earlier experience in patients with non-Hodgkin lymphoma, it appears that less than 1.0% of patients evaluated for HACA were positive.14 18
Although monitoring of PAIgG did not allow a correlation with clinical response, the importance of the quantity of PAIgG in chronic ITP is controversial. Results are obviously dependent on the sensitivity and specificity of the assay19 and are complicated by the fact that antiplatelet antibodies are a pool of immunoglobulins including different isotypes with varying affinities.20 In this study, we used a “second-generation” assay, based on the detection of the total IgG content of lysed platelets, that is not able to discriminate antiplatelet antibodies from immune complexes that bind to platelets via the Fc receptor. Therefore, it is clear that this assay does not have a specific diagnostic value and should be used in conjunction with clinical findings and other test results. Another weak point is that this technique does not provide comparable results with respect to the amount of measured PAIgG.19
Rituximab therapy in our series gave results that compare favorably with those of standard secondary ITP agents such as vinca alkaloids, danazol, oral cyclophosphamide, and azathioprine. All of these agents are expected to have, at best, response rates in the range of 40% to 50%, but sustained-remission rates are usually well below 20%.2-5 Data in the literature suggest that intensive chemotherapy, particularly pulse cyclophosphamide, may produce better results by virtue of the suppression of lymphocyte numbers and function.21 22 In our series, however, 4 patients had received pulse cyclophosphamide with no response. Besides, 2 of these patients had a complete and sustained response with rituximab, indicating either different immunologic targets or a greater efficacy of the antibody in eliminating autoreactive clones.
Several patient characteristics were examined for an association with rituximab treatment outcome. Because of the small number of patients studied, none of the associations reached statistical significance. There is a suggestion, however, that women and younger patients respond better to rituximab. The biologic significance of these findings, if confirmed, remains to be determined.
In conclusion, our results indicate that rituximab therapy has a limited but valuable effect in patients with chronic ITP. In view of its mild toxicity and the lack of effective alternative treatments, it should be strongly considered in severely affected patients who do not respond to standard therapy. Some issues about this agent, such as the optimum dose and treatment schedule, the exact mechanisms of action, and long-term side effects, remain to be explored and warrant further investigation.
We acknowledge Roche Company for supplying, in part, the rituximab monoclonal antibody used in this study. We also thank Franco Nasella for bibliographic assistance.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Roberto Stasi, Department of Medical Sciences, Regina Apostolorum Hospital, Via S. Francesco, 50, 00041 Albano Laziale, Italy; e-mail: roberto.stasi@schering-plough.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal